Abstract
Despite striking insights on lung cancer progression, and cutting-edge therapeutic approaches the survival of patients with lung cancer, remains poor. In recent years, targeted gene therapy with nanoparticles is one of the most rapidly evolving and extensive areas of research for lung cancer. The major goal of targeted gene therapy is to bring forward a safe and efficient treatment to cancer patients via specifically targeting and deterring cancer cells in the body. To achieve high therapeutic efficacy of gene delivery, various carriers have been engineered and developed to provide protection to the genetic materials and efficient delivery to targeted cancer cells. Nanoparticles play an important role in the area of drug delivery and have been widely applied in cancer treatments for the purposes of controlled release and cancer cell targeting. Nanoparticles composed of artificial polymers, proteins, polysaccharides and lipids have been developed for the delivery of therapeutic deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) sequences to target cancer. In addition, the effectiveness of cancer targeting has been enhanced by surface modification or conjugation with biomolecules on the surface of nanoparticles. In this review article we provide an overview on the latest developments in nanoparticle-based targeted gene therapy for lung cancers. Firstly, we outline the conventional therapies and discuss strategies for targeted gene therapy using nanoparticles. Secondly, we provide the most representative and recent researches in lung cancers including malignant pleural mesothelioma, mainly focusing on the application of Polymeric, Lipid-based, and Metal-based nanoparticles. Finally, we discuss current achievements and future challenges.
Keywords: Lung cancer, nanoparticles, targeted gene therapy, malignant mesothelioma, EphrinA1, EphA2
Introduction
In the last five years, an estimated 1.6 million new cases and almost 600,000 deaths from all cancer were reported in the United States [4]. In 2012 alone about 14 million new cases and 8.2 million cancer related deaths were reported by World Health Organization Worldwide. Among all cancers, lung cancer is the leading cause of cancer-related deaths worldwide. The number of new cases is the second highest in all cancers only behind prostate and breast cancers. Generally lung cancer is mainly classified as small cell (13%) and non-small cell (87%) carcinomas for their different histology and purposes of treatment. Due to the lack of effective therapeutic methods, the 5-year survival rate in all stages of non-small cell lung cancer (NSCLC) patients has been remained below 20% and this high rate of mortality has not been significantly improved over the years. In addition to small and NSCLC, malignant pleural mesothelioma (MPM) is a rare form of lethal cancer that develops on the protective linings of the lungs. The MPM currently has a relatively low occurrence rate in the United States, which is around 3,000 new cases are being diagnosed each year, however, the 5-year survival rate of MPM is lower than 10% and there is currently no cure for mesothelioma reported. The global incidence of MPM, especially in the developing countries, is predicted to increase in the following years.
Conventional treatments for lung cancers include surgical resection, chemotherapy, radiation therapy and targeted drug therapy. Although surgical resection might still be considered the most effective therapeutic strategy to cure NSCLC, sometimes the operability of surgery is limited. Thus expanding the applicability of surgical resection or applying systemic therapy become critical to improve the survival rate of patients. For the non-surgical treatment, targeted drugs such as afatinib (Gilotrif), ramucirumab (Cyramza), and bevacizumab (Avastin), have provided a new treatment option for lung cancer patients due to their lower adverse effects and promising therapeutic potential to inhibit tumors and metastasis [5-7]. The afatinib, which has been approved by FDA in 2013, targets on the epidermal growth factor receptor (EGFR) and erbB-2 via irreversible covalent inhibition to inhibit tumor growth and metastasis in EGFR mutant positive NCSLC patients [8,9]. The ramucirumab, approved by FDA in 2014, is a monoclonal antibody which acts as an angiogenesis inhibitor through binding the vascular endothelial growth factor receptor 2 (VEGFR2) [6]. Though conventional therapies are applied there is a potential risk of recurrence and side effects. Hence there is an urgent need to develop a novel state-of-the-art approach towards this fatal cancer.
Gene therapy in lung cancer treatment
As the researchers have learned more about the genetic pathways involved in cancer development and progression, these genetic events in the complex process during cancer occurrence, such as activation of dominant oncogenes and inactivation of tumor suppressor genes, may present as novel targets for effective cancer treatments [10-12]. Gene therapy involves using oligonucleotides to specifically target and regulate the abnormal genetic expressions which are related to cancer development in cancer cells. Compared to the conventional therapeutic approaches, gene therapy is generally expected to provide higher efficiency on cancer treatments and minimize the systematic cytotoxicity in cancer patients.
Most strategies proposed for lung cancer gene therapy requires in vivo gene delivery. In most of the cases, due to their negative charge, the gene molecules such as DNA and RNA biopolymers lack the ability to penetrate the cell membrane and effectively enter into the nucleus. Thus various kinds of vectors and vehicles have been developed to deliver the genetic materials into target cells which showed limited success. With the progression of nanotechnology, nanoparticles composed of various materials have been widely applied in the field of gene delivery for lung cancer treatment, as listed in Table 1. The nanoparticles not only provide protection to the encapsulated nucleotides but also improve the uptake by target cells. In the past decades, nanoparticles with different properties and functionalities have been designed and developed to efficiently deliver therapeutic gene molecules into the target cells directly or via the circulatory system.
Table 1.
Nanoparticles applied in gene therapy for lung cancers
|
Therapeutic strategies using nanoparticles
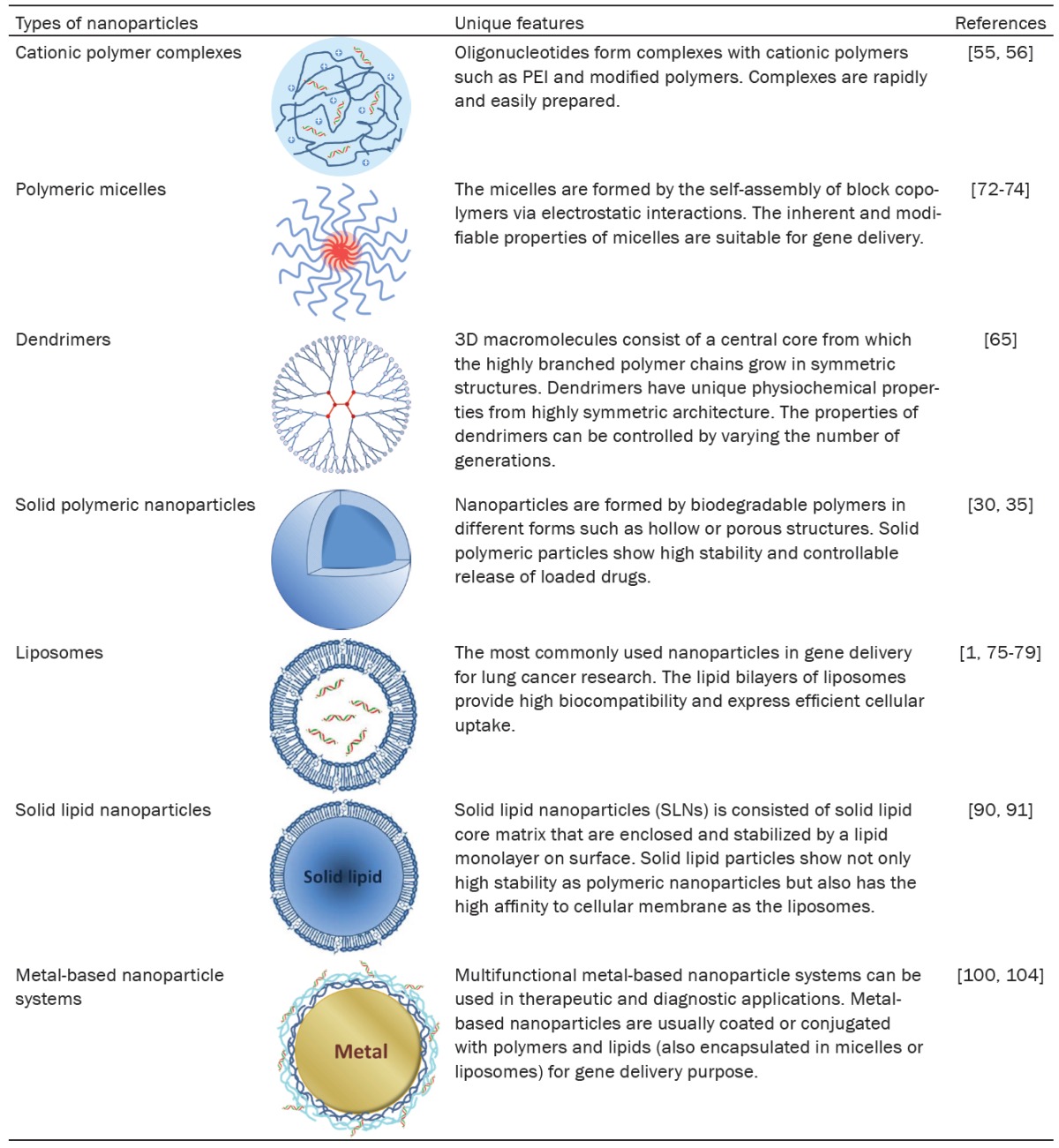
The lung cancer targeting strategies applying particulate delivery systems can be mainly classified into two different strategies: passive targeting and active targeting, as shown in Figure 1. In 1986, a new concept called enhanced permeation and retention (EPR) effect has been brought up by Matsumura and Maeda [13,14]. The EPR effect is the basis of passive cancer targeting and which has been widely applied in numerous drug delivery systems for cancer targeting since then. In this strategy, the cancer targeting drug delivery system benefits from the EPR effect in which the newly and fast growing tumor tissues and vessels interrupt the integrity of normal tissue and vessel wall and result in higher local permeability and retention of delivered drug loaded particles. The passive cancer targeting benefits from defective vasculature, fenestrations and poor lymphatic drainage caused by angiogenesis in tumor sites. The accumulation level of drug loaded particles in the tumor tissues may be influenced by the properties and characteristics of the particulate carriers, such as the materials, particle size and surface charge [15,16]. In general, compared to the cationic nanoparticles, the neutral or negatively charged nanoparticles have higher stability against the enzymatic attacks and longer circulation time in bloodstream; however, the cationic nanoparticles show higher cellular uptake due to the negative charges on cancer cells. Whereas, in active targeting, the basic concept is to utilize molecular targeting agent to specifically target the biomarkers or receptors on the cancer cells. Active cancer targeting has been widely studied and applied in the drug delivery systems to target tumor cells actively and minimized the systematic toxicity [17-20]. One of the examples for the active targeting is to decorate targeting ligands on the surface of nanoparticles for the specific targeting toward the membrane receptors on cancer cells. The binding of the targeting ligands with the membrane receptors trigger the receptor-mediated endocytosis leading to cellular uptake of nanoparticles [21,22], as shown in Figure 1D.
