Abstract
Hereditary nonpolyposis colorectal cancer (HNPCC) is caused by functional defects in mismatch repair (MMR) genes, including mutL homolog 1 (MLH1) and mutS homolog 2 (MSH2). This study aimed to assess whether the mRNA expression of MLH1 in peripheral blood could be used as a biomarkers for the diagnosis of HNPCC. The mRNA level of MLH1 was determined in 19 HNPCC families (46 members) using real-time quantitative polymerase chain reaction (qPCR). The mRNA levels of MLH1 in HNPCC were significantly lower than controls (P < 0.001). Receiver operating characteristic (ROC) curve showed a high diagnostic value of the mRNA level of MLH1 for the diagnosis of HNPCC with the area under curve of 0.858. At an optimal cut-off value (0.511), the mRNA level of MLH1 had a sensitivity of 81.3% and a specificity of 86.7% for distinguishing HNPCC from controls. In conclusion, the mRNA expression of MLH1 in peripheral blood may serve as a biomarker for the diagnosis of HNPCC.
Keywords: Hereditary nonpolyposis colorectal cancer, mismatch repair gene, MLH1, mutation, diagnosis
Introduction
Hereditary nonpolyposis colorectal cancer (HNPCC), also known as Lynch Syndrome, has a high penetrance (80-90%) and is the most common inherited cancer of the digestive system, accounting for 3-5% of all colorectal cancer (CRC) cases [1,2]. HNPCC is caused by functional defects in mismatch repair (MMR) genes, including mutS homolog 2 (MSH2), mutL homolog 1 (MLH1), mutS homolog 6 (MSH6), postmeiotic segregation increased 2 (PMS2), postmeiotic segregation increased 1 (PMS1)[3-6]. Although HNPCC has a significant family history, diagnosis of this disease depends on detection of germline mutation of MMR genes in HNPCC patients and their kindred. Genetic studies have been showed that germline mutations of MLH1 and MSH2 account for approximately 90% of detected mutations in HNPCC [1,7]. Therefore, most mutational analyses of family CRC are focused primarily on the MLH1 and MSH2 genes [8-14].
Previous studies on HNPCC focused on detecting MMRs mutation, protein expression, and microsatellite instability [10,15,16]. However, genetic transmission is a serial step including DNA duplication, gene transcription and protein translation, namely, an expressional procedure of cell phenotype through corresponding mRNA transmission and special protein or enzymes translation. Although mutations and promoter hypermethylation of MMRs can lead to reduced MMR protein expression, the effect of MMR mutations on its mRNA levels in peripheral blood lymphocytes remains largely unknown. There is no study evaluating the role of mRNA levels of MMRs in screening for HNPCC.
In this study, we used real-time quantitative polymerase chain reaction (qPCR) to investigate peripheral mRNA level of MLH1 in HNPCC families according to Amsterdam Criteria II, and to explore the feasibility of the mRNA level of MLH1 in peripheral mRNA level blood for screening for HNPCC.
Material and methods
HNPCC families
We selected pedigrees according to Amsterdam Criteria II [17], which principally includes: 1) there should be at least three relatives with an HNPCC-associated tumor such as CRC, endometrial, small bowel, and ureter/renal pelvis cancer; 2) at least two successive generations should be affected; 3) at least one should be diagnosed before age 50 years. Nineteen Chinese HNPCC families (totally 46 family participants) fulfilling above mentioned clinical criteria were collected in Shanghai Cancer Center between August 1998 and March 2009. Among them, 12 HNPCC families were described in previous works [13,14]. For the remaining 7 families, MLH1 mutation screening was performed as previously described [13,14]. Informed consent was obtained from each participant before drawing peripheral blood.
