Abstract
Toxocariasis is an anthropozoonosis that occurs in all parts of the world. In particular, this disease can often be found in developing countries and in regions, where basic sanitation conditions are poor. However, industrialized countries have reported seroprevalence rates as high as 14.2% in humans. The definitive hosts of the disease are dogs and cats, whereas humans are a paratenic host. To determine the burden of toxocariasis in Brazil, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to conduct a systematic review of the literature. Using keywords and applying the established criteria, we identified 160 publications and selected 22 articles for further analysis. The seroprevalence of toxocariasis in various regions of the country ranged from 4.2% to 65.4%. The highest prevalence was found in the northeast region, although the majority of the studies identified were from the southeast region. The findings suggest the importance of raising awareness among health professionals and public authorities about the fact that toxocariasis is a health problem.
Introduction
The term “toxocariasis” is used in medical practice to designate the human infection produced by the roundworms Toxocara canis and Toxocara cati. Toxocara canis is the nematode most frequently found in Canidae, which are accidental hosts of this roundworm. Other animal species, such as rats, birds, and humans, are accidental hosts and act as reservoirs for these parasites.1
The infection of humans occurs by ingestion of eggs that are present in the soil via foods such as vegetables and by consumption of the meat and/or raw or undercooked viscera of chicken, ducks, or cattle infected with T. canis larvae.2–4 Once ingested, the eggs hatch in the intestinal lumen, and the larvae are then released into the circulatory system and settle in various organs and tissues, including the eye, brain, lungs, liver, and muscles.5 The host immune response involves both the innate and the adaptive systems. Th2 lymphocytes are specifically produced, including interleukins 4, 5, 10, and 13, and lecithins are released.6
The hygiene hypothesis implies that a helminth infection will decrease allergic reactions and diseases, including obesity, by interfering with the immune response.7–9 However, Maizels6 notes that this allergic modulation does not occur with the infections produced by T. canis in rats. One reason may be that these animals are not the definitive hosts of the parasite, so there may be another type of inflammatory modulation in this host. In any case, Fialho and Corrêa10 showed that asthmatic children infected with Toxocara have a higher body mass index than do asthmatic children not infected with the parasite. The association between asthma and toxocariasis has been noted by various articles published in several countries, although the studies were all based on cross-sectional designs. Even though these studies proposed valid hypotheses, a cross-sectional design is a substantial limitation for determining a causal relationship in associations between factors (in this case, asthma and obesity) because no cause–effect correlation can be determined between variables if they are measured at the same time.11
Human infections can cause serious clinical disease, with various levels of severity and possible chronicity. The diagnosis of toxocariasis is performed using an enzyme-linked immunosorbent assay (ELISA) specific for excretion–secretion antigens,12 and the classic treatment is antihelminthic drugs.13
Toxocariasis has always been present but has had varying prevalence rates. A study conducted by Campos Júnior and Elefant14 showed a significant difference between the prevalence of seropositivity for T. canis among children from poor neighborhoods in Brasilia (21.8%) and children living in the wealthiest sectors of the city (3%). The prevalence in Brasilia can be compared with the reported prevalences of 37.9% in subtropical Argentina,15 39% in the city of La Plata,16 29.6% in Nigeria,17 22% in western France,18 30% in the outskirts of Caracas,19 and 27.2% in a school population in Trinidad.20
Toxocariasis is an important but neglected tropical disease with a worldwide distribution and a high prevalence in both developing and developed countries. This disease is considered to be one of the most prevalent helminthiases in endemic areas in America. Human toxocariasis is also associated with important morbidities that are of public health concern and has been included on lists of neglected zoonoses.21–23 The present study is a systematic review that aims to understand how studies on the prevalence of toxocariasis in humans are being conducted in Brazil.
Based on the magnitude of its prevalence and its association with various diseases and clinical manifestations, toxocariasis is a relevant disease that should receive attention from public health systems. Accordingly, the specific aim of the present study is to verify, using a systematic review, the study populations and references of existing studies and the prevalence or incidence rates described in each of the investigations.
Methods
Data and search strategy.
Articles were selected from the following databases: PubMed (www.ncbi.nlm.nih.gov/PubMed/), Latin American and Caribbean Health Sciences Literature, Brazil (http://www.bireme.br), and Embase. The articles were published between January 2008 and October 2014. The descriptors and medical subject heading search terms all included “toxocariasis Brazil” in the bibliographic data, title field, and/or abstract and keywords. Articles were restricted to English and Portuguese.