Figure 1.
Nanoparticle targeting mechanisms. A. Nanoparticles perform nonspecific passive targeting by diffusing and aggregating through leaky vessel in solid tumors via EPR effect. B. Nanoparticles modified with targeting agents perform active targeting on tumor cells expressing specific receptors. Nanoparticles specifically target on metastatic cancer cells. C. Fate of nanoparticles in non-specific gene delivery: phagocytosis, decomposition of nanoparticles outside of cells, and for the lipid or surfactant-based nanoparticles, the nanoparticles infuse into the cell membrane bilayers for drug release. D. Nanoparticles modified with targeting ligands specifically bind onto the membrane receptor and induce endocytosis. Higher proportion of gene molecules may eventually enter into nucleus.
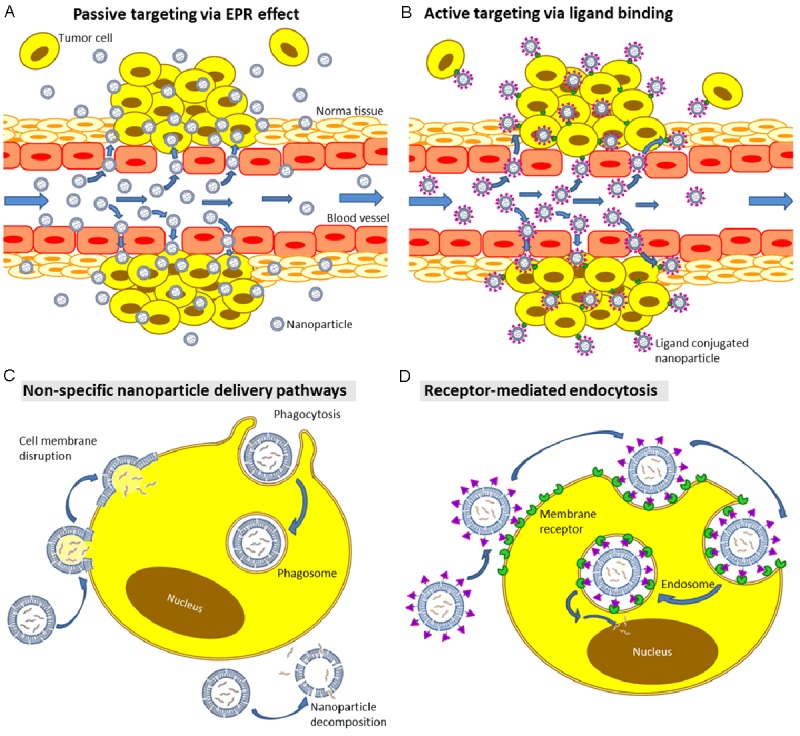
Gene molecules are usually carried by the nanoparticles for gene delivery in three different ways: encapsulated inside nanoparticles, forming complexes through ionic interactions with nanoparticles, and loaded on surface via conjugation or modified-polymer trapping as shown in Figure 2. Various kinds of nanoparticles have been designed and developed for the purpose of gene delivery in cancer treatment. The nanoparticles applied in gene delivery can be classified into two major types: solid and liquid forms of nanoparticles. The solid nanoparticles used in gene delivery have been composed of various materials including polysaccharides (e.g. chitosan, alginate and etc.), lipids, proteins, biodegradable polymers (e.g. poly (lactic-co-glycolic acid (PLGA), polycaprolactone (PCL), and magnetic metal oxides [23-35]. Cationic polymers such as polyethylenimine (PEI) have also been incorporated into the delivery system to enhance encapsulation efficiency and cellular uptake. The solid nanoparticles have been developed in different structures such as bulk, hydrogel, core-shell hollow and porous, due to their proposed properties and functions [36,37]. The nanoparticles in liquid form are majorly liposomes, micelles or the emulsion systems composed of amphiphilic molecules or polymers [38]. The liposomal nanoparticles containing artificial cationic lipids (e.g. 1, 2-dioleoyl-3-trimethylammonium-propane (DOTAP), 1,2-di-O-octadecenyl-3-trimethylammonium propane (DOTMA) etc. and are one of the most commonly used carriers in gene delivery. The advantages of using cationic liposomes as the gene carrier include high encapsulating efficiency of gene molecules, high stability and compatibility in biological environment. In addition, high cellular uptake, ease of preparation, and modification. Nanoliposomes are often physically or chemically modified with targeting ligands and polymers like polyethylene glycol (PEG) to enhance their cancer targeting ability [39,40].
Figure 2.
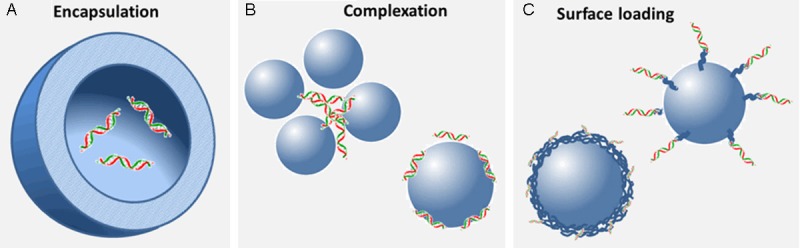
Gene molecules carried by nanoparticles in three different forms: Gene molecules are (A) encapsulated inside nanoparticles, (B) forming complexes through ionic interactions with nanoparticles, and (C) loaded on the surface via conjugation or modified-polymer trapping.
Recent development in nanoparticle-based gene delivery for lung cancer
In lung cancer therapy, nanoparticles have been utilized for the targeted delivery of gene molecules including DNA, plasmid DNA (pDNA), messenger RNA (mRNA), small interfering RNA (siRNA), microRNA (miRNA), RNA precursors, and etc. The development of nanoparticles for the delivery of DNA or pDNA has been a highly investigated area in cancer therapy in the last two decades. Current ongoing studies suggest that the targeted delivery of small RNAs such as siRNA and miRNA is gaining momentum in the field of cancer therapy due to their high gene transfection and silencing efficiency [41,42]. In general, compared to the DNA molecules, except for the smaller size which is generally between 10 to 20 KDa, the small RNA molecules are less stable and more easily to be attacked by the enzymes in the microenvironments. It has been reported that using RNA is more efficient than using DNA in the lipid-mediated delivery in non-dividing cells [43]. The experimental results suggested that RNA delivery might be more suitable for short-term transient gene expression due to its rapid onset, shorter duration of expression and greater efficiency. In addition, the exploration of the regulatory roles of mRNA and miRNA in the development of lung cancers trigger a lot of research interest in the delivery of small RNA molecules for cancer treatment.