RNA isolation
Seven ml of peripheral blood samples were collected from each patients and their family members in 10 ml sterile centrifuge tube containing 200 μl of 0.5 mol/L EDTA, and then centrifuged at 3000 r/min for 10 min. After upper stratum of plasma were removed, medium stratum of karyocytes and lower stratum of erythrocytes were mixed uniformly with 8 ml of hypotonic solution and then set aside at room temperature for 45 min. After centrifuged at 3000 r/min for 2 min, the supernatant of lysed erythrocytes were discarded and then washed out completely as far as possible with hypotonic solution. The precipitate of karyocytes was resuspended with 1 ml of Trizol (Life Technologies, CA, USA) and then incubated at 4°C for 10 min. Subsequently, the mixture was added into 200 μl of chloroform, mixed thoroughly, incubated at 4°C for 10 min and centrifuged at 12,000 r/min for 10 min at 4°C in order. After that, the supernatant was gently pipette, transferred to another sterile centrifuge tube and mixed with an equal volume of isopropanol thoroughly. After incubation at 4°C for 10 min, the mixture was centrifuged at 12,000 r/min for 15 min and the supernatant was then discarded. The pellets were rinsed with pre-chilled 80% ethanol, air-dried for 5 min at room temperature, dissolved with 30-100 μl of DEPC-treated water. Total RNA was stored at -80°C for later use.
qPCR
Complementary DNA was synthesized with 1 μg of total RNA in a 10 μl of a reaction mixture containing 10 units of MMLV reverse transcriptase (Promega, WI, USA), 1 × transcriptor reverse buffer, and 500 M dNTPs. The reaction mixture was incubated at 55°C for 45 min, and then be stored at -20°C for later use. The qPCR was performed using PCR Master Mix (Promega, WI, USA) in a 20 μl of a reaction mixture containing 10 μl of PCR Master Mix, 10 pmol of primers, and 2 μl of cDNA. The PCR amplification conditions were as follows: denaturation at 94°C for 5 min, followed by 40 cycles of denaturation at 95°C for 45 s, annealing at 58 for 45 s, and extension at 72°C for 45 s. The qPCR assays were conducted on a DNA Engine Opticon™ 2 Continuous Fluorescence Detection System (MJ research, USA). The nucleotide sequences of primers and probes were as follows: MLH1, 5’-GTTCTCCGGGAGATGTTGCATA-3’ (forward), 5’-TGGTGGTGTTGAGAAGGTATAACTTTG-3’ (reverse), and FAM-CCTCAGTGGGCCTTGGCACAGC-TAMARA (probe); HBA2, 5’-CCGTCTTCCCCTCCATCG-3’ (forward), 5’-GTCCCAGTTGGTGACGATGC-3’ (reverse), F-CCAGGGCGTGATGGTGGGCAT-P (probe).
Statistical analyses
All statistical analyses were performed using SPSS 11.5 (SPSS Inc., Chicago, IL, USA) and Graphpad Prism 5 (GraphPad, CA, USA). Non-parametric test was used to examine the statistical significance. The area under the receiver operating characteristic (ROC) curve (AUC) was used for the evaluation of the sensitivity and specificity of the mRNA level of MLH1 from peripheral blood as a diagnostic marker for the detection of HNPCC. A P value < 0.05 was considered statistically significant.
Results
Standard template, obtained from serially tenfold diluted standard sample, was subjected to qPCR. To produce a standard curve, log value of the template was plotted vs. the cycle threshold (Ct) value. Figure 1A is the standard curve of MLH1. The standard curve shows a linear relationship between the templates and Ct values with a strong correlation coefficient of r2 = 0.996. The range of detection for MLH1 is from 108 to 100. Figure 1B is the standard curve of HBA2 and its correlation coefficient is 0.996. The upper and lower limit of detection for HBA2 is 107 and 101 copies, respectively.
Figure 1.
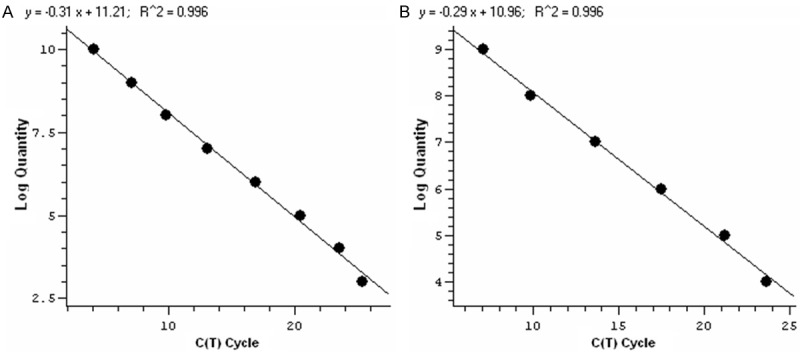
The qPCR standard curve for MLH1 (A) and HBA2 (B) genes. The imput amount is 108, 107, 106, 105, 104, 103, 102, 101 copies of MLH1, and 107, 106, 105, 104, 103, 102, 101 copies of HBA2, respectively.