Selection criteria.
We used the following selection criteria: scientific articles originally published in national or international journals, studies with a date of publication between January 2008 and October 2014, studies on human beings, studies with no age limits on the study population, and studies reporting the prevalence or incidence of toxocariasis. As the main purpose was to determine the current prevalence of toxocariasis in several regions of Brazil, the selected studies could have been conducted in any Brazilian city, and the search period started with 2008.
Data extraction.
The selection of articles and the data extraction were independently performed by two reviewers using a standardized instrument that collected the following information: study region, sample size, total number of seropositive individuals, study design, variables (risk factors, symptoms, morbidity, sociodemographic characteristics), main results, and study limitations. The data were compiled in Microsoft Excel and were analyzed by comparing categorical variables.
Results and Discussion
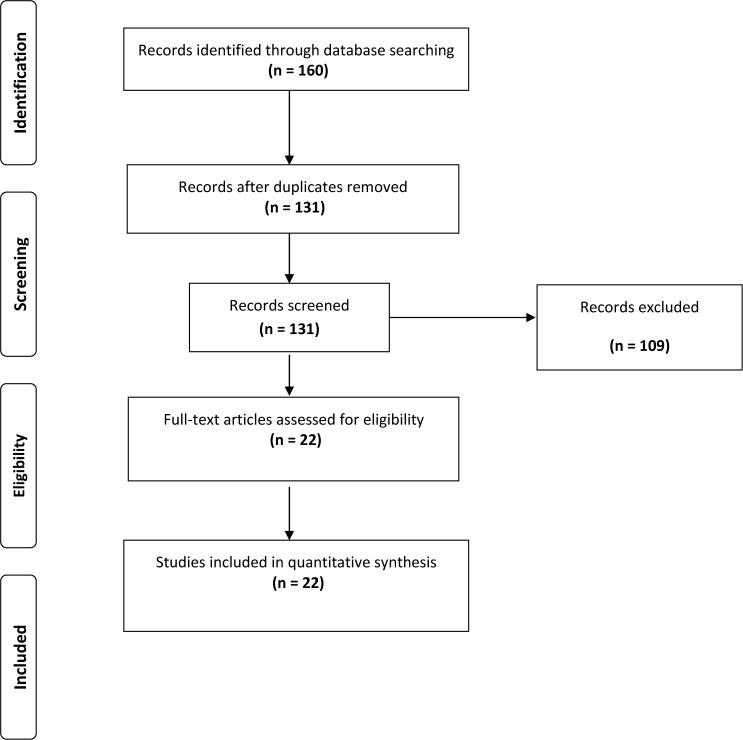
After the bibliographic search, we identified 160 publications. Among the 160 publications, 29 duplicates from two or more databases were discarded. After reading the titles and abstracts, 109 were excluded because they were not studies on human beings (they were experimental studies) or because there was no mention of the prevalence or incidence of toxocariasis. Therefore, a total of 22 complete articles were eligible for analysis. Figure 1 shows the flowchart of the study selection.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. (Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097.)
We selected 22 articles published between January 2008 and October 2014 that described the current prevalence of toxocariasis in various regions of the country; the values ranged from 4.2% to 65.4% (Table 1). The articles considered the epidemiological characterization of toxocariasis in Brazil over the last 6 years. Most studies used a cross-sectional design and presented the prevalence of the disease as a measure of frequency. One study described the incidence of toxocariasis, which was seven of 100 children in the year surveyed.35
Table 1.
