Abstract
Accumulating evidences suggest that large-scale loss of 5-hydroxymethylcytosine (5-hmC) is an epigenetic hallmark in different cancers. However, the levels of 5-hmC in laryngeal squamous cell carcinoma (LSCC) and its prognostic value in this cancer remain largely unknown. Using dot-blot and quantitative RT-PCR assays, we investigate 5-hmC levels and expression of TET-1, -2 and -3 in LSCCs and explore the association of 5-hmC levels with clinicopathological characteristics and clinical outcome of LSCC patients. Our data showed that 5-hmC was significantly decreased in LSCCs as compared with matched normal tissues. We also found a strong link between decreased 5-hmC and the reduction of TET-1 gene expression, but not TET-2 or -3, suggesting that decreased TET-1 expression was implicated in 5-hmC loss in LSCC. Moreover, Mann-Whitney U tests showed that 5-hmC content was significantly associated with smoking (P = 0.039) and tumor invasion (P = 0.004). Importantly, we found that decreased 5-hmC was significantly associated with poor survival of early-stage LSCC patients (P = 0.043). Altogether, our findings implicate that decreased 5-hmC probaly caused by the reduction of TET-1 is crucial to the clinical pathology of LSCC and is a poor prognostic factor in ealry-stage LSCC patients.
Keywords: Laryngeal squamous cell carcinoma (LSCC), 5-hydroxymethylcytosine (5-hmC), TET-1, poor survival
Introduction
Head and neck squamous cell carcinoma is the most frequently cancer in the world [1]. Of which, laryngeal squamous cell carcinoma (LSCC) possess the second position [2]. Despite important advances in the understanding and management of LSCC, the 5-year survival rates have not increased over the last 20 years [3]. Thus, a better understanding of the pathogenesis and molecular mechanisms of LSCC may lead to new diagnostic, therapeutic and preventive strategies to this disease.
Epigenetics formally refers to heritable changes in gene expression and in phenotype occurring without changes in the underlying DNA sequence. DNA methylation is an extensively characterized mechanism for epigenetic regulation and plays an important role in the regulation of gene expression. It occurs at the cytosine residues of CpG dinucleotides by an enzymatic reaction that produces 5-methycytosine (5-mC), which is catalyzed by DNA methyltransferases (DNMTs) [4]. Aberrant DNA methylation at the gene promotor is a well-known epigenetic feature of cancer cells [5]. The modifications impact upon gene expression and promote tumourigenesis predominantly by silencing of tumour-suppressor and/or apoptotic pathway genes [6]. Promoter methylation of tumor-suppressor genes has been widely studied in human cancers including LSCC [7]. Thus, enzymes of with opposing function protect cells from becoming malignant is much concerned in recent years.
Increasing evidences have demonstrated that ten-eleven translocation (TET) family of 5-mC hydroxylases may reverse DNA methylation in mammalian cells [8-10]. TET family comprise TET-1, -2 and -3, which progressively oxidizes 5-mC to afford 5-hydroxymethylcytosine (5-hmC) [8]. The exciting discovery of 5-hmC sheds light on the dynamic nature of 5-mC, and emerging evidence has shown that TET family proteins and 5-hmC play critical roles in normal development as well as in many diseases, which adds another dimension of complexity to our understanding of DNA methylation [11]. It will be potentially helpful to investigate the roles of TET and 5-hmC contributing to tumorigenesis, as the global DNA hypomethylation and locus-specific DNA hypermethylation have been identified as vital proterties of many cancers [12,13]. Although previous studies have showed that loss of TET and 5-hmC are present in a broad spectrum of solid tumors, such as gastric carcinoma, hepatocellular carcinoma, colon cancer and hematopoietic malignancy [14-17], the role of TETs and 5-hmC in diagnostic and prognostic evaluation in LSCC remains elusive.
In this study, we used quantitative dot-blot assay to determine 5-hmC levels in LSCCs and explore its association with clinicopathological characteristics and clinical outcome of LSCC patients. Our data showed that 5-hmC was significantly decreased in LSCCs compared with matched normal tissues. Importantly, decreased 5-hmC was associated with poor prognosis of early-stage LSCC patients.
Materials and methods
Patients and tissue samples
With the approval of our institutional review board and human ethics committee, a total of 123 paraffin-embedded LSCC tissues were randomly obtained from the First Affiliated Hospital of Xi’an Jiaotong University. None of these patients received chemotherapy and radiotherapy and informed consent was obtained from each patient prior to surgery. All samples were histologically examined by a senior pathologist in the Department of Pathology of the Hospital based on the World Health Organization (WHO) criteria. Clinicopathological data were obtained from the patients’ files or by telephone interviews with the patients or their relatives, and were summarized in Table 1.