The mRNA level of MLH1 was measured in all 46 HNPCC family members selected according to Amsterdam Criteria II. Changes in mRNA quality and reverse transcription efficiency were normalized to HBA2. Relative MLH1 gene expression was determined by copies ratio of MLH1 to HBA2 and expressed as MLH1/HBA2 × 105. Relative mRNA expression of MLH1 in HNPCC family ranged from 2.98 × 10-6~64.32 (Table 1) and its mean is 10.91. There was significant difference in mRNA level of MLH1 between HNPCC family members with and without MLH1 mutation (P < 0.001). The levels of MLH1 in members with MLH1 or MSH2 mutation were lower than those without mutation (Figure 2).
Table 1.
The mRNA level of MLH1 in HNPCC family members
| Family number | Mutation | Expression level of MLH1* |
|---|---|---|
| H2-1 | MLH1 mutation | 4.3436 × 10-3 |
| -2 | MLH1 mutation | 5.4375 × 10-3 |
| -3 | MLH1 mutation | 4.6078 × 10-1 |
| -4 | No mutation | 6.4324 × 10-1 |
| H11-1 | MSH2 mutation | 7.3112 × 10-3 |
| -2 | No mutation | 2.3719 × 10-1 |
| H17 | No mutation | NR |
| H21-1 | No mutation | 4.1852 |
| -2 | No mutation | 27.351 |
| H27-1 | No mutation | NA |
| -2 | No mutation | NA |
| -3 | No mutation | 51.671 |
| H28-1 | No mutation | 37.964 |
| -2 | No mutation | 1.9153 × 10-5 |
| -3 | No mutation | 46.278 |
| H31-1 | MLH1 mutation | 3.1153 × 10-1 |
| -2 | MLH1 mutation | 3.2621 × 10-1 |
| -3 | No mutation | 4.7874 |
| H32-1 | No mutation | 29.436 |
| -2 | No mutation | 3.3179 |
| -3 | No mutation | 18.794 |
| H38 | MSH2 mutation | 8.7201 |
| H45-1 | MLH1 mutation | 2.9774 × 10-6 |
| -2 | MLH1 mutation | 2.9723 × 10-5 |
| -3 | No mutation | NA |
| -4 | MLH1mutation | NA |
| H65-1 | MSH2 mutation | 2.5410 × 10-3 |
| -2 | MSH2 mutation | 3.3278 × 10-1 |
| H68-1 | MLH1 mutation | 4.4365 × 10-1 |
| -2 | No mutation | NR |
| H99-1 | MSH2 mutation | 8.8604 × 10-3 |
| -2 | MSH2 mutation | 3.4362 × 10-2 |
| -3 | No mutation | 5.6140 × 10-1 |
| H111-1 | MLH1 mutation | 7.4573 × 10-1 |
| -2 | No mutation | 6.3657 |
| H114-1 | MLH1 mutation | 3.2365 |
| -2 | No mutation | 4.7321 |
NR, no result. NA, sample absence.
Figure 2.