Description of articles published from 2008 to 2014 that satisfied the search criteria
| Article | Region | State | n | Total positives | Seroprevalence (%) | Main studied characteristics | Statistical evaluation |
|---|---|---|---|---|---|---|---|
| Marchioro and others, 201424 | Southeast | Paraná | 544 | 136 | 25.0 | 1–12 years; Toxoplasma gondii; contact with dogs; contact with cats; eosinophilia; playing in the sand | Bivariate analysis; multiple logistic regression |
| Cassenote and others, 201425 | Southeast | São Paulo | 252 | 39 | 15.5 | 1–12 years; sociodemographic characteristics; geophagy; onychophagia; hand washing habits; eosinophilia; intestinal parasites | Poisson multiple regression |
| Fialho and Corrêa, 201410 | Southeast | São Paulo | 116 | 63 | 54.3 | 2–14 years; asthma; eosinophilia; height; weight; BMI | Descriptive analysis; Wilcoxon test for two samples |
| Negri and others, 201326 | Southeast | São Paulo | 253 | 22 | 8.7 | 19–65 years (blood donor); education; income; house location; dog; cat; garden; contact with soil; geophagy; onychophagia; raw meat consumption | Bivariate analysis; logistic regression |
| Guilherme and others, 201327 | Southeast | Paraná | 167 | 7 | 4.2 | 1–15 years; eosinophilia; allergies; rhinitis; asthma; bronchitis; domestic dog; domestic cat | Comparison of frequencies; ratio between the outer diameter of the sample and the optical density of the cutoff value |
| Schoenardie and others, 201328 | Southeast | Rio Grande do Sul | 427 | 216 | 50.6 | 1–12 years | chi-square test; Mantel–Haenszel test; ORs |
| Prestes-Carneiro and others, 201329 | Southeast | São Paulo | 194 | 28 | 14.4 | 5–73 years; contact with dogs and cats; education; family income; eosinophilia; anemia; T. gondii; Taenia solium metacestode | Chi-square test; Fisher's exact test; regression coefficients |
| Manini and others, 201230 | Southeast | Paraná | 90 | 16 | 17.8 | 1–12 years; asthma; bronchitis; skin allergies; eosinophilia | Mean and standard deviation; relative and absolute frequencies; Fisher's exact test |
| Mattia and others, 201231 | Southeast | Paraná | 353 | 130 | 36.8 | 0–12 years; recurrent wheezing; headache; fever; abdominal pain; eosinophilia; contact with dogs; contact with cats; geophagy; onychophagia | Bivariate analysis; logistic regression |
| Fragoso and others, 201132 | Southeast | Espírito Santo | 391 | 202 | 51.6 | 7 years; parasitological; eosinophilia; asthma history; skin allergy history; sociodemographic variables | Frequencies; 95% CIs |
| Marchioro and others, 201133 | Southeast | Paraná | 1,199 | 386 | 32.2 | 7 months to 12 years; eosinophilia | Descriptive analysis; chi-square test |
| Santarém and others, 201134 | Southeast | São Paulo | 126 + 126 Total = 252 | 12 + 16 Total = 28 | 9.5 + 12.7 Accumulated = 11.1 | 10 months to 15 years; eosinophilia; dog at home; domestic cat; onychophagia; social class (two-class comparison) | Mann–Whitney test; chi-square test or Fisher's exact test; multivariate logistic regression; ORs; 95% CIs |
| Correa and Bismarck, 201035 | Southeast | São Paulo | 100 | 28 | 28.0 | 6–14 years; toxocariasis incidence | Calculation of incidence and prevalence |
| Colli and others, 201036 | Southeast | Paraná | 376 | 194 | 51.6 | 1–12 years; eosinophilia; abdominal pain; headache; recurrent wheezing; parasitological (parasites not found); onychophagia; geophagy | Multiple logistic regression |
| Prestes-Carneiro and others, 200937 | Southeast | São Paulo | 182 | 25 | 13.7 | 4–84 years (≤ 15 vs. ≥ 15 years); risk factor (dog breeding, cat breeding, health conditions, level of education, clinical symptoms); anemia; leucopenia; neutropenia; lymphocytosis; monocytosis; eosinophilia | t-test for independent samples; chi-square test; Fisher's exact test; correlation; coefficient test; ORs; multiple logistic regression |
| Prestes-Carneiro and others, 200838 | Southeast | São Paulo | 79 | 17 | 21.5 | ≤ 15 vs. ≥ 15 years; eosinophilia; education; helminths; house with dogs and cats; anemia; health conditions; family income | t-test for independent samples; chi-square test; Fisher's exact test; correlation coefficients (r) |
| Mendonça and others, 201339 | Northeast | Bahia | 1,309 | 633 | 48.4 | 4–11 years; maternal education; going to school; paved street; domestic dog; domestic cat | Univariate analysis; multivariate logistic regression; ORs; 95% CIs |
| Mendonça and others, 201240 | Northeast | Bahia | 1,148 | 540 | 47.0 | 4–11 years (1.445); wheezing; allergies; parasitological findings; eosinophilia | Univariate analysis; multivariate logistic regression |
| Souza and others, 201141 | Northeast | Bahia | 150 + 188 | 78 + 123 | 52.0 + 65.4 | ≤ 15 vs. ≥ 15 years; comparison of two population groups; contact with dogs; contact with cats; social class | Chi-square test; univariate and multivariate logistic regression |
| Dattoli and others, 201142 | Northeast | Bahia | 268 | 124 | 46.3 | 31–40 years (blood donor); eosinophilia; intestinal helminths (not found in samples); education | Univariate analysis; multivariate logistic regression; ORs; 95% CIs |
| Oliart-Guzmán and others, 201443 | North | Amazonas | 182 + 357 | 41 + 65 | 28.0 + 23.3 | 6 months to 59 months; socioeconomic status and demographic variables; wheezing; asthma; helminths; domestic dog; domestic cat | Variance analysis; chi-square test or Fisher's exact test |
| Rubinsky-Elefant and others, 200844 | North | Amazonas | 403 | 108 | 26.8 | 5–90 years; education of the family provider; presence of dogs; presence of cats; sector of residence; index of wealth; intestinal parasites | Prevalence rate; 95% CIs; chi-square test; Fisher's exact test; Mann–Whitney test; ORs; logistic regression |
BMI = body mass index; CI = confidence interval; OR = odds ratio.