Since the first synthesized cationic lipid N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA) was developed for DNA transfection in late 80’s [44], various synthetic lipids and lipid-based nanoparticles have been developed for efficient gene delivery. To date, the commercial products of liposomes composed of cationic lipids, Lipofectamine, is still one of the most commonly used transfection reagents in the laboratories. In lung cancer treatment, liposomal nanoparticle is the most commonly used in-vitro or in-vivo gene delivery carriers. Hundreds of studies have been reported using liposomal nanoparticles for gene delivery of small oligonucleotides. Two major encapsulation strategies have been reported for the gene delivery using liposomes, one is to encapsulate the gene molecules inside the aqueous phase in liposomes, and the other one is to encapsulate among liposomes through the formation of complex, which is also called lipoplex [45,46]. To discuss the nanoparticle-based gene delivery systems that have been applied on lung cancer therapy, we categorize the gene carriers into polymeric and lipid-based nanoparticles in different forms such as solid particles, micelles, liposomes, etc.
However, researches in nanoparticle-mediated gene therapy for MPM are not as prosperous as that for lung cancer. Only a few research studies have been conducted in MPM treatment by using gene delivery systems based on nanoparticles [1,47]. Although targeted drug delivery by functional nanoparticles for MPM treatment has been studied more widely by researchers recently, the gene therapy using nanoparticles is scarce [48-50] to nanoparticle albumin-bound paclitaxel and carboplatin in malignant pleural mesothelioma [51].
Polymeric nanoparticles
PEI-based polymeric nanoparticles
Polymeric carriers have received wide attention in gene delivery due to their outstanding characteristics including high stability, biocompatibility and diverse modifications to adapt desirable properties in particle forms. Among the polymers used in gene delivery carriers, the cationic polymer, PEI, is the most widely used polymer for the delivery of DNA and RNA molecules [52,53]. Carriers incorporated with PEI generally shows high encapsulation efficiency of bio-macromolecules with negative charges and high in-vitro transfection efficiency due to the positive charges on polymer chains. However, the cytotoxicity induced by the high positive charges of PEI has always been a concern while applying PEI in the biological systems and a major hurdle for clinical use, moreover, PEI itself is non-biodegradable. It has also been reported that the polymer structure, such as liner or branched, may influence the cytotoxicity and transfection efficiency in various environments [54]. To be used as an effective in-vivo gene delivery carrier, PEI has been modified or copolymerized with other neutral or biodegradable polymers such as polyethylene (PEG), PLGA or biopolymers to minimize the cytotoxicity and enzymatic attacks in the body [35].
In recent reports for the gene delivery on lung cancers, Zamora-Avila et al. used branched PEI (25 KDa) to form a complex with Wilms’ tumor gene (WT1) DNA for gene delivery to the lungs of mice bearing B16F10 lung metastasis [55]. Through aerosol administration, the WT1 DNA complexes effectively reduce the expression of WT1 mRNA and inhibited lung metastases growth in a 4-week treatment. For the RNA delivery for lung cancer, Bonnet et al. have shown the use of jetPEITM to form the complex with cyclin-B1 and survivin sticky siRNA (ssiRNA) for the targeting systemic delivery for lung tumor metastasis in the mice bearing aggressive adenocarcinoma cells (TSA-Luc) [56]. The particle size of the PEI/ssiRNA complex ranged from 40 to 70 nm and the zeta potential was around +35 mv. It has been shown that a mean of 64.0 ± 17.0% and 67.0.0 ± 7.5% of lung tumor metastasis inhibition were achieved by the treatments of cyclin-B1 and survivin ssiRNA/PEI complexes; however, the cytotoxicity of this PEI/ssiRNA complex was not able to be neglected although the LD50 was 25-fold greater than IC50.
Nanoparticle-based gene therapy has been combined with chemotherapy for enhanced therapeutic efficacy. Su et al. developed a gene delivery system which is combined with controlled release of chemotherapeutic drugs for the in-vitro studies of NSCLC treatment (A549) [35]. The PEI was coated on the paclitaxel loaded PLGA nanoparticles to carry signal transducer and activator of transcription-3 (Stat-3) siRNA on particle surface through electrostatic interactions. Stat-3 has been known as the regulator of cellular resistance of anti-cancer agents, the results show that the combination system suppressed Stat-3 expression and induced higher cellular apoptosis by paclitaxel in A549 cells. Another combination therapy involving chemotherapy with gene silencing using PEI complex has been shown by Chen et al [57]. The complex of vascular endothelial growth factor receptor 2 (VEGFR2), epidermal growth factor receptor (EGFR) siRNA and PEI was injected intratumorally into the NSCLC (A549) tumor xenografts with cisplatin administration. Enhanced treatment efficacy was observed with lower dose of siRNA and cisplatin combination, and which may result in reduced systemic toxicity of chemotherapy.
PEI has been incorporated and modified with a wide range of biocompatible or biodegradable biomaterials to improve its delivery efficacy and minimized the potential cytotoxicity. In a recent study, Guo et al. reported the alkane-modified PEI displayed enhanced efficiency in siRNA-mediated gene silencing [58]. The hydrophobically modified PEI showed reduced siRNA binding affinity but expressed tendency to self-assemble into nanoparticles which leads to enhanced cellular uptake. These nanoparticles showed similar in-vitro transfection efficiency as LipofectamineTM 2000 in aqueous solution. Based on the in-vitro studies, these hydrophobically modified PEI nanoparticles may have a high potential to be used as the gene carrier for intravenous injection due to the high stability contributed by the hydrophobic segments.
Although polysaccharides such as chitosan and hyaluronic acid (HA) are very widely used in biomaterials and drug delivery systems, their application in gene delivery is quite limited due to their inefficient encapsulation of nucleotides and majorly the low transfection efficiency. However, incorporating these highly biocompatible natural biopolymers into the PEI-based gene delivery systems can significantly enhance the stability and reduce the cytotoxicity of delivery systems. Ganesh et al. have published a self-assembling nanoparticle system based on HA grafted with PEI and PEG (HA-PEI/PEG) for siRNA delivery to SCLC (H69) solid NSCLC (A549) tumors [59]. The SSB/PLK1 siRNA were complexed with the PEI-grafted HA and formed the siRNA encapsulated nanoparticles to A549 cells overexpressing CD44 receptors. The particle size is in the range of 50 to 80 nm and surface charge was measured to be -15 mv. Through intravenous injection, the SSB siRNA in PEI incorporated HA nanoparticle system showed target specific gene silencing in the subcutaneous A549 tumors (~55%), but relatively low in the metastatic tumors (~25%). PEI has also been incorporated with inorganic magnetic nanoparticles for specific targeting on lung cancer. Another siRNA delivery system developed by Li et al. is composed of siRNA loaded magnetic mesoporous silica nanoparticles and an outer layer of PEI conjugated with fusogenic peptide (KALA) to enhance the cellular uptake efficiency by NSCLC (A549) tumor [60]. After a 30-days treatment of VEGF-siRNA encapsulated nanoparticles via intratumoral injection, significant inhibition (~70%) on tumor volume has been shown in the mice bearing A549 tumors.
Dendrimeric nanoparticles
Dendrimers are the three-dimensional macromolecules consist of a central core acting as the root from which the highly branched, tree-like polymer chains grow in an ordered and symmetric structure. Since the concept has been brought out in the late 1970s and early 1980s, the applications of dendrimers have attracted great attention from a wide range of research areas including drug and gene delivery [61-63]. Dendrimers show unique chemical and physical properties due to their highly symmetric molecular architecture. The size and surface properties of dendrimers can be controlled by varying the number of generations in synthesis. In gene delivery, the dendrimeric nanoparticles based on cationic or amide-rich polymers have been used to complex with nucleotides via electrostatic interactions to provide protection and delivery.
Because of the abundant amide and amine groups on polymer chains, the poly(amidoamine) (PAMAM) or poly(propyleneimine) (PPI) based dendrimeric nanoparticles are one of the most commonly used carriers in gene delivery [64]. The use of PAMAM dendrimers for gene delivery was first reported in 1991 by Haensler et al. It has been shown that the sixth generation dendrimers has the highest gene transfection efficiency compared to higher and lower generation dendrimers. In addition, the degradability of amide linkage in PAMAM dendrimers enhances its transfection effectiveness in gene delivery.