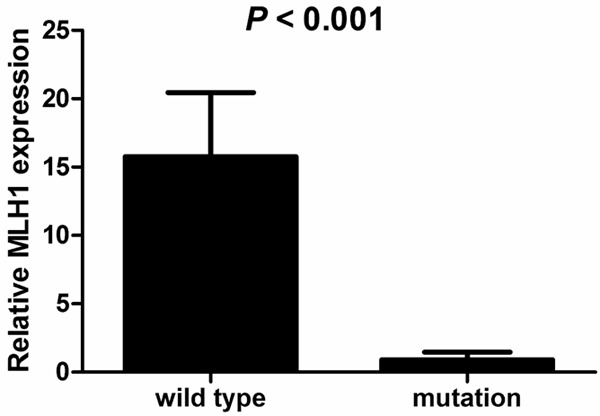
The relative mRNA level of MLH1 in HNPCC family members with or without MLH1 or MSH2 mutation. The levels of MLH1 in HNPCC family members with MLH1 mutation were lower than those with wild type MLH1 (P < 0.001).
The ROC curve was constructed to determine which value of the mRNA level of MLH1 can distinguish HNPCC from controls. ROC analysis showed that AUC for the mRNA level of MLH1 was 0.858 (95% confidence interval 0.714 to 1.003) (Figure 3). At an optimal cut-off value of 0.511, the sensitivity and specificity was 81.3% and 86.7%, respectively.
Figure 3.
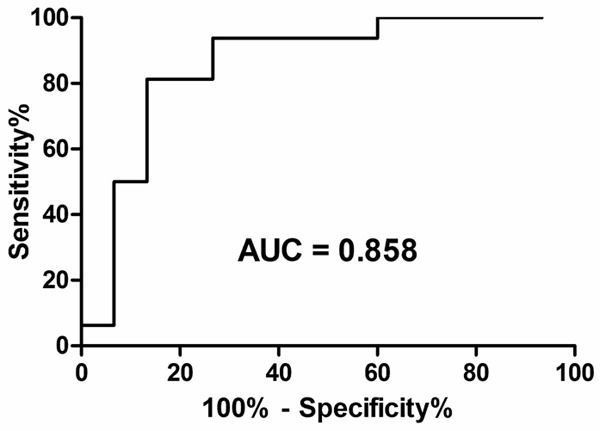
ROC curve of the relative mRNA level of MLH1 in distinguishing HNPCC family members with or without MLH1 or MSH2 mutation. The relative mRNA level of MLH1 was a significant predictor of HNPCC status (cut off: 0.511; Sensitivity: 81.3%, Specificity: 86.7%. AUC = 0.858; P < 0.001).
Discussion
The incidence of colorectal cancer (CRC) is increasingly worldwide. However, therapeutic efficacy for CRC is not very optimistic since its ten-year survival rate is still being stagnated at about 50%. Early diagnosis and treatment is crucial to improve survival rates of CRC. As far as tumor treatment and prevention is concerned, it is potential and fundamental to develop effective means from etiopathogenisis to interfere and interrupt the development and progression of tumor. CRC can be classified into two types, sporadic and hereditary CRC, according to its pathogenesis. HNPCC is a typical kind of inherited CRC and differs greatly from sporadic CRC on treatment and follow-up. Thereafter, it will benefit not only clinical treatment but also genetic consultation to distinguish HNPCC from sporadic CRC [18,19].
Many studies suggested that functional defect of MMR, which at least includes five genes (MLH1, MSH2, PMS1, PMS2, MSH6), lead to the development of HNPCC [19]. The evolutionary conserved mismatch repair proteins correct a wide range of DNA replication errors. Their importance as guardians of genetic integrity is reflected by the tremendous decrease of replication fidelity conferred by their loss. In 1993, microsatellite instability was observed in HNPCC by three uncorrelated study groups at almost the same time, which immediately made biogenetic consciousness of the causal relationship between mutation phenotype and MMR function defect. Studies in yeast and bacteria had shown that microsatellite instability resulted from mutations in so-called postreplicative DNA MMR genes can cause genome instability. It was hypothesized that a similar mechanism might underlie the observed microsatellite instability in HNPCC. Though several methods was used to distinguish HNPCC from sporadic CRC, including different clinical criteria, microsatellite instability detection, immunohisochemistry and sequencing, no strategy and device with high efficiency were established up to now [15,20]. Although next generation sequencing (NGS) can be used to detect all CRC-associated genes [21], even whole genome, its high cost makes it unsuitable for CRC screening in China.