Geographical areas and socioeconomic class.
Among the 22 articles analyzed, the most commonly studied Brazilian geographical regions were the southeast (41%), south (32%), northeast (18%), and north (9%). We did not find any study of the population in the midwest region.
In the northeast region, a study of 1,309 children aged 4–11 years was conducted in the city of Salvador, Bahia. The study investigated the possible association between seropositivity for Toxocara, atopy, and childhood wheezing in a population of children in poor areas of the city.40 Also in the city of Salvador, another study, which examined 338 middle- and lower-class individuals, found a higher prevalence of toxocariasis among those of the lower class and those with greater contact with dogs and cats. The study also showed that being in the lower class was associated with a higher risk of infection with T. canis. This association may be related to the lack of knowledge of the population in relation to forms of infection, as well as to contact with dogs and cats that have not been dewormed.41
Another study, conducted by Santarém and others in 2011 in the municipality of Presidente Prudente, São Paulo, also examined the association between social class and seropositivity for Toxocara. The results showed that being in the upper middle class was a protective factor both for the total population and for subgroups (middle class and lower class).34
Study population.
The age of the subjects who participated in the studies ranged from 6 months to 90 years; however, the majority of the studies were conducted in children up to 15 years of age (68%) (Table 1).
The studies assessed in this review included sample sizes that ranged from 79 to 1,309 subjects, with 55% of the studies including 250–500 individuals (Table 1).
Three studies described the calculation of the sample size but did not describe their method of random sampling. In 86% of the studies, the authors relied on nonrandomized sampling.
Seroprevalence.
The 22 studies analyzed in this review included 8,980 individuals who were evaluated for the presence of toxocariasis in four out of five Brazilian regions.
Due to the methodology used in the studies, the overall prevalence of toxocariasis in Brazil could not be calculated. However, most of the studies showed prevalence rates greater than 20%. Additionally, most studies evaluated the association of toxocariasis with other clinical manifestations.
The seroprevalence of the disease ranged from 4.2% to 65.4%. In 45% of the studies, the prevalence was higher than 50%. The highest prevalences were found in the northeast region (Table 1).
In the south region of the country, a study was conducted among 1,199 children aged 7 months to 12 years. The children resided in the urban areas of nine municipalities in the northwest region of Parana and were receiving assistance from the Unified Health System. The authors found a prevalence of 32.2%.33 In contradiction to other studies,45–50 the majority of the children (80.4%) showed no eosinophilia.
In regard to the association between toxocariasis and other variables, 36% of the studies described a relationship with asthma or wheezing.10,27,30–32,36,40 In all of these studies, a higher proportion of patients with asthma was found among those infected with Toxocara, which lends this infection greater relevance in terms of public health.
The majority of studies (95.4%) described the diagnostic method for the detection of toxocariasis, which was ELISA.10,24–29,31–34,36–44 One study did not describe the diagnostic methodology applied.35 As T. canis and T. cati cannot be distinguished serologically, we identified the study population as having toxocariasis.