The use of dendrimers in gene delivery for lung cancer is scarce. In the recently reported in-vivo gene delivery studies for lung cancer, Taratula et al. used the generation 5 PPI dendrimeric nanoparticles to form the complexes with siRNA for intratumoral gene delivery [65]. The siRNA-nanoparticle complexes were modified with functional PEG polymers and a synthetic analog of Luteinizing Hormone-Releasing Hormone (LHRH) peptide was conjugated on the distal end of PEG polymers to specifically direct the siRNA-nanoparticle complexes to the lung cancer cells. The BCL-2 siRNA complexes prepared by Taratula et al. showed specific uptake, enhanced accumulation and efficient gene silencing in A549 cells. In the in-vivo studies, the siRNA-nanoparticle complexes were injected intravenously for systemic delivery in nude mice bearing subcutaneous xenografts of A549 NSCLC tumors. The complexes conjugated with targeting LHRH peptides showed high specific targeting on the tumor sites (from 0 to 300 a.u.) via the systemic delivery, whereas the unspecific distribution of nanoparticle complexes in liver and kidney were significantly reduced (from 300 and 600 to <50 a.u.). In addition, Taratula et al. also showed that the accumulation of siRNA in xeno-grafted tumors was greatly enhanced (from 0 to 400 a.u.) after 72 hours of injection. In another case of specifically targeting on NSCLC cells using dendrimeric nanoparticles, PAMAM was conjugated with S6 aptamer, a single-stranded RNA molecule specifically binds to A549 cells, to form the dendrimeric nanoparticles for the delivery of mirco-RNA-34a (miR-34a), a transcriptional target of tumor suppressor p53 [66]. It was revealed that aptamer conjugation on the dendrimeric nanoparticles significantly enhances cellular uptake as well as gene transfection efficiency in the in-vitro studies.
Polymeric micelles
Polymeric micelles applied in drug delivery are normally composed of the functional inner core and outer shell. The inner core can be the hydrophobic segments of amphiphilic block copolymer, and the outer shell can be the hydrophilic segments. The polymeric micelles can also be composed of totally water-soluble block copolymers containing neutral polymer chain and charged segment. The micelles formed by the self-assembly of block copolymers via electrostatic interactions are called polyion complex micelles. The electrostatic interaction between the ionic segments of copolymer and oppositely charged species, negatively charged nucleotides in gene delivery, lead to neutralization of ionic chains and change the ionic hydrophilic segment to hydrophobic. Polymeric micelles have been widely applied in gene delivery [67-69]. The DNA or RNA nucleotides are generally loaded or complexed inside inner core, and the outer shell controls the pharmacokinetic behavior [70].
Since 1980s, self-assembled polymeric micelles have been developed as carrier systems for delivering various bioactive molecules, such as nucleotides, proteins, peptides and chemotherapeutic drugs [71]. Micelles have also been widely applied in the co-delivery of drugs and gene molecules. Shen et al. published a new micelle complex system for co-delivery of drug and oligonucleotides for overcoming drug resistance in lung cancer [72,73]. The co-delivery complex nanoparticles were composed of Pluronic P85 conjugated PEI and D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS), the derivative of the natural Vitamin E (atocopherol) and PEG, which is used as solubilizer or absorption enhancer for increasing micelle stability and encapsulation efficiency. The micelle systems were complexed with paclitaxel and survivin shRNA to overcome the paclitaxel resistance in A549 cancer tumors. In has been shown that via the intravenous injection into the mice bearing subcutaneous A549 tumors, the complex nanoparticles effectively down-regulate survivin protein and increase paclitaxel accumulation in A549 tumors. The IC50 of micelle complex system against A549 cells was reported to be 360-fold lower than that of free paclitaxel treatment. Hao et al., developed a multifunctional polymer-drug conjugate, which contains PEI and candesartan conjugated via amide bonds, as the co-delivery micelle of drug and VEGF siRNA for lung cancer therapy [74]. The conjugates self-assembled into 100nm core-shell structure micelles with positive surface charge. The drug conjugates complexing with siRNA showed high efficacy on suppressing the expression of VEGF mRNA and displayed ~70% antitumor activity through intravenous injection in nude mice bearing A549 xenografts.
Lipid-based nanoparticles
Cationic lipid-based liposomes
Nanoliposome-based gene carriers have been the most widely used gene delivery system in lung cancer treatment. In the last five years, more than 100 publications have explored the application of modified or functionalized nanoliposomes in the field of targeted gene delivery for lung cancer therapy. Among all the artificial cationic lipids, DOTAP is the most commonly used transfection agent to form complex with oligonucleotides in gene delivery carriers. Novel nanoliposome-based gene delivery systems have been recently developed for in-vitro and in-vivo gene therapy in lung cancer cells or tumors. Ito et al., has demonstrated the in-vivo antitumor activity of FUS1 DNA complexed to DOTAP/cholesterol (Chol) nanoliposomes in the mice bearing A549 and H1299 NSCLC tumors and metastasis [75]. In the in-vivo results, intravenous injection of the FUSI DNA/nanoliposome complex showed ~70% significant inhibition on the number of metastatic tumor nodules in the mice bearing A549 metastasis. Intratumoral injection of the FUSI DNA complex showed up to 80% suppression on tumor growth, and induced 18% and 33% (7-folds higher than control) apoptosis in A549 and H1299.
Although DOTAP provides high encapsulation of oligonucleotides and high cellular uptake, it has been reported that DOTAP-based liposomes accumulate near the vasculature without perinuclear punctuations and is preferentially taken up by the liver and spleen while injected intravenously, which limits its therapeutic effectiveness in cancer or systemic treatments. Compared to the DOTAP liposomes, neutral liposomes based on DOPC (1,2-dioleoylsn-glycero-3-phosphatidylcholine) showed deep delivery of siRNA in the tumor tissue, instead of clustering near the vasculature [76]. Zhu et al. developed a neutral nanoparticle, with surface charge of 10 to 15 mV, consisting of a solid PLGA/cationic lipid hybrid core containing siRNA and a lipid-PEG shell for systemic gene delivery [77]. The solid core coated with PEG modified DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanolamine) displayed long blood circulation, high tumor accumulation and efficient gene silencing in the mice bearing NSCLC A549 tumors. The neutrally modified nanoparticles exhibited long blood circulation, with about 8 hours of half-time in circulation, and showed high accumulation of nanoparticles in liver, spleen and targeted NSCLC tumors. The nanoparticles loaded with therapeutic PHB1 (Prohibitin 1) siRNA showed drastic inhibition of tumor growth in the first 12 days of treatment including three intravenous injections; with the combination with intraperitoneal injection of cisplatin, PHB1 siRNA loaded nanoparticles exhibited complete suppression of tumor growth up to 40 days.
Nanoliposomes have been modified with polymers, peptides, biomolecules or receptor ligands for the enhancement of cancer targeting ability in systemic circulation. Hattori et al., recently reported that the anionic polymer-coated cationic lipoplexes (DOTAP/Chol) may be safe systemic carriers for gene delivery [78]. Via intravenous injection in the mice bearing 100-200 mm3 Lewis lung carcinoma (LLC) tumors subcutaneously, the cationic lipoplexes coated with chondroitin sulfate and poly-L-glutamic acid showed same gene transfection in tumor, compared to the unmodified cationic lipoplex. In the results, cationic lipoplex showed high accumulation in lung, whereas the anionic lipoplex accumulated in liver. More importantly, the anionic polymer-coatings significantly reduced liver toxicity which was induced by cationic lipoplex. To actively target lung cancer cells that overly express EphA2, Lee et al. developed a modified cationic liposomal nanoparticle system for the targeted delivery of let-7a miRNA precursors to MPM and NSCLC cells, as shown in Figure 3 [1]. This nanoliposome in the size range of 100 to 200 nm is composed of lipids including DOTAP, Cholesterol and the DSPE modified with reactive cyanur groups on the end of PEG polymer chain. The let-7a miRNA was encapsulated in the nanoliposomes through film hydration method and the ligand protein, eprhin-A1, was chemically conjugated via the reaction with the caynur chloride on the particle surface, for specific targeting on the over-expressed EphA2 membrane receptors on NSCLC (A549) and MPM (CRL-2081 and -5830) cells. In this targeted delivery system prepared by Lee et al., the highly positive surface charge of DOTAP-based nanoliposomes was neutralized by the surface conjugated ephrin-A1, from +45 to -15 mv, and the encapsulation of miRNA was highly endured. This miRNA targeted delivery system showed enhanced cellular uptake and transfection efficiency in the cell lines expressing high level of EphA2. In the in-vitro studies, this combination system effectively suppressed RAS signaling and inhibited proliferation, migration and tumorosphere growth [2]. In another report of using liposomal nanoparticles for gene therapy in MPM, Qu et al. used the dimethyldioctadecyl-ammonium bromide (DDAB) based cationic liposomes for co-delivery of siRNA and chemotherapeutic drug for synergistic treatment of NSCLC and MPM cells [47]. In the in-vitro results, the co-delivery of BCL-2 siRNA and docetaxel inhibited cell proliferation of MPM cell line (CRL-5826) and showed increasing hypodiploids and apoptotic cells.
Figure 3.