It is hitherto unknown whether there is abnormal MMR expression of peripheral blood at the mRNA level in HNPCC. This will also help better understand and elucidate the pathogenesis of HNPCC. In a previous study, we investigated mutational genotype and phenotype of MLH and MSH2 by peripheral blood cDNA sequencing analysis in different family selected according to Amsterdam II Criteria of HNPCC and found two novel MLH1 and three new MSH2 missense mutation [13,14]. In this study, we evaluated the mRNA level of MLH1 in HNPCC family fulfilling Amsterdam II Criteria and found a significant difference in mRNA level of MLH1 between members with and without mutation. Our results indicated that HNPCC patients had an abnormal MMR expression at the mRNA level. Moreover, other studies showed that hypermethylation of the MLH1 promoter region was found in 10-15% of sporadic CRC, which inhibited MLH1 expression [15,22]. Therefore, hypermethylation of MLH1 should be excluded before clarifying the relationship between abnormal mRNA expression and MMR mutations.
The definition of normal range of the mRNA expression of MMR, depending on detection of large scale non-HNPCC, is certainly required to judge whether the mRNA level of MMR is normal or not. Therefore, further studies are required to validate whether the mRNA level of MMR in peripheral blood detected by real-time PCR could be applied as a simple technique to clinically screen or even diagnose HNPCC.
Acknowledgements
This study was supported by Taizhou Society Development Project, Jiangsu, China (grant No. TS028), The 333 project of scientific research project Foundation, Jiangsu, China (grant No. BRA2015224), and Clinical Medicine Science and Technology Development Fund of Jiangsu University, Jiangsu, China (grant No. JLY20140136).
References
- 1.Lynch HT, de la Chapelle A. Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet. 1999;36:801–818. [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 3.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 4.Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, Lescoe MK, Kane M, Earabino C, Lipford J, Lindblom A, et al. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994;368:258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- 5.Nicolaides NC, Papadopoulos N, Liu B, Wei YF, Carter KC, Ruben SM, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994;371:75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- 6.Miyaki M, Konishi M, Tanaka K, Kikuchi-Yanoshita R, Muraoka M, Yasuno M, Igari T, Koike M, Chiba M, Mori T. Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat Genet. 1997;17:271–272. doi: 10.1038/ng1197-271. [DOI] [PubMed] [Google Scholar]
- 7.Marra G, Boland CR. Hereditary nonpolyposis colorectal cancer: the syndrome, the genes, and historical perspectives. J Natl Cancer Inst. 1995;87:1114–1125. doi: 10.1093/jnci/87.15.1114. [DOI] [PubMed] [Google Scholar]
- 8.Liu F, Yang L, Zhou X, Sheng W, Cai S, Liu L, Nan P, Xu Y. Clinicopathological and genetic features of Chinese hereditary nonpolyposis colorectal cancer (HNPCC) Med Oncol. 2014;31:223. doi: 10.1007/s12032-014-0223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei W, Liu F, Liu L, Li Z, Zhang X, Jiang F, Shi Q, Zhou X, Sheng W, Cai S, Li X, Xu Y, Nan P. Distinct mutations in MLH1 and MSH2 genes in hereditary non-polyposis colorectal cancer (HNPCC) families from China. BMB Rep. 2011;44:317–322. doi: 10.5483/BMBRep.2011.44.5.317. [DOI] [PubMed] [Google Scholar]