Table 1.
Association of 5-hmC levels with clinicopathological factors of LSCC patients (75th interquartile as a cutoff point)
| Variables | Cases (n = 114) | Low 5-hmC | High 5-hmC | P |
|---|---|---|---|---|
| Age, years | 0.997 | |||
| ≤ 60 | 59 | 44 | 15 | |
| > 60 | 55 | 41 | 14 | |
| Gender | 0.144 | |||
| Male | 108 | 79 | 29 | |
| Female | 6 | 6 | 0 | |
| Smoking | 0.039 | |||
| No | 23 | 21 | 2 | |
| Yes | 91 | 64 | 27 | |
| TNM stage | 0.602 | |||
| I/II | 44 | 34 | 10 | |
| III/IV | 70 | 51 | 19 | |
| Tumor invasion | 0.004 | |||
| T1/T2 | 65 | 55 | 10 | |
| T3/T4 | 49 | 30 | 19 | |
| LNM | 0.388 | |||
| N | 75 | 54 | 21 | |
| Y | 39 | 31 | 8 | |
| Tumor grade | 0.718 | |||
| 1 | 18 | 15 | 3 | |
| 2 | 82 | 57 | 25 | |
| 3 | 14 | 13 | 1 | |
| Survival status | 0.452 | |||
| Alive | 38 | 30 | 8 | |
| Dead | 76 | 55 | 21 |
DNA preparation and dot blot assay
Genomic DNA was extracted from frozen surgical tissues using standard phenol/chloroform protocol. For paraffin-embedded sections, the protocol of DNA extraction was as described previously [18]. Briefly, tissues were digested with 1% sodium dodecyl sulfate (SDS) and 0.5 mg/ml proteinase K at 48°C for 48 h after a treatment for overnight at room temperature with xylene to remove paraffin. DNA was then isolated using phenol/chloroform method, and was dissolved in distilled water and stored at -80°C until use. The dot-blot assay was performed as described previously [14]. In brief, genomic DNA was prepared in 2×SSC buffer and denatured for 10 min at 95°C. Samples were rapidly chilled for 5 min and then spotted on a positive charged nylon membrane (Roche Diagnostics, Mannheim, Germany). The membrane was washed twice in 2×SSC buffer, UV crosslink and dried for 1 h at 70°C, and then blocked with 5% casein buffer and incubated with anti-5-hmC antibody (Active Motif, Cat. 39769, dilution at 1:10000) at 4°C overnight. After incubation with species-specific HRP-conjugated secondary antibody (ZSGB-BIO, Cat. ZB-23012, dilution at 1:5000), dot signal was visualized with the Western Bright ECL detection system (Advansta, CA) by exposing to X-ray film. To ensure equal spotting of total DNA on the membrane, the same blot was stained with 0.02% methylene blue in 0.3 M sodium acetate (pH 5.2). The 5-hmC intensity was quantified using ImageJ image processing and analysis software (ImageJ version 1.44p, NIH, MD).
Reverse transcription quantitative PCR (RT-qPCR)
Total RNA was extracted from frozen surgical tissues using TRIzol (Takara Inc., Dalian, P. R. China) according to the protocols supplied by the manufacturers. Complementary DNA (cDNA) was synthesized using a PrimeScript 1st strand cDNA Synthesis Kit (Takara Inc., Dalian, P. R. China) according to the instructions of the manufacturer. RT-qPCR was then performed to evaluate the expression of TET-1, -2 and -3 on a CFX96 Thermal Cycler DiceTM real-time PCR system (Bio-Rad Laboratories, Inc., CA), using SYBR Premix ExTaq II (Takara Inc., Dalian, P. R. China) with 18S rRNA cDNA as endogenous control. Each sample was run in triplicate. The primer sequences were presented in Table 2.
Table 2.