Although the Centers for Disease Control and Prevention in the United States acknowledges that toxocariasis is one of five neglected parasitic diseases for which there should be investment in diagnostic and therapeutic methods,21,23,51 the disease has not been widely recognized as a public health concern.
Toxocara canis infection is known to be associated with clinical polymorphism that varies from asymptomatic infection to asthmatic bronchitis and meningoencephalitis. Therefore, health professionals and the public health system should be aware that toxocariasis is a health problem. The present study provides relevant information for reflection on and review of public policies regarding toxocariasis.
The current burden and prevalence of disease due to toxocariasis in Brazil are largely unknown. We conducted this review to determine both the prevalence of toxocariasis and the amount of available data measuring the burden of toxocariasis in Brazil and to identify areas needing future research. Another salient point made by this review is that preventive efforts, such as prevention of soil contamination by dog and cat feces in public areas, hand washing after soil contact, and preventive anthelmintic treatment of puppies and kittens, can help to minimize exposure to Toxocara spp. and control potential morbidity associated with Toxocara infection.21,52
Footnotes
Financial support: We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (grant no. 2012/14134-6) for the PhD scholarship for PMMF.
Authors' addresses: Paula Mayara Matos Fialho and Carlos Roberto Silveira Corrêa, Departamento de Saúde Coletiva, Faculdade de Ciências Médicas, Universidade Estadual de Campinas, Campinas, São Paulo, Brazil, E-mails: paulamayara2@gmail.com and ccorrea@fcm.unicamp.br.
References
- 1.Andrade LD. Aspectos clinico-epidemiológicos da toxocaríase humana. Rev Patol Trop. 2000;29:147–159. [Google Scholar]
- 2.Morimatsu Y, Akao N, Akiyoshi H, Kawazu T, Okabe Y, Aizawa H. A familial case of visceral larva migrans after ingestion of raw chicken livers: appearance of specific antibody in bronchoalveolar lavage fluid of the patients. Am J Trop Med Hyg. 2006;75:303–306. [PubMed] [Google Scholar]
- 3.Choi D, Lim JH, Choi D-C, Paik SW, Kim S-H, Huh S. Toxocariasis and ingestion of raw cow liver in patients with eosinophilia. Korean J Parasitol. 2008;46:139–143. doi: 10.3347/kjp.2008.46.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010;104:3–23. doi: 10.1179/136485910X12607012373957. [DOI] [PubMed] [Google Scholar]
- 5.Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003;16:265–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maizels RM. Exploring the immunology of parasitism—from surface antigens to the hygiene hypothesis. Parasitology. 2009;136((Special Issue 12)):1549–1564. doi: 10.1017/S0031182009006106. [DOI] [PubMed] [Google Scholar]
- 7.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann GG, Bergmann ML de A, Pinheiro E dos S, Moreira RB, Marques AC, Garlipp DC, Gaya DC. Índice de massa corporal: tendência secular em crianças e adolescentes brasileiros. Rev Bras Cineantropometria Desempenho Hum. 2009;11:280–285. [Google Scholar]
- 9.Terres NG, Pinheiro RT, Horta BL, Pinheiro KAT, Horta LL. Prevalência e fatores associados ao sobrepeso e à obesidade em adolescentes. Rev Saude Publica. 2006;40:627–633. doi: 10.1590/s0034-89102006000500011. [DOI] [PubMed] [Google Scholar]
- 10.Fialho PMM, Corrêa CRS. Toxocaríase, asma e índice de massa corporal em crianças e adolescentes em Campinas-SP, 1996 a 1998. Epidemiol E Serviços Saúde. 2014;23:361–368. [Google Scholar]
- 11.Medronho RA. Epidemiologia. São Paulo, Brazil: Atheneu; 2009. [Google Scholar]