Schematic presentation of Combination therapy of ligand protein and miRNA using nanoliposomes for NSCLC and MPM over-expressing EphA2. A. The ligand proteins, ephrin-A1, were conjugated with miR-Let-7a encapsulated nanoliposomes via the reaction between amines on proteins and cyanuric chlorides on PEGylated lipids. The ephrin-A1, ligand of EphA2 receptor acts as targeting and therapeutic agents in the complex system. B. The miR-Let-7a/liposome/ephrinA1 complex system suppresses tumor growth through ephrin-A1/EphA2 binding and intracellular release of miR-Let-7a. The binding of ephrin-A1 leads to phosphorylation of EphA2 and inhibits the RAS pathway. In one of the signaling pathways, the activation of EphA2 induces miR-Let-7a which inhibits RAS signaling. Combined with ligand proteins, the extrinsic miR-Let-7a were delivered intracellularly majorly through endocytosis of nanoliposomes to enhance therapeutic effectiveness. BBS= borate buffered saline; TK= tyrosine kinase domain; PDZ= PDZ-binding motif; P= phosphorylation; ┴= inhibition [1-3].
In addition to DOTAP, novel cationic lipids have been developed for efficient transfection and reduced cytotoxicity. For example, a novel single-tailed cationic lipid, 6-lauroxyhexyl lysinate (LHLN), was synthesized to be used in the nanoliposomes for safer and efficient gene delivery of plasmid DNA [79]. The LHLN showed a high DNA binding capability, lower cytotoxicity and similar transfection efficiency in A549 NSCLC cells, compared to the conventional Lipofectamin-2000. In the in-vivo evaluation, the LHLN lipid-based nanoliposomes showed higher pulmonary transfection efficiency than Lipofectamin-2000, ~28% to ~11%, while administered by intra-tracheal instillation into rat lungs.
Anionic liposomes
Due to their high safety and stability, anionic liposomes have been developed for gene delivery other than the liposomes based on cationic lipids [80-82]. Unlike the cationic lipids, cytotoxicity of anionic lipids has never been a major concern since various kinds of anionic lipids can be found in nature and generally not cytotoxic. Furthermore, high positive charges on cationic liposomes could cause molecular condensation of DNA or RNA oligonucleotides and lead to loss of function of the gene molecules via the strong interaction. Although it has been reported that cationic liposomes exhibited a high accumulation in tumor tissue and tumor vasculature via intravenous injection, the accumulation of cationic liposomes in the surrounding normal tissues is still unneglectable and higher than the anionic liposomes [83]. However, in that study, the anionic liposomes showed homogeneous distribution without specific binding and displayed clear extravasation from the tumor vasculature. It reveals that the anionic liposomes may be used as carriers for extravascular applications and actively targeting the metastatic cancer cells via conjugating with targeting agents.
In anionic liposomes for gene delivery, low encapsulation efficiency of DNA or RNA nucleotides is generally encountered in the preparation of liposome complexing or nucleotides encapsulated liposome systems. To enhance the encapsulation of nucleotides, cationic helper molecules such as calcium chloride, poly (amino acid) or some arginine-rich oligomers are usually used to complex with nucleotides encapsulated in or among anionic liposomes to form the lipoplex. The transfection efficiency can also be enhanced by the involvement of cationic molecules in the gene delivery system. However, these cationic helper molecules still have potential cytotoxicity while applied in the gene delivery systems. High cytotoxicity can be observed as well in high concentration of calcium chloride in the microenvironment of cells. In addition to these cationic helper molecules which have been widely studied, It has been recently reported that using the Octa-ammonium polyhedral oligomeric silsesquioxane (OA-POSS), a hybrid nano-cage oligomer with eight ammonium groups on the cage corners, as the cationic helper molecule may form a low-cytotoxic, stable and highly efficient ternary complex with siRNA and anionic liposomes [84]. The OA-POSS oligomers showed similar cytotoxicity level with calcium chloride; however, the complexation ability with nucleotides is much stronger than calcium ions. This 100 to 200 nm ternary lipoplex showed stable zeta potential around -25 mv. With encapsulated with EphA2 siRNA, the lipoplex system showed high in-vitro silencing efficiency in the MPM cells in microenvironment containing serum.
Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) are typically spherical and consisted of solid lipid core matrix that are enclosed and stabilized by a lipid monolayer on the surface. Like the liposomes, the SLNs have high biocompatibility and superior cell membrane affinity while used as the drug delivery carriers; however, the chemical and physical stability of SLNs in ambient and biological environments are higher than that of the liposomes. Although using SLNs for drug delivery and controlled release has been widely reported since early 1990’s, the development of SLNs for gene delivery has been seldom published, compared to the usage of liposomes in gene therapy [85,86].
Various kinds of modified SLNs based on cationic lipids for in-vivo gene transfection have been lately published [87-89]. It has been reported that using dextran and protamine based SLNs as the vector, the protein expression was maintained for at least 7 days after intravenous administration in mice. As for the SLN systems which have been applied in lung cancer gene therapy, in 2008, Choi et al. used the SLNs composed of 3β-[N-(N’,N’-dimethylaminoethane)-carbamoyl]cholesterol hydrochloride (DC-Chol), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and Tween 80 to complex with p53-EGFP plasmid DNA for gene transfection in H1299 NSCLC cell line [90]. The SLNs mediated transfection of p53 gene resulted in higher transfection efficiency than that of the commercial Lipofectamine®, and restored the apoptotic pathway in H1299 cells. Recently Han et al., reported a combination therapy of EGFP plasmid DNA and doxorubicin by using lecithin-based SNLs for in-vivo transfection in mice bearing A549 tumors [91]. The targeting ligand, transferrin (Tf), was conjugated on surface of SNLs for specifically targeting the Tf receptors found in tumor cells. The SNL complexes showed a narrow particle size around 200nm and the zeta potential was neutralized from +40 to +19 mv after ligand conjugation. The combination therapy using SNL delivery system showed enhanced antitumor effects via intratumoral injection in subcutaneous tumors.
Metal-based nanoparticles
Metallic nanoparticles including gold and iron oxide nanoparticles have been widely studied for their applications in imaging and drug delivery. Advantageous properties of gold nanoparticles (AuNPs), such as non-toxicity, high bio-inertness, and ease of preparation and functionalization, enable them to be ideal alternative delivery carriers in gene delivery systems [92]. The high surface reactivity of AuNPs allows the particle surface to be easily modified or conjugated with functional polymers or biomolecules. AuNPs have also been applied in functional complex drug delivery systems with other carriers. In a recent study, AuNPs were encapsulated in liposomes, and drug release can be triggered through the light-induced heat generated on AuNPs [93]. For purpose of efficient gene delivery, AuNPs have been conjugated with nucleotides, coated with polymer layers or utilized as the core for dendrimers [94-98]. In a pH-dependent gene delivery system, AuNPs were chemically modified with carboxylic acid chains and coated with PEI, the pH-dependent charge-reversal polyelectrolyte, for controlled release of siRNA and plasmid DNA after pH change in endosome [99]. In an in-vivo study, Conde et al., prepared the PEG modified AuNPs and conjugated with RGD and c-myc siRNA on the surface for targeted gene delivery via intra-tracheal instillation [100]. The therapeutic siRNA/RGD AuNPs treatment resulted in efficient c-myc oncogene down-regulation and tumor growth inhibition in mice bearing CMT/167 mouse lung carcinoma tumors. In addition, the AuNPs conjugates were demonstrated to trigger the complex inflammatory and immune responses that might enhance the effectiveness of treatment in the tumor-bearing mice.
Iron oxide nanoparticles have also been widely applied in biomedical fields due to their superparamagnetic properties. Various iron oxide-based nanoparticle systems have been applied in medical diagnosis and therapeutics such as magnetic resonance imaging (MRI) and magnetic field-induced drug delivery, although the potential cytotoxicity of iron oxide nanoparticles might be a concern [101]. Dual purpose iron oxide based magnetic nanoparticles have been developed for gene delivery and as MRI probe [102]. In the gene delivery systems, the iron oxide nanoparticles were usually utilized as the core and cationic polymers or lipids were coated on the surface to complex with nucleotides [103]. The poly-L-lysine modified iron oxide nanoparticles had been shown to be a promising therapeutic gene delivery system for lung cancer tumor [104]. Xiang et al. used the cationic poly-amino acid conjugated magnetic nanoparticles to complex with NM230HA-GFP plasmid DNA for gene transfection in mice bearing B16F10 melanoma tumors. The intravenous injection of the nanoparticle/DNA complex resulted in longer survival times and suppression of metastasis. The number of nodules from lung tumor metastases was shown to be reduced to 25% by the treatment of the iron-oxide based nanoparticles carrying NM230HA-GFP, and the number further decreased to 10% by the combination treatment with the chemotherapeutic drug, cyclophosphamide.
