Abstract
Sporotrichosis is a polymorphic disease of humans and animals, which is acquired via traumatic inoculation of Sporothrix propagules into cutaneous or subcutaneous tissue. The etiological agents are in a clinical complex, which includes Sporothrix brasiliensis, Sporothrix schenckii, Sporothrix globosa, and Sporothrix luriei, each of which has specific epidemiological and virulence characteristics. Classical manifestation in humans includes a fixed localized lesion