- 12.Smith H, Holland C, Taylor M, Magnaval J-F, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009;25:182–188. doi: 10.1016/j.pt.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Lescano SZ, Chieffi PP, Amato Neto V, Ikai DK, Ribeiro MCSA. Anti-helmínticos na toxocaríase experimental: efeito na recuperação de larvas de Toxocara canis e na resposta humoral. J Bras Patol E Med Lab. 2005;41:21–24. [Google Scholar]
- 14.Campos Júnior D, Elefant GR, de Melo e Silva EO, Gandolfi L, Jacob CMA, Tofeti A, Pratesi R. Freqüência de soropositividade para antígenos de Toxocara canis em crianças de classes sociais diferentes. Rev Soc Bras Med Trop. 2003;36:509–513. [PubMed] [Google Scholar]
- 15.Alonso JM, Bojanich MVI, Chamorro M, Gorodner JO. Toxocara seroprevalence in children from a subtropical city in Argentina. Rev Inst Med Trop Sao Paulo. 2000;42:235–237. doi: 10.1590/s0036-46652000000400010. [DOI] [PubMed] [Google Scholar]
- 16.Shields JA. Ocular toxocariasis. A review. Surv Ophthalmol. 1984;28:361–381. doi: 10.1016/0039-6257(84)90242-x. [DOI] [PubMed] [Google Scholar]
- 17.Ajayi O, Duhlinska D, Agwale S, Njoku M. Frequency of human toxocariasis in Jos, Plateau State, Nigeria. Mem Inst Oswaldo Cruz. 2000;95:147–149. doi: 10.1590/S0074-02762000000200002. [DOI] [PubMed] [Google Scholar]
- 18.Gueglio B, de Gentile L, Nguyen JM, Achard J, Chabasse D, Marjolet M. Epidemiologic approach to human toxocariasis in western France. Parasitol Res. 1994;80:531–536. doi: 10.1007/BF00932703. [DOI] [PubMed] [Google Scholar]
- 19.Lynch N, Hagel I, Vargas V, Rotundo A, Varela M, Di Prisco M, Hodgen AN. Comparable seropositivity for ascariasis and toxocariasis in tropical slum children. Parasitol Res. 1993;79:547–550. doi: 10.1007/BF00932238. [DOI] [PubMed] [Google Scholar]
- 20.Baboolal S, Rawlins SC. Seroprevalence of toxocariasis in schoolchildren in Trinidad. Trans R Soc Trop Med Hyg. 2002;96:139–143. doi: 10.1016/s0035-9203(02)90281-6. [DOI] [PubMed] [Google Scholar]
- 21.Lee RM, Moore LB, Bottazzi ME, Hotez PJ. Toxocariasis in North America: a systematic review. PLoS Negl Trop Dis. 2014;8:e3116. doi: 10.1371/journal.pntd.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008;2:e256. doi: 10.1371/journal.pntd.0000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parise ME, Hotez PJ, Slutsker L. Neglected parasitic infections in the United States: needs and opportunities. Am J Trop Med Hyg. 2014;90:783–785. doi: 10.4269/ajtmh.13-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchioro AA, Colli CM, Ferreira ÉC, Viol BM, Araújo SM, Falavigna-Guilherme AL. Risk factors associated with toxoplasmosis and toxocariasis in populations of children from nine cities in southern Brazil. J Helminthol. 2014;89:428–432. doi: 10.1017/S0022149X14000212. [DOI] [PubMed] [Google Scholar]
- 25.Cassenote AJF, de Abreu Lima AR, Pinto Neto JM, Rubinsky-Elefant G. Seroprevalence and modifiable risk factors for Toxocara spp. in Brazilian schoolchildren. PLoS Negl Trop Dis. 2014;8:e2830. doi: 10.1371/journal.pntd.0002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negri EC, Santarém VA, Rubinsky-Elefant G, Giuffrida R. Anti-Toxocara spp. antibodies in an adult healthy population: serosurvey and risk factors in Southeast Brazil. Asian Pac J Trop Biomed. 2013;3:211–216. doi: 10.1016/S2221-1691(13)60052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilherme EV, Marchioro AA, Araujo SM, Falavigna DL, Adami C, Falavigna-Guilherme G, Rubinsky-Elefant G, Falavigna-Guilherme AL. Toxocariasis in children attending a Public Health Service Pneumology Unit in Paraná State, Brazil. Rev Inst Med Trop Sao Paulo. 2013;55:189–192. doi: 10.1590/S0036-46652013000300009. [DOI] [PubMed] [Google Scholar]