Conclusion and future challenges
Nanoparticles based on different materials and functions have been developed and applied in the therapeutic treatment of lung cancers in the last two decades. The utilization of polymer, lipid or metal-based nanoparticle systems in the field of targeted gene delivery has grew tremendously and showed promising in-vitro or in-vivo experimental results in therapeutic efficacy. In the treatment of lung cancers, nanoparticles carrying gene molecules showed high transfection efficiency and targeting ability on lung cancer tumors through systematic or localized administrations. In addition, therapeutic efficiency of gene therapy can be improved by active targeting on specific lung cancer tumors or metastases through modification or conjugation of targeting agents on the surface of the nanoparticles. However, translating these novel nanoparticle-mediated gene delivery techniques into clinical practice is a huge challenge. The challenges of applying nanoparticle-mediated gene delivery within the body, such as maintaining the stability of nanoparticles and gene molecules during delivery, controlling the bio-distribution and pharmacokinetics, penetrating biological barriers and minimizing the potential cytotoxicity of the nanoparticles, need to be considered and overcome before entering into clinical trials. To expand the application of nanoparticle systems in gene therapy in clinics, standards in the examination of nanoparticle safety and evaluation of therapeutic efficacy should be established to guide the direction of research and intervention in gene therapy using nanoparticles. In addition, nanoparticle-based targeted gene delivery can be more widely applied in MPM treatment for improved therapeutics.
Acknowledgements
The authors would like to acknowledge the financial support from Florida Department of Health research grants (#00083831), and 4BB02 to NN; and the Department of Veterans Affairs, VA Merit Review to MKA.
Disclosure of conflict of interest
None.
References
- 1.Lee HY, Mohammed KA, Kaye F, Sharma P, Moudgil BM, Clapp WL, Nasreen N. Targeted delivery of let-7a microRNA encapsulated ephrin-A1 conjugated liposomal nanoparticles inhibit tumor growth in lung cancer. International Journal of Nanomedicine. 2013;8:4481–4493. doi: 10.2147/IJN.S41782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khodayari N, Mohammed KA, Goldberg EP, Nasreen N. EphrinA1 inhibits malignant mesothelioma tumor growth via let-7 microRNA-mediated repression of the RAS oncogene. Cancer Gene Ther. 2011;18:806–816. doi: 10.1038/cgt.2011.50. [DOI] [PubMed] [Google Scholar]
- 3.Nasreen N, Khodayari N, Mohammed KA. Advances in malignant pleural mesothelioma therapy: targeting EphA2 a novel approach. Am J Cancer Res. 2012;2:222–234. [PMC free article] [PubMed] [Google Scholar]
- 4.American cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 5.Dungo RT, Keating GM. Afatinib: First Global Approval. Drugs. 2013;73:1503–1515. doi: 10.1007/s40265-013-0111-6. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu YZ, Liepa AM, Gao L, Schwartz JD, Tabernero J REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 7.Molina JR, Yang PG, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clinic Proceedings. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson V, Ziehr J, Agulnik M, Johnson M. Afatinib: emerging next-generation tyrosine kinase inhibitor for NSCLC. Oncotargets and Therapy. 2013;6:135–143. doi: 10.2147/OTT.S23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popat S, Mok T, Yang JCH, Wu YL, Lungershausen J, Stammberger U, Griebsch I, Fonseca T, Paz-Ares L. Afatinib in the treatment of EGFR mutation-positive NSCLC - A network meta-analysis. Lung Cancer. 2014;85:230–238. doi: 10.1016/j.lungcan.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Kasinski AL, Slack FJ. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nature Reviews Cancer. 2011;11:849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downward J. Targeting ras signalling pathways in cancer therapy. Nature Reviews Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 12.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nature Reviews Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 13.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 14.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 15.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular Pharmaceutics. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albanese A, Tang PS, Chan WCW. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu Rev Biomed Eng. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 17.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Advanced Drug Delivery Reviews. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Karra N, Benita S. The Ligand Nanoparticle Conjugation Approach for Targeted Cancer Therapy. Current Drug Metabolism. 2012;13:22–41. doi: 10.2174/138920012798356899. [DOI] [PubMed] [Google Scholar]
- 19.Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141:769–784. doi: 10.1007/s00432-014-1767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirollo KF, Chang EH. Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends in Biotechnology. 2008;26:552–558. doi: 10.1016/j.tibtech.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Advanced Drug Delivery Reviews. 2013;65:121–138. doi: 10.1016/j.addr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yameen B, Choi WI, Vilos C, Swami A, Shi JJ, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. Journal of Controlled Release. 2014;190:485–499. doi: 10.1016/j.jconrel.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endres TK, Beck-Broichsitter M, Samsonova O, Renette T, Kissel TH. Self-assembled biodegradable amphiphilic PEG-PCL-lPEI triblock copolymers at the borderline between micelles and nanoparticles designed for drug and gene delivery. Biomaterials. 2011;32:7721–7731. doi: 10.1016/j.biomaterials.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 24.Sun TM, Du JZ, Yan LF, Mao HQ, Wang J. Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials. 2008;29:4348–4355. doi: 10.1016/j.biomaterials.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 25.Karimi M, Avci P, Mobasseri R, Hamblin MR, Naderi-Manesh H. The novel albumin-chitosan core-shell nanoparticles for gene delivery: preparation, optimization and cell uptake investigation. J Nanopart Res. 2013;15:1651. doi: 10.1007/s11051-013-1651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang JS, Kim SY, Lee SB, Kim KO, Han JS, Lee YM. Poly(ethylene glycol)/poly(epsilon-caprolactone) diblock copolymeric nanoparticles for non-viral gene delivery: the role of charge group and molecular weight in particle formation, cytotoxicity and transfection. J Control Release. 2006;113:173–182. doi: 10.1016/j.jconrel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Wang K, Kievit FM, Florczyk SJ, Stephen ZR, Zhang M. 3D Porous Chitosan-Alginate Scaffolds as an In Vitro Model for Evaluating Nanoparticle-Mediated Tumor Targeting and Gene Delivery to Prostate Cancer. Biomacromolecules. 2015;16:3362–3372. doi: 10.1021/acs.biomac.5b01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young TJ, Johnson KP, Pace GW, Mishra AK. Phospholipid-stabilized nanoparticles of cyclosporine A by rapid expansion from supercritical to aqueous solution. Aaps Pharmscitech. 2004;5:E11. doi: 10.1208/pt050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su WP, Cheng FY, Shieh DB, Yeh CS, Su WC. PLGA nanoparticles codeliver paclitaxel and Stat3 siRNA to overcome cellular resistance in lung cancer cells. Int J Nanomedicine. 2012;7:4269–4283. doi: 10.2147/IJN.S33666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bordelon H, Biris AS, Sabliov CM, Monroe WT. Characterization of Plasmid DNA Location within Chitosan/PLGA/pDNA Nanoparticle Complexes Designed for Gene Delivery. Journal of Nanomaterials. 2011 [Google Scholar]
- 31.Endres TK, Beck-Broichsitter M, Samsonova O, Renette T, Kissel TH. Self-assembled biodegradable amphiphilic PEG-PCL-lPEI triblock copolymers at the borderline between micelles and nanoparticles designed for drug and gene delivery. Biomaterials. 2011;32:7721–7731. doi: 10.1016/j.biomaterials.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 32.Sun TM, Du JZ, Yan LF, Mao HQ, Wang J. Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials. 2008;29:4348–4355. doi: 10.1016/j.biomaterials.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 33.Jang JS, Kim SY, Lee SB, Kim KO, Han JS, Lee YM. Poly(ethylene glycol)/poly(epsilon-caprolactone) diblock copolymeric nanoparticles for non-viral gene delivery: The role of charge group and molecular weight in particle formation, cytotoxicity and transfection. Journal of Controlled Release. 2006;113:173–182. doi: 10.1016/j.jconrel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 34.You JO, Peng CA. Calcium-alginate nanoparticles formed by reverse microemulsion as gene carriers. Macromolecular Symposia. 2004;219:147–153. [Google Scholar]