qRT-PCR primers used in the present study
| Genes | Forward primer (5’→3’) | Reverse primer (5’→3’) |
|---|---|---|
| TET1 | TCTTCCCCATGACCACATCT | GAGGGAAAAGAAGCCCAAAG |
| TET2 | ACGCTTGGAAGCAGGAGAT | CACAAGGCTGCCCTCTAGTT |
| TET3 | CCCACAAGGACCAGCATAAC | CCATCTTGTACAGGGGGAGA |
| 18S | CGCCGCTAGAGGTGAAATTC | CTTTCGCTCTGGTCCGTCTT |
Statistical analysis
Linear regression analysis was used to test the possible association of 5-hmC intensity with the expression of TET-1, -2 and -3 using GraphPad Prism (GraphPad Prism version 5.01, SD). In this study, all samples were compared using two-tailed Mann-Whitney U test. To divide the patients into high and low 5-hmC groups, we defined the 75th percentile of 5-hmC quantities from 114 LSCC DNA samples as the cut-off point. Association of 5-hmC levels with clinicopathological characteristics of tumors were assessed univariately using the SPSS statistical package (version 11.5, Chicago, IL, USA). Multivariate models were then developed that adjusted for the most important covariates, including age, tumor stage, lymph node metastasis and survival status. Survival length was determined from the day of primary tumor surgery to the day of death or last clinical follow-up. The survival analysis grouping with 5-hmC levels were performed by using the Kaplan-Meier curves. the log-rank test were used to analyze differences between curves. All statistical analyses were performed using the SPSS statistical package (11.5, Chicago, IL, USA). A P < 0.05 was considered to be statistically significant.
Results
5-hmC is decreased in LSCCs
Given that 5-hmC is an antigen present in genomic DNA, we used dot blot assay to assess 5-hmC levels in genome DNA in LSCCs and matched normal tissues in the present study. To compare 5-hmC levels between LSCCs and matched normal tissues, 250 ng, 100 ng and 50 ng of genome DNAs from each sample were spotted on the same membrane, respectively. 5-hmC levels were strikingly decreased in LSCCs as compared with matched normal tissues when 100 ng and 50 ng of DNA were used (Figure 1A, left panel). Unlike previous studies in breast, lung and liver cancers [19], it was more sensitive for detecting 5-hmC in LSCCs when lower amount of DNA was used, such as 100 ng and 50 ng. Our data demonstrated that 5-hmC was significantly decreased in LSCCs as compared with matched normal tissues when 100 ng of DNA was used (Figure 1B, right panel).
Figure 1.
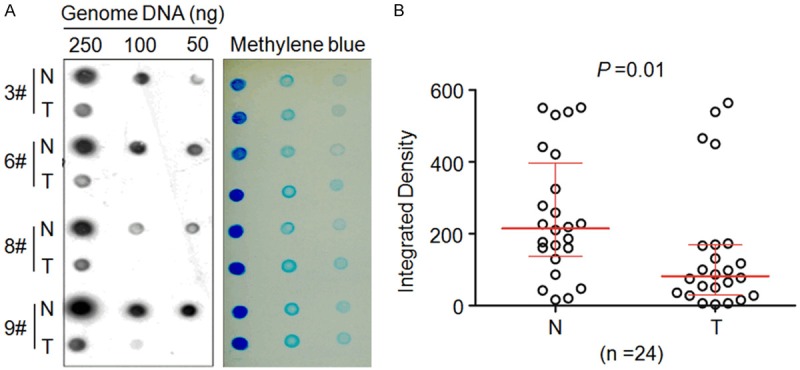
Decreased 5-hmC levels in LSCCs. Dot-blot assay showed significantly lower 5-hmC levels in LSCCs (T) than matched normal tissues (N). Dot-blot images of four representative LSCC patients were shown here from 24 pairs of cases (left panel). Meanwhile, equal amounts of genomic DNA were verified by methylene blue staining of the blot. The imageJ software was used to quantify the 5-hmC signal intensities of DNA samples (250 ng/each) obtained from 24 pairs of cases, and data were presented as median ± interquartile (right panel).
Decreased 5-hmC content is closely associated with the reduction of TET1 gene expression in LSCCs
Given the TET family of 5-mC DNA hydroxylases are directly responsible for the generation of 5-hmC, we investigated the expression of TET genes and their impact on 5-hmC levels in LSCCs. As shown in Figure 2A, we found that TET-1 expression was significantly reduced in LSCCs compared with matched normal tissues. However, TET-2 expression was not different between LSCCs and matched normal tissues. Conversely, we found that TET-3 was significantly up-expressed in LSCCs compared with matched normal tissues. Notably, a significantly positive correlation was found between TET-1 expression and 5-hmC levels in LSCCs (P = 0.0006, R = 0.502; Pearson’s correlation coefficient). However, there was no correlation between the expression of TET-2 (P = 0.329, R = 0.144; Pearson’s correlation coefficient) or TET-3 (P = 0.115, R = 0.231; Pearson’s correlation coefficient) and 5-hmC levels in LSCCs (Figures 2B and 3). These observations provide a potential molecular mechanism, which decreased 5-hmC is mainly caused by the reduction of TET-1 gene expression in LSCCs.
Figure 2.