- 28.Schoenardie ER, Scaini CJ, Brod CS, Pepe MS, Villela MM, McBride AJA, Borsuk S, Berne MEA. Seroprevalence of Toxocara infection in children from southern Brazil. J Parasitol. 2013;99:537–539. doi: 10.1645/GE-3182. [DOI] [PubMed] [Google Scholar]
- 29.Prestes-Carneiro LE, Rubinsky-Elefant G, Ferreira AW, Araujo PR, Troiani C, Zago SC, Kaiahara M, Sasso L, Iha A, Vaz A. Seroprevalence of toxoplasmosis, toxocariasis and cysticercosis in a rural settlement, São Paulo State, Brazil. Pathog Glob Health. 2013;107:88–95. doi: 10.1179/2047773213Y.0000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manini MP, Marchioro AA, Colli CM, Nishi L, Falavigna-Guilherme AL. Association between contamination of public squares and seropositivity for Toxocara spp. in children. Vet Parasitol. 2012;188:48–52. doi: 10.1016/j.vetpar.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Mattia S, Colli CM, Adami CM, Guilherme GF, Nishi L, Rubinsky-Elefant G, Marchioro AA, Gomes ML, Falavigna-Guilherme AL. Seroprevalence of Toxocara infection in children and environmental contamination of urban areas in Paraná State, Brazil. J Helminthol. 2012;86:440–445. doi: 10.1017/S0022149X11000666. [DOI] [PubMed] [Google Scholar]
- 32.Fragoso RP, Monteiro MBM, Lemos EM, Pereira FEL. Anti-Toxocara antibodies detected in children attending elementary school in Vitoria, State of Espírito Santo, Brazil: prevalence and associated factors. Rev Soc Bras Med Trop. 2011;44:461–466. doi: 10.1590/s0037-86822011000400012. [DOI] [PubMed] [Google Scholar]
- 33.Marchioro AA, Colli CM, Mattia S, Paludo ML, de Melo GC, Adami CM, Pelloso SM, Guilherme ALF. Avaliação eosinofílica e soropositividade para anticorpos IgG anti-Toxocara em crianças atendidas pelo Sistema Único de Saúde. Rev Paul Pediatr. 2011;29:80–84. [Google Scholar]
- 34.Santarém VA, Leli FNC, Rubinsky-Elefant G, Giuffrida R. Protective and risk factors for toxocariasis in children from two different social classes of Brazil. Rev Inst Med Trop Sao Paulo. 2011;53:66–72. doi: 10.1590/s0036-46652011000200002. [DOI] [PubMed] [Google Scholar]
- 35.Correa CRS, Bismarck CM. Toxocariasis: incidence, prevalence and the time serum remains positive in school children from Campinas, SP, Brazil. J Trop Pedriatrics. 2010;56:215–216. doi: 10.1093/tropej/fmp095. [DOI] [PubMed] [Google Scholar]
- 36.Colli CM, Rubinsky-Elefant G, Paludo ML, Falavigna DLM, Guilherme EV, Mattia S, Araujo SM, Ferreira É, Previdelli ITS, Falavigna-Guilherme AL. Serological, clinical and epidemiological evaluation of toxocariasis in urban areas of south Brazil. Rev Inst Med Trop Sao Paulo. 2010;52:69–74. doi: 10.1590/s0036-46652010000200002. [DOI] [PubMed] [Google Scholar]
- 37.Prestes-Carneiro LE, Souza DHP, Moreno GC, Troiani C, Santarém V, Zago SCS, Miguel NA, Freitas SBZ, Faria R, Martini L, Rubinsky-Elefant G, Iha A, Vaz AJ. Toxocariasis/cysticercosis seroprevalence in a long-term rural settlement, São Paulo, Brazil. Parasitology. 2009;136:681–689. doi: 10.1017/S0031182009005769. [DOI] [PubMed] [Google Scholar]
- 38.Prestes-Carneiro LE, Santarém V, Zago SCS, Miguel NA, Zambelli S de F, Villas R, Vaz AJ, Rubinsky-Elefant G. Sero-epidemiology of toxocariasis in a rural settlement in São Paulo state, Brazil. Ann Trop Med Parasitol. 2008;102:347–356. doi: 10.1179/136485908X278801. [DOI] [PubMed] [Google Scholar]