- 35.Su WP, Cheng FY, Shieh DB, Yeh CS, Su WC. PLGA nanoparticles codeliver paclitaxel and Stat3 siRNA to overcome cellular resistance in lung cancer cells. Int J Nanomedicine. 2012;7:4269–4283. doi: 10.2147/IJN.S33666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu YL, Wu YH, Tsai WB, Tsai CC, Chen WS, Wu CS. Core-shell silica@chitosan nanoparticles and hollow chitosan nanospheres using silica nanoparticles as templates: Preparation and ultrasound bubble application. Carbohydrate Polymers. 2011;84:770–774. [Google Scholar]
- 37.Ma X, Zhao Y, Ng KW, Zhao YL. Integrated Hollow Mesoporous Silica Nanoparticles for Target Drug/siRNA Co-Delivery. Chemistry-a European Journal. 2013;19:15593–15603. doi: 10.1002/chem.201302736. [DOI] [PubMed] [Google Scholar]
- 38.Liu CH, Yu SY. Cationic nanoemulsions as non-viral vectors for plasmid DNA delivery. Colloids Surf B Biointerfaces. 2010;79:509–515. doi: 10.1016/j.colsurfb.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Cai LL, Wang XH, Wang WW, Qiu N, Wen JL, Duan XM, Li X, Chen X, Yang L, Qian ZY, Wei YQ, Chen LJ. Peptide ligand and PEG-mediated long-circulating liposome targeted to FGFR overexpressing tumor in vivo. Int J Nanomedicine. 2012;7:4499–4510. doi: 10.2147/IJN.S32817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kibria G, Hatakeyama H, Ohga N, Hida K, Harashima H. Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J Control Release. 2011;153:141–148. doi: 10.1016/j.jconrel.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Muthiah M, Park IK, Cho CS. Nanoparticle-mediated delivery of therapeutic genes: focus on miRNA therapeutics. Expert Opinion on Drug Delivery. 2013;10:1259–1273. doi: 10.1517/17425247.2013.798640. [DOI] [PubMed] [Google Scholar]
- 42.Tong AW. Small RNAs and non-small cell lung cancer. Curr Mol Med. 2006;6:339–349. doi: 10.2174/156652406776894554. [DOI] [PubMed] [Google Scholar]
- 43.Zou S, Scarfo K, Nantz MH, Hecker JG. Lipid-mediated delivery of RNA is more efficient than delivery of DNA in non-dividing cells. Int J Pharm. 2010;389:232–243. doi: 10.1016/j.ijpharm.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wasungu L, Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release. 2006;116:255–264. doi: 10.1016/j.jconrel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 46.Ma BC, Zhang SB, Jiang HM, Zhao BD, Lv HT. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J Control Release. 2007;123:184–194. doi: 10.1016/j.jconrel.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 47.Qu MH, Zeng RF, Fang S, Dai QS, Li HP, Long JT. Liposome-based co-delivery of siRNA and docetaxel for the synergistic treatment of lung cancer. Int J Pharm. 2014;474:112–122. doi: 10.1016/j.ijpharm.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 48.Lei H, Hofferberth SC, Liu R, Colby A, Tevis KM, Catalano P, Grinstaff MW, Colson YL. Paclitaxel-loaded expansile nanoparticles enhance chemotherapeutic drug delivery in mesothelioma 3-dimensional multicellular spheroids. J Thorac Cardiovasc Surg. 2015;149:1417–1424. doi: 10.1016/j.jtcvs.2015.02.020. discussion 1424-1425 e1411. [DOI] [PubMed] [Google Scholar]
- 49.Mayol L, Serri C, Menale C, Crispi S, Piccolo MT, Mita L, Giarra S, Forte M, Saija A, Biondi M, Mita DG. Curcumin loaded PLGA-poloxamer blend nanoparticles induce cell cycle arrest in mesothelioma cells. Eur J Pharm Biopharm. 2015;93:37–45. doi: 10.1016/j.ejpb.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Kanai O, Fujita K, Nakatani K, Mio T. Repetitive responses to nanoparticle albumin-bound paclitaxel and carboplatin in malignant pleural mesothelioma. Respirol Case Rep. 2016;4:28–31. doi: 10.1002/rcr2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iyer AK, Su Y, Feng J, Lan X, Zhu X, Liu Y, Gao D, Seo Y, Vanbrocklin HF, Courtney Broaddus V, Liu B, He J. The effect of internalizing human single chain antibody fragment on liposome targeting to epithelioid and sarcomatoid mesothelioma. Biomaterials. 2011;32:2605–2613. doi: 10.1016/j.biomaterials.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nimesh S. Polyethylenimine as a promising vector for targeted siRNA delivery. Current clinical pharmacology. 2012;7:121–130. doi: 10.2174/157488412800228857. [DOI] [PubMed] [Google Scholar]
- 53.Patnaik S, Gupta KC. Novel polyethylenimine-derived nanoparticles for in vivo gene delivery. Expert Opin Drug Deliv. 2013;10:215–228. doi: 10.1517/17425247.2013.744964. [DOI] [PubMed] [Google Scholar]
- 54.Kafil V, Omidi Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in a431 cells. BioImpacts: BI. 2011;1:23–30. doi: 10.5681/bi.2011.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zamora-Avila DE, Zapata-Benavides P, Franco-Molina MA, Saavedra-Alonso S, Trejo-Avila LM, Resendez-Perez D, Mendez-Vazquez JL, Isaias-Badillo J, Rodriguez-Padilla C. WT1 gene silencing by aerosol delivery of PEI-RNAi complexes inhibits B16-F10 lung metastases growth. Cancer Gene Therapy. 2009;16:892–899. doi: 10.1038/cgt.2009.35. [DOI] [PubMed] [Google Scholar]
- 56.Bonnet ME, Gossart JB, Benoit E, Messmer M, Zounib O, Moreau V, Behr JP, Lenne-Samuel N, Kedinger V, Meulle A, Erbacher P, Bolcato-Bellemin AL. Systemic delivery of sticky siRNAs targeting the cell cycle for lung tumor metastasis inhibition. J Control Release. 2013;170:183–190. doi: 10.1016/j.jconrel.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 57.Chen S, Liu XX, Gong WF, Yang H, Luo DF, Zuo XL, Li WS, Wu P, Liu L, Xu Q, Ji AM. Combination therapy with VEGFR2 and EGFR siRNA enhances the antitumor effect of cisplatin in non-small cell lung cancer xenografts. Oncol Rep. 2013;29:260–268. doi: 10.3892/or.2012.2097. [DOI] [PubMed] [Google Scholar]
- 58.Guo GY, Zhou L, Chen ZF, Chi WL, Yang XQ, Wang W, Zhang BL. Alkane-modified low-molecular-weight polyethylenimine with enhanced gene silencing for siRNA delivery. Int J Pharm. 2013;450:44–52. doi: 10.1016/j.ijpharm.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 59.Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34:3489–3502. doi: 10.1016/j.biomaterials.2013.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Chen YJ, Wang MQ, Ma YJ, Xia WL, Gu HC. A mesoporous silica nanoparticle - PEI - Fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials. 2013;34:1391–1401. doi: 10.1016/j.biomaterials.2012.10.072. [DOI] [PubMed] [Google Scholar]
- 61.Bai SH, Thomas C, Rawat A, Ahsan F. Recent progress in dendrimer-based nanocarriers. Criti Rev Ther Drug Carrier Syst. 2006;23:437–495. doi: 10.1615/critrevtherdrugcarriersyst.v23.i6.10. [DOI] [PubMed] [Google Scholar]
- 62.Shcharbin D, Shakhbazau A, Bryszewska M. Poly(amidoamine) dendrimer complexes as a platform for gene delivery. Expert Opinion on Drug Delivery. 2013;10:1687–1698. doi: 10.1517/17425247.2013.853661. [DOI] [PubMed] [Google Scholar]
- 63.Kesharwani P, Lyer AK. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discovery Today. 2015;20:536–547. doi: 10.1016/j.drudis.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 65.Taratula O, Garbuzenko OB, Kirkpatrick P, Pandya I, Savla R, Pozharov VP, He H, Minko T. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J Control Release. 2009;140:284–293. doi: 10.1016/j.jconrel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H, Zhao X, Guo C, Ren D, Zhao Y, Xiao W, Jiao W. Aptamer-Dendrimer Bioconjugates for Targeted Delivery of miR-34a Expressing Plasmid and Antitumor Effects in Non-Small Cell Lung Cancer Cells. PLoS One. 2015;10:e0139136. doi: 10.1371/journal.pone.0139136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu XQ, Sun CY, Yang XZ, Wang J. Polymeric-Micelle-Based Nanomedicine for siRNA Delivery. Particle & Particle Systems Characterization. 2013;30:211–228. [Google Scholar]