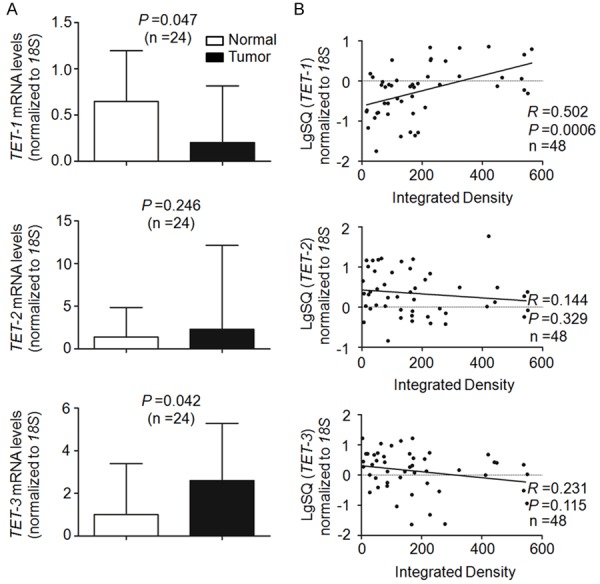
Association of 5-hmC levels with the expression of TET genes. A. qRT-PCR assay was performed to assess the expression of TET-1, -2 and -3 in LSCCs and match normal tissues. Expression levels of these genes were normalized with 18S rRNA levels. Data were presented as median ± interquartile. B. Linear regression analysis was performed to assess the correlations between 5-hmC levels and mRNA expression of TET-1, -2 and -3 in 24 pairs of LSCC and matched normal tissues. 18S rRNA was used as a normalized control.
Figure 3.
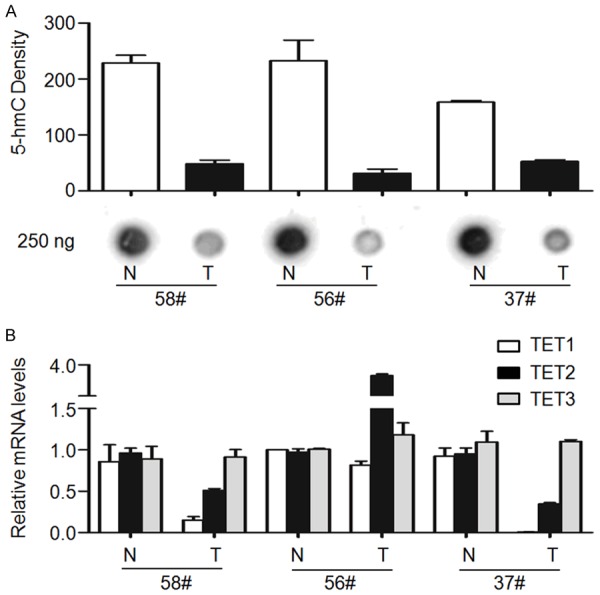
Association of loss of 5-hmC with the reduction of TET-1 expression. Dot-blot and qRT-PCR assays were used to examine 5-hmC levels and the expression of TET genes, respectively. A. Dot-blot assay showed that 5-hmC was significantly decreased in LSCCs (T) compared with matched normal tissues (N). The images shown are representative of three pairs of tumor and matched normal tissues. B. qRT-PCR assay was performed to the expression of TET-1, -2 and -3 in these samples. 18S rRNA was used as an endogenous control. Data were presented as mean ± SD.
Association of 5-hmC levels with clinicopathological characteristics in LSCC
To determine 5-hmC levels in clinical specimens, serial dilutions of genomic DNA extracted from a normal laryngeal tissue, in which a high level of 5-hmC was detected, were subjected to dot-blot assay for establishing a calibration curve. As shown in Figure 4A, non-linear regression indicated broad dynamic range and high goodness of fit (R = 0.98), suggesting that, under optimal experimental condition, this assay can be applied for quantification of 5-hmC in clinical samples. 5-hmC levels of a total of 123 LSCC patients were quantified using calibration curve, ranging from 178 to 19901 (median, 5388) units (Figure 4B). According to the value of 75th percentile (10029 units), we divided the patients into high and low 5-hmC groups.
Figure 4.