- 39.Mendonça LR, Figueiredo CA, Esquivel R, Fiaccone RL, Pontes-de-Carvalho L, Cooper P, Barreto ML, Alcantara-Neves NM. Seroprevalence and risk factors for Toxocara infection in children from an urban large setting in northeast Brazil. Acta Trop. 2013;128:90–95. doi: 10.1016/j.actatropica.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendonça LR, Veiga RV, Dattoli VCC, Figueiredo CA, Fiaccone R, Santos J, Cruz ÁA, Rodrigues LC, Cooper PJ, Pontes-de-Carvalho LC, Barreto ML, Alcantara-Neves NM. Toxocara seropositivity, atopy and wheezing in children living in poor neighbourhoods in urban Latin American. PLoS Negl Trop Dis. 2012;6:e1886. doi: 10.1371/journal.pntd.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souza RF, Dattoli VCC, Mendonça LR, Jesus JR, Baqueiro T, Santana Cde C, Santos NM, Barrouin-Melo SM, Alcantara-Neves NM. Prevalência e fatores de risco da infecção humana por Toxocara canis em Salvador, Estado da Bahia. Rev Soc Bras Med Trop. 2011;44:516–519. doi: 10.1590/s0037-86822011000400024. [DOI] [PubMed] [Google Scholar]
- 42.Dattoli VCC, Freire SM, Mendonça LR, Santos PC, Meyer R, Alcantara-Neves NM. Toxocara canis infection is associated with eosinophilia and total IgE in blood donors from a large Brazilian centre. Trop Med Int Health. 2011;16:514–517. doi: 10.1111/j.1365-3156.2010.02719.x. [DOI] [PubMed] [Google Scholar]
- 43.Oliart-Guzmán H, Delfino BM, Martins AC, Mantovani SAS, Braña AM, Pereira TM, Branco FLCC, Ramalho AA, Campos RG, Fontoura PS, de Araujo TS, de Oliveira CSM, Muniz PT, Rubinsky-Elefant G, Codeço CT, da Silva-Nunes M. Epidemiology and control of child toxocariasis in the western Brazilian Amazon—a population-based study. Am J Trop Med Hyg. 2014;90:670–681. doi: 10.4269/ajtmh.13-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubinsky-Elefant G, da Silva-Nunes M, Malafronte RS, Muniz PT, Ferreira MU. Human toxocariasis in rural Brazilian Amazonia: seroprevalence, risk factors, and spatial distribution. Am J Trop Med Hyg. 2008;79:93–98. [PubMed] [Google Scholar]
- 45.Núñez CR, Martínez GDM, Arteaga SY, Macotela MP, Montes PB, Durán NR. Prevalence and risk factors associated with Toxocara canis infection in children. Scientific World J. 2013;2013:572089. doi: 10.1155/2013/572089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim Y-H, Huh S, Chung Y-B. Seroprevalence of toxocariasis among healthy people with eosinophilia. Korean J Parasitol. 2008;46:29–32. doi: 10.3347/kjp.2008.46.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roldán WH, Espinoza YA, Atúncar A, Ortega E, Martinez A, Saravia M. Frequency of eosinophilia and risk factors and their association with Toxocara infection in schoolchildren during a health survey in the north of Lima, Peru. Rev Inst Med Trop Sao Paulo. 2008;50:273–278. doi: 10.1590/s0036-46652008000500005. [DOI] [PubMed] [Google Scholar]
- 48.Paludo ML, Falavigna DLM, Elefant GR, Gomes ML, Baggio MLM, Amadei LB, Falavigna-Guilherme AL. Frequency of Toxocara infection in children attended by the health public service of Maringá, south Brazil. Rev Inst Med Trop Sao Paulo. 2007;49:343–348. doi: 10.1590/s0036-46652007000600002. [DOI] [PubMed] [Google Scholar]
- 49.Figueiredo SDP, Taddei JAAC, Menezes JJC, Novo NF, Silva EOM, Cristóvão HLG, Cury MCFS. Estudo clínico-epidemiológico da toxocaríase em população infantil. J Pediatr (Rio J) 2005;81:126–132. [PubMed] [Google Scholar]
- 50.Jacob CMA, Pastorino AC, Peres BA, Mello EO, Okay Y, Oselka GW. Clinical and laboratorial features of visceral toxocariasis in infancy. Rev Inst Med Trop Sao Paulo. 1994;36:19–26. doi: 10.1590/s0036-46651994000100004. [DOI] [PubMed] [Google Scholar]
- 51.Woodhall DM, Eberhard ML, Parise ME. Neglected parasitic infections in the United States: toxocariasis. Am J Trop Med Hyg. 2014;90:810–813. doi: 10.4269/ajtmh.13-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Won KY, Kruszon-Moran D, Schantz PM, Jones JL. National seroprevalence and risk factors for zoonotic Toxocara spp. infection. Am J Trop Med Hyg. 2008;79:552–557. [PubMed] [Google Scholar]