- 10.Marques-Lespier JM, Diaz-Algorri Y, Gonzalez-Pons M, Cruz-Correa M. Report of a Novel Mutation in MLH1 Gene in a Hispanic Family from Puerto Rico Fulfilling Classic Amsterdam Criteria for Lynch Syndrome. Gastroenterol Res Pract. 2014;2014:527946. doi: 10.1155/2014/527946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangold E, Pagenstecher C, Friedl W, Mathiak M, Buettner R, Engel C, Loeffler M, Holinski-Feder E, Muller-Koch Y, Keller G, Schackert HK, Kruger S, Goecke T, Moeslein G, Kloor M, Gebert J, Kunstmann E, Schulmann K, Ruschoff J, Propping P. Spectrum and frequencies of mutations in MSH2 and MLH1 identified in 1,721 German families suspected of hereditary nonpolyposis colorectal cancer. Int J Cancer. 2005;116:692–702. doi: 10.1002/ijc.20863. [DOI] [PubMed] [Google Scholar]
- 12.Rajender S, Pooja S, Kumar MV, Karwasra R, Singh L, Thangaraj K. R659X mutation in the MLH1 gene in hereditary non-polyposis colorectal cancer (HNPCC) in an Indian extended family. Indian J Med Res. 2010;131:64–70. [PubMed] [Google Scholar]
- 13.Wang CF, Zhou XY, Sun MH, Cai Q, Zhang TM, Xu Y, Cai SJ, Shi DR. [Two novel germline mutations of MLH1 in hereditary nonpolyposis colorectal cancer family] . Zhonghua Bing Li Xue Za Zhi. 2006;35:68–72. [PubMed] [Google Scholar]
- 14.Wang CF, Zhou XY, Zhang TM, Sun MH, Xu Y, Shi DR. [The analysis for mRNA mutation of MLH1, MSH2 genes and the gene diagnosis for hereditary nonpolyposis colorectal cancer] . Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2006;23:32–36. [PubMed] [Google Scholar]
- 15.Lynch PM. The hMSH2 and hMLH1 genes in hereditary nonpolyposis colorectal cancer. Surg Oncol Clin N Am. 2009;18:611–624. doi: 10.1016/j.soc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Coolbaugh-Murphy MI, Xu JP, Ramagli LS, Ramagli BC, Brown BW, Lynch PM, Hamilton SR, Frazier ML, Siciliano MJ. Microsatellite instability in the peripheral blood leukocytes of HNPCC patients. Hum Mutat. 2010;31:317–324. doi: 10.1002/humu.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieber OM, Heinimann K, Gorman P, Lamlum H, Crabtree M, Simpson CA, Davies D, Neale K, Hodgson SV, Roylance RR, Phillips RK, Bodmer WF, Tomlinson IP. Analysis of chromosomal instability in human colorectal adenomas with two mutational hits at APC. Proc Natl Acad Sci U S A. 2002;99:16910–16915. doi: 10.1073/pnas.012679099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drost M, Zonneveld J, van Dijk L, Morreau H, Tops CM, Vasen HF, Wijnen JT, de Wind N. A cell-free assay for the functional analysis of variants of the mismatch repair protein MLH1. Hum Mutat. 2010;31:247–253. doi: 10.1002/humu.21180. [DOI] [PubMed] [Google Scholar]
- 19.Shin YK, Heo SC, Shin JH, Hong SH, Ku JL, Yoo BC, Kim IJ, Park JG. Germline mutations in MLH1, MSH2 and MSH6 in Korean hereditary non-polyposis colorectal cancer families. Hum Mutat. 2004;24:351. doi: 10.1002/humu.9277. [DOI] [PubMed] [Google Scholar]
- 20.Shia J, Klimstra DS, Nafa K, Offit K, Guillem JG, Markowitz AJ, Gerald WL, Ellis NA. Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol. 2005;29:96–104. doi: 10.1097/01.pas.0000146009.85309.3b. [DOI] [PubMed] [Google Scholar]
- 21.Kraus C, Rau TT, Lux P, Erlenbach-Wunsch K, Lohr S, Krumbiegel M, Thiel CT, Stohr R, Agaimy A, Croner RS, Sturzl M, Hohenberger W, Hartmann A, Reis A. Comprehensive screening for mutations associated with colorectal cancer in unselected cases reveals penetrant and nonpenetrant mutations. Int J Cancer. 2015;136:E559–568. doi: 10.1002/ijc.29149. [DOI] [PubMed] [Google Scholar]
- 22.Kim HC, Kim CN, Yu CS, Roh SA, Kim JC. Methylation of the hMLH1 and hMSH2 promoter in early-onset sporadic colorectal carcinomas with microsatellite instability. Int J Colorectal Dis. 2003;18:196–202. doi: 10.1007/s00384-002-0445-0. [DOI] [PubMed] [Google Scholar]