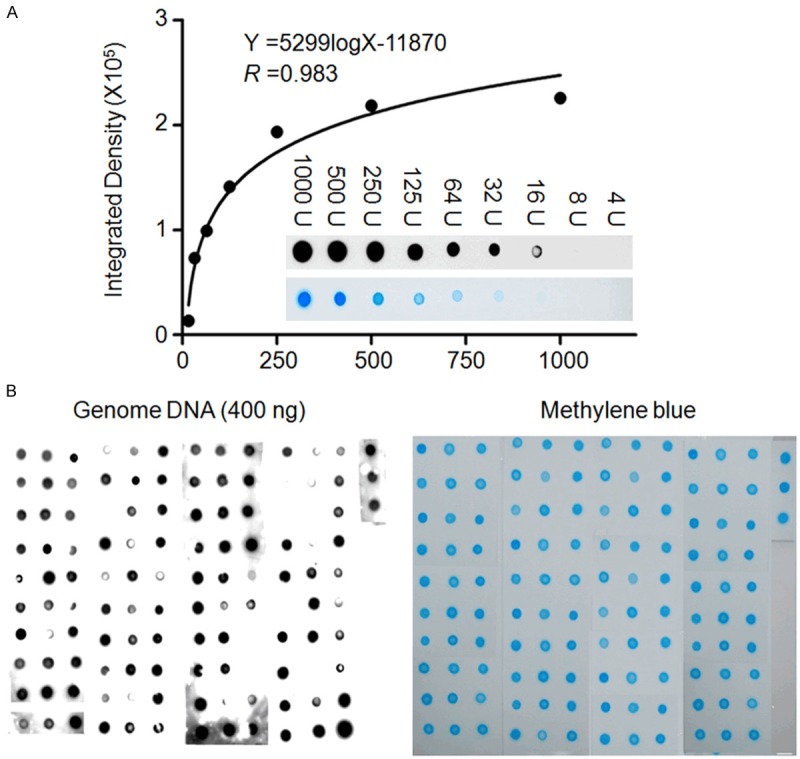
Quantification of genomic 5-hmC in LSCCs. A. Gradient-diluted genomic DNAs including 1000, 500, 250, 125, 64, 32, 16, 8 and 4 units (U) from a normal laryngeal tissue with high 5-hmC levels were subjected to dot-blot to establish a calibration curve. A high goodness of fit (R = 0.983) between signal densities and 5-hmC relative quantities in a sufficient dynamic range supported the usefulness of this method to quantify 5-hmC in genomic DNA. Insert was dot-blot images from a serial dilution of genomic DNAs (upper, dot-blot images of 5-hmC; lower, images of methylene blue staining). B. Genomic DNAs (400 ng/each sample) from a total of 123 LSCCs were spotted randomly on the two positive charged nylon membranes. One was incubated with anti-5-hmC antibody for dot-blot of 5-hmC (left panel). Another was stained with methylene blue as loading control (right panel). Details are described in the Materials and Methods.
We next investigated the correlation of 5-hmC levels with clinicopathological characteristics, including age, gender, smoking, TNM stage, tumor invasion, lymph node metastasis and survival status, in a cohort of 114 LSCCs with complete follow-up data. As shown in Table 1, we found a significantly negative association of 5-hmC levels with smoking (P = 0.039) and tumor invasion (P = 0.004).
Association of 5-hmC levels with poor survival of early-stage LSCC patients
In order to identify whether decreased 5-hmC is associated with poor prognosis of LSCC patients, the association of 5-hmC content with clinicopathological factors of LSCC patients was subsequently investigated in unvariate survival analysis. Similarly, the 75 percentile of 5-hmC levels was used as a reference in this study. As shown in Table 3, 5-hmC levels did not affect overall survival of LSCC patients. Next, we used Kaplan-Meier survival curves to further determine the effect of 5-hmC on the survival of LSCC patients. Similar to the findings in Table 3, 5-hmC levels did not still affect median survival times of LSCC patients (101 months vs. 106 months, P = 0.787) (Figure 5A). Cox multivariate repression showed that 5-hmC levels (HR = 0.953, 95% CI = 0.574-1.582, P = 0.853) is not a predictor of poor survival for LSCC patients as an independently variable with respect to age, TNM stage, lymph node metastasis (Table 3).
Table 3.
Prognostic value of clinicopathological factors and 5-hmC content in univariate and multivariate Cox regression analysis (n = 114)
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | |
| Age, years | ||||
| ≤ 60 | 1.00 (reference) | 1.00 (reference) | ||
| > 60 | 1.309 (0.825-2.076) | 0.253 | 1.133 (0.682-1.884) | 0.630 |
| TNM stage | ||||
| I/II | 1.00 (reference) | 1.00 (reference) | ||
| III/IV | 1.593 (0.946-2.682) | 0.080 | 1.437 (0.802-2.575) | 0.223 |
| LNM | ||||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 1.349 (0.836-2.177) | 0.219 | 1.259 (0.775-2.046) | 0.352 |
| 5-hmC | ||||
| High | 1.00 (reference) | 1.00 (reference) | ||
| Low | 0.936 (0.563-1.555) | 0.798 | 0.953 (0.574-1.582) | 0.853 |
Figure 5.