- 68.Jhaveri AM, Torchilin VP. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front Pharmacol. 2014;5:77. doi: 10.3389/fphar.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Navarro G, Pan JY, Torchilin VP. Micelle-like Nanoparticles as Carriers for DNA and siRNA. Molecular Pharmaceutics. 2015;12:301–313. doi: 10.1021/mp5007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gong J, Chen MW, Zheng Y, Wang SP, Wang YT. Polymeric micelles drug delivery system in oncology. J Control Release. 2012;159:312–323. doi: 10.1016/j.jconrel.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 71.Cabral H, Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Release. 2014;190:465–476. doi: 10.1016/j.jconrel.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 72.Shen JA, Yin Q, Chen LL, Zhang ZW, Li YP. Co-delivery of paclitaxel and survivin shRNA by pluronic P85-PEI/TPGS complex nanoparticles to overcome drug resistance in lung cancer. Biomaterials. 2012;33:8613–8624. doi: 10.1016/j.biomaterials.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 73.Shen JA, Sun HP, Xu PF, Yin Q, Zhang ZW, Wang SL, Yu HJ, Li YP. Simultaneous inhibition of metastasis and growth of breast cancer by co-delivery of twist shRNA and paclitaxel using pluronic P85-PEI/TPGS complex nanoparticles. Biomaterials. 2013;34:1581–1590. doi: 10.1016/j.biomaterials.2012.10.057. [DOI] [PubMed] [Google Scholar]
- 74.Hao SH, Yan Y, Ren X, Xu Y, Chen LL, Zhang HB. Candesartan-graft-polyethyleneimine Cationic Micelles for Effective Co-delivery of Drug and Gene in Anti-angiogenic Lung Cancer Therapy. Biotechnology and Bioprocess Engineering. 2015;20:550–560. [Google Scholar]
- 75.Ito I, Ji L, Tanaka F, Saito Y, Gopalan B, Branch CD, Xu K, Atkinson EN, Bekele BN, Stephens LC, Minna JD, Roth JA, Ramesh R. Liposomal vector mediated delivery of the 3p FUS1 gene demonstrates potent antitumor activity against human lung cancer in vivo. Cancer Gene Therapy. 2004;11:733–739. doi: 10.1038/sj.cgt.7700756. [DOI] [PubMed] [Google Scholar]
- 76.Landen CN, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 77.Zhu X, Xu YJ, Solis LM, Tao W, Wang LZ, Behrens C, Xu XY, Zhao LL, Liu D, Wu J, Zhang N, Wistuba II, Farokhzad OC, Zetter BR, Shi JJ. Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment. Proc Natl Acad Sci U S A. 2015;112:7779–7784. doi: 10.1073/pnas.1505629112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hattori Y, Yamasaku H, Maitani Y. Anionic polymer-coated lipoplex for safe gene delivery into tumor by systemic injection. J Drug Target. 2013;21:639–647. doi: 10.3109/1061186X.2013.789035. [DOI] [PubMed] [Google Scholar]
- 79.Li P, Liu D, Sun X, Liu C, Liu Y, Zhang N. A novel cationic liposome formulation for efficient gene delivery via a pulmonary route. Nanotechnology. 2011;22:245104. doi: 10.1088/0957-4484/22/24/245104. [DOI] [PubMed] [Google Scholar]
- 80.Lee RJ, Huang L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J Biol Chem. 1996;271:8481–8487. doi: 10.1074/jbc.271.14.8481. [DOI] [PubMed] [Google Scholar]
- 81.Patil SD, Rhodes DG, Burgess DJ. Anionic liposomal delivery system for DNA transfection. AAPS J. 2004;6:e29. doi: 10.1208/aapsj060429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kapoor M, Burgess DJ. Physicochemical characterization of anionic lipid-based ternary siRNA complexes. Biochim Biophys Acta. 2012;1818:1603–1612. doi: 10.1016/j.bbamem.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 83.Krasnici S, Werner A, Eichhorn ME, Schmitt-Sody M, Pahernik SA, Sauer B, Schulze B, Teifel M, Michaelis U, Naujoks K, Dellian M. Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels. Int J Cancer. 2003;105:561–7. doi: 10.1002/ijc.11108. [DOI] [PubMed] [Google Scholar]
- 84.Targeted SiRNA Delivery Using A Novel Anionic Lipid/Polyhedral Oligomeric Silsesquioxane Complex System For Malignant Pleural Mesothelioma. Am J Respir Crit Care Med. 2015;191:A1123. [Google Scholar]
- 85.Das S, Chaudhury A. Recent Advances in Lipid Nanoparticle Formulations with Solid Matrix for Oral Drug Delivery. AAPS PharmSciTech. 2011;12:62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bummer PM. Physical chemical considerations of lipid-based oral drug delivery - Solid lipid nanoparticles. Crit Rev Ther Drug Carrier Syst. 2004;21:1–19. [PubMed] [Google Scholar]
- 87.Delgado D, del Pozo-Rodriguez A, Angeles Solinis M, Bartkowiak A, Rodriguez-Gascon A. New gene delivery system based on oligochitosan and solid lipid nanoparticles: ‘In vitro’ and ‘in vivo’ evaluation. Eur J Pharm Sci. 2013;50:484–491. doi: 10.1016/j.ejps.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 88.He SN, Li YL, Yan JJ, Zhang W, Du YZ, Yu HY, Hu FQ, Yuan H. Ternary nanoparticles composed of cationic solid lipid nanoparticles, protamine, and DNA for gene delivery. Int J Nanomedicine. 2013;8:2859–2869. doi: 10.2147/IJN.S47967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Delgado D, Gascon AR, del Pozo-Rodriguez A, Echevarria E, de Garibay APR, Rodriguez JM, Solinis MA. Dextran-protamine-solid lipid nanoparticles as a non-viral vector for gene therapy: In vitro characterization and in vivo transfection after intravenous administration to mice. Int J Pharm. 2012;425:35–43. doi: 10.1016/j.ijpharm.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 90.Choi SH, Jin SE, Lee MK, Lim SJ, Park JS, Kim BG, Ahn WS, Kim CK. Novel cationic solid lipid nanoparticles enhanced p53 gene transfer to lung cancer cells. Eur J Pharm Biopharm. 2008;68:545–554. doi: 10.1016/j.ejpb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 91.Han Y, Zhang P, Chen Y, Sun J, Kong F. Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy. Int J Mol Med. 2014;34:191–196. doi: 10.3892/ijmm.2014.1770. [DOI] [PubMed] [Google Scholar]
- 92.Mieszawska AJ, Mulder WJ, Fayad ZA, Cormode DP. Multifunctional Gold Nanoparticles for Diagnosis and Therapy of Disease. Mol Pharm. 2013;10:831–847. doi: 10.1021/mp3005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lajunen T, Viitala L, Kontturi LS, Laaksonen T, Liang H, Vuorimaa-Laukkanen E, Viitala T, Le Guevel X, Yliperttula M, Murtomaki L, Urtti A. Light induced cytosolic drug delivery from liposomes with gold nanoparticles. J Control Release. 2015;203:85–98. doi: 10.1016/j.jconrel.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 94.Ding Y, Jiang Z, Saha K, Kim CS, Kim ST, Landis RF, Rotello VM. Gold Nanoparticles for Nucleic Acid Delivery. Mol Ther. 2014;22:1075–1083. doi: 10.1038/mt.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tiwari PK, Lee YS. Gene delivery in conjunction with gold nanoparticle and tumor treating electric field. J Applied Physics. 2013:114. [Google Scholar]
- 96.Ryou SM, Kim S, Jang HH, Kim JH, Yeom JH, Eom MS, Bae J, Han MS, Lee K. Delivery of shRNA using gold nanoparticle-DNA oligonucleotide conjugates as a universal carrier. Biochem Biophys Res Commun. 2010;398:542–546. doi: 10.1016/j.bbrc.2010.06.115. [DOI] [PubMed] [Google Scholar]
- 97.Bishop CJ, Tzeng SY, Green JJ. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater. 2015;11:393–403. doi: 10.1016/j.actbio.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shan Y, Luo T, Peng C, Sheng R, Cao A, Cao X, Shen M, Guo R, Tomas H, Shi X. Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors. Biomaterials. 2012;33:3025–3035. doi: 10.1016/j.biomaterials.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 99.Guo S, Huang Y, Jiang Q, Sun Y, Deng L, Liang Z, Du Q, Xing J, Zhao Y, Wang PC, Dong A, Liang XJ. Enhanced Gene Delivery and siRNA Silencing by Gold Nanoparticles Coated with Charge-Reversal Polyelectrolyte. ACS Nano. 2010;4:5505–5511. doi: 10.1021/nn101638u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conde J, Tian F, Hernandez Y, Bao C, Cui D, Janssen KP, Ricardo Ibarra M, Baptista PV, Stoeger T, de la Fuente JM. In vivo tumor targeting via nanoparticle-mediated therapeutic siRNA coupled to inflammatory response in lung cancer mouse models. Biomaterials. 2013;34:7744–7753. doi: 10.1016/j.biomaterials.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 101.Choi SJ, Oh JM, Choy JH. Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. J Inorg Biochem. 2009;103:463–471. doi: 10.1016/j.jinorgbio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 102.Wang C, Ravi S, Martinez GV, Chinnasamy V, Raulji P, Howell M, Davis Y, Mallela J, Seehra MS, Mohapatra S. Dual-purpose magnetic micelles for MRI and gene delivery. J Control Release. 2012;163:82–92. doi: 10.1016/j.jconrel.2012.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG. Lipidoid-Coated Iron Oxide Nanoparticles for Efficient DNA and siRNA delivery. Nano Lett. 2013;13:1059–1064. doi: 10.1021/nl304287a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Z, Xiang J, Zhang W, Fan S, Wu M, Li X, Li G. Nanoparticle delivery of anti-metastatic NM23-H1 gene improves chemotherapy in a mouse tumor model. Cancer Gene Ther. 2009;16:423–429. doi: 10.1038/cgt.2008.97. [DOI] [PubMed] [Google Scholar]