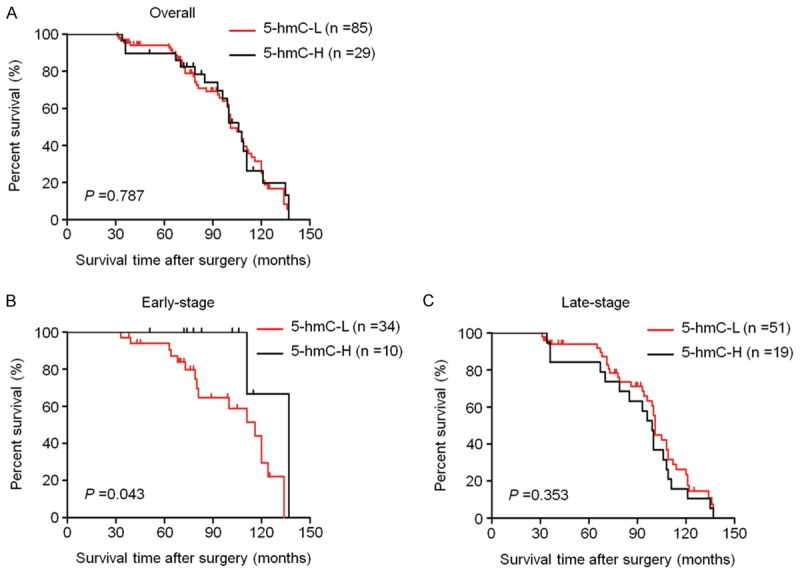
The effect of 5-hmC levels on poor survival of LSCC patients. Kaplan-Meier analysis of survival was performed according to 5-hmC levels in a cohort of LSCCs. (A) Kaplan-Meier survival curves showed that 5-hmC content was not associated with overall survival of the patients. When the data were stratified based on the TNM tumor stage, decreased 5-hmC was strongly associated with poor survival in the patients who had early-stage tumors (B). However, no significant difference was seen in late-stage LSCC patients (C).
Next, the data were stratified further based on the TNM stage. In the early-stage LSCC patients, 5-hmC content was significantly associated with patient survival. The patients with low 5-hmC content had significantly shorter median survival times than those with high 5-hmC content (116 months vs. 137 months; P = 0.043) (Figure 5B). However, 5-hmC content was not associated with poor survival in the patients who had late-stage tumors (Figure 5C). Altogether, these data suggest that decreased 5-hmC content may play a role in early tumorigenesis of LSCC.
Discussion
Expanded knowledge of epigenetic events occurring in cancers has improved our understanding of carcinogenesis. Methylation of cytosine residues in CpG islands of promoter regions leads to epigenetic gene silencing of gene [20,21]. In recent years, the discovery of oxidation of 5-mC to 5-hmC by enzymes of TET family in mammalian genomes has raised many questions regarding the role of this type of DNA modification in epigenetic regulation during human carcinogenesis. The balance of DNA methylation and demethylation in epigenetic modification has become a hot topic in cancer research. It has been revealed that loss of function mutations of TET2 with decreased 5-hmC occur in various myeloid leukemias [22,23]. Meanwhile, TET dysfunction and decreased 5-hmC have been also reported in various solid tumors [14,19,24]. Notably, several recent studies has revealed that loss of 5-hmC may be served as a poor prognostic marker in gastric cancer, hepatocellular carcinoma and breast cancer [14,15,25]. However, the levels of TET genes and 5-hmC and prognosis value of 5-hmC in LSCC patients remain totally unknown.
In this study, we investigated 5-hmC levels in a cohort of LSCCs by using dot-blot assays. Our data showed 5-hmC was significantly decreased in LSCCs as compared with matched normal tissues, which was consistent with the findings in other solid tumors [14,15]. It has been well documented that 5-mC is converted to 5-hmC by the enzymes of TET family [8,26]. The previous studies have identified that all three TET genes were uniformly reduced in malignant tissues such as melanoma, breast and liver cancers as compared with normal tissues, which may explain in part the loss of 5-hmC in these cancers [19,24,27]. To further investigate the possible mechanism underlying 5-hmC loss in LSCCs, we determined the mRNA expression of three TET genes in LSCCs and matched normal tissues. Surprisingly, unlike the findings in other tumors such as melanoma, breast and liver cancers, our data showed that only TET-1 expression was significantly decreased in LSCCs compared to matched normal tissues. In contrast, TET-3 expression was significantly increased in LSCCs compared with matched normal tissues. TET-2 expression was not different between LSCCs and control subjects. This was supported by the findings in gastric cancer [14]. Thus, we speculate that loss of 5-hmC in LSCCs is caused, at least in part by the decreased expression of TET-1, as supported by our findings that there was a significantly positive correlation between TET-1 expression and 5-hmC levels, but not TET-2 or -3.
The hydroxymethylase TET-1 catalyzing 5 mC to 5 hmC plays a critical role in early embryo preimplantation development, somatic cell reprogramming, ESCs self-renewal and tumorigenesis [19,28-30]. In addition, a previous study and the present study showed that TET-1 mRNA level was enriched in many human tissues such as prostate, breast, ovary, lung, liver, colon and gastric tissues [14,31]. Thus, loss of TET-1 may also contribute to the development of tumors derived from these TET-1-rich tissues including laryngeal. This hypothesis is supported by a recent study that TET-1 is substantially down-expressed in gastric cancers and ectopic expression of TET-1 is associated with an increase in 5-hmC and reduced gastric carcinogenesis [32]. Similarly, in the present study, we also found loss of TET-1 expression and 5-hmC in LSCCs. Thus, it is plausible to speculate that loss of TET-1-mediated carcinogenesis suppression is most likely universal among human cancers including LSCC, which need to be defined in further studies.
To explore the association of 5-hmC levels with clinicopathological characteristics and clinical outcome of LSCC patients, we categorized the patients into high and low 5-hmC groups based on 75th percentile of 5-hmC levels. Our findings showed that decreased 5-hmC was significantly associated with smoking and tumor invasion particularly the latter, suggesting that decreased 5-hmC may contribute to the progression of LSCC. However, there was no significant association of 5-hmC content with patient survival. It is noteworthy that loss of 5-hmC is found in each of tumor stage and decreased 5-hmC content affects the overall prognosis in LSCC patients with early-stage tumors, suggesting that it is likely an early event in laryngeal tumorigenesis, and plays a role in the initiation of this cancer. In brief, our study provides strong evidences to support that genome-wide loss of 5-hmC is an epigenetic hallmark in LSCC.
In summary, our data showed that TET-1 expression and 5-hmC was decreased in LSCCs, and indicated that loss of 5-hmC was probably caused by the reduction of TET-1 expression. To our knowledge, the present study is the first to demonstrate the association of decreased 5-hmC levels with poor survival of early-stage of LSCC patients. Collectively, loss of TET-1 and 5-hmC is a fundamental epigenetic event in laryngeal tumorigenesis, and genes affecting the generation of 5-hmC such as TET-1 may be therapeutically targeted to restore 5-hmC in LSCC, thus revealing a new strategy for the treatment of this cancer.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81402340 and 81472622), and the Fundamental Research Funds for the Central Universities.
Disclosure of conflict of interest
None.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Fleming JL, Huang TH, Toland AE. The role of parental and grandparental epigenetic alterations in familial cancer risk. Cancer Res. 2008;68:9116–21. doi: 10.1158/0008-5472.CAN-08-2184. [DOI] [PubMed] [Google Scholar]
- 6.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 7.López F, Sampedro T, Llorente JL, Domínguez F, Hermsen M, Suárez C, Alvarez-Marcos C. Utility of MS-MLPA in DNA methylation profiling in primary laryngeal squamous cell carcinoma. Oral Oncol. 2014;50:291–7. doi: 10.1016/j.oraloncology.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Wu F, Tan L, Kong L, Xiong L, Deng J, Barbera AJ, Zheng L, Zhang H, Huang S, Min J, Nicholson T, Chen T, Xu G, Shi Y, Zhang K, Shi YG. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol Cell. 2011;42:451–64. doi: 10.1016/j.molcel.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao H, Chen T. Tet family of 5-methylcytosine dioxygenases in mammalian development. J Hum Genet. 2013;58:421–7. doi: 10.1038/jhg.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development. 2012;139:1895–902. doi: 10.1242/dev.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–76. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 13.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Wu K, Ji M, Jin W, He N, Shi B, Hou P. Decreased 5-hydroxymethylcytosine (5-hmC) is an independent poor prognostic factor in gastric cancer patients. J Biomed Nanotechnol. 2013;9:1607–16. doi: 10.1166/jbn.2013.1713. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Liu L, Chen X, Shen J, Shan J, Xu Y, Yang Z, Wu L, Xia F, Bie P, Cui Y, Bian XW, Qian C. Decrease of 5-hydroxymethylcytosine is associated with progression of hepatocellular carcinoma through downregulation of TET1. PLoS One. 2013;8:e62828. doi: 10.1371/journal.pone.0062828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman CG, Mariani CJ, Wu F, Meckel K, Butun F, Chuang A, Madzo J, Bissonette MB, Kwon JH, Godley LA. TET-catalyzed 5-hydroxymethylcytosine regulates gene expression in differentiating colonocytes and colon cancer. Sci Rep. 2015;5:17568. doi: 10.1038/srep17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cimmino L, Dawlaty MM, Ndiaye-Lobry D, Yap YS, Bakogianni S, Yu Y, Bhattacharyya S, Shaknovich R, Geng H, Lobry C, Mullenders J, King B, Trimarchi T, Aranda-Orgilles B, Liu C, Shen S, Verma AK, Jaenisch R, Aifantis I. TET1 is a tumor suppressor of hematopoietic malignancy. Nat Immunol. 2015;16:653–62. doi: 10.1038/ni.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi J, Yao D, Liu W, Wang N, Lv H, Zhang G, Ji M, Xu L, He N, Shi B, Hou P. Highly frequent PIK3CA amplification is associated with poor prognosis in gastric cancer. BMC Cancer. 2012;12:50. doi: 10.1186/1471-2407-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Liu Y, Bai F, Zhang JY, Ma SH, Liu J, Xu ZD, Zhu HG, Ling ZQ, Ye D, Guan KL, Xiong Y. Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation. Oncogene. 2013;32:663–9. doi: 10.1038/onc.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53–65. doi: 10.1016/j.cca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Ji M, Guan H, Gao C, Shi B, Hou P. Highly frequent promoter methylation and PIK3CA amplification in non-small cell lung cancer (NSCLC) BMC Cancer. 2011;11:147. doi: 10.1186/1471-2407-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, Kosmider O, Le Couedic JP, Robert F, Alberdi A, Lecluse Y, Plo I, Dreyfus FJ, Marzac C, Casadevall N, Lacombe C, Romana SP, Dessen P, Soulier J, Viguie F, Fontenay M, Vainchenker W, Bernard OA. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360:2289–301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 23.Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS, An J, Lamperti ED, Koh KP, Ganetzky R, Liu XS, Aravind L, Agarwal S, Maciejewski JP, Rao A. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–43. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudo Y, Tateishi K, Yamamoto K, Yamamoto S, Asaoka Y, Ijichi H, Nagae G, Yoshida H, Aburatani H, Koike K. Loss of 5-hydroxymethylcytosine is accompanied with malignant cellular transformation. Cancer Sci. 2012;103:670–76. doi: 10.1111/j.1349-7006.2012.02213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai KW, Li GC, Chen CH, Yeh MH, Huang JS, Tseng HH, Fu TY, Liou HH, Pan HW, Huang SF, Chen CC, Chang HY, Ger LP, Chang HT. Reduction of global 5-hydroxymethylcytosine is a poor prognostic factor in breast cancer patients, especially for an ER/PR-negative subtype. Breast Cancer Res Treat. 2015;153:219–34. doi: 10.1007/s10549-015-3525-x. [DOI] [PubMed] [Google Scholar]
- 26.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–30. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lian CG, Xu Y, Ceol C, Wu F, Larson A, Dresser K, Xu W, Tan L, Hu Y, Zhan Q, Lee CW, Hu D, Lian BQ, Kleffel S, Yang Y, Neiswender J, Khorasani AJ, Fang R, Lezcano C, Duncan LM, Scolyer RA, Thompson JF, Kakavand H, Houvras Y, Zon LI, Mihm MC Jr, Kaiser UB, Schatton T, Woda BA, Murphy GF, Shi YG. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150:1135–46. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–33. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, Jiao Y, Li C, Liu S, Wu D, Gu W, Yang YG, Xu GL, Wang H. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc. 2013;135:10396–403. doi: 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- 30.Cimmino L, Abdel-Wahab O, Levine RL, Aifantis I. TET family proteins and their role in stem cell differentiation and transformation. Cell Stem Cell. 2011;9:193–204. doi: 10.1016/j.stem.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu CH, Peng KL, Kang ML, Chen YR, Yang YC, Tsai CH, Chu CS, Jeng YM, Chen YT, Lin FM, Huang HD, Lu YY, Teng YC, Lin ST, Lin RK, Tang FM, Lee SB, Hsu HM, Yu JC, Hsiao PW, Juan LJ. TET1 suppresses cancer invasion by activating the tissue inhibitors of metalloproteinases. Cell Rep. 2012;2:568–79. doi: 10.1016/j.celrep.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Park JL, Kim HJ, Seo EH, Kwon OH, Lim B, Kim M, Kim SY, Song KS, Kang GH, Kim HJ, Choi BY, Kim YS. Decrease of 5hmC in gastric cancers is associated with TET1 silencing due to with DNA methylation and bivalent histone marks at TET1 CpG island 3’-shore. Oncotarget. 2015;6:37647–62. doi: 10.18632/oncotarget.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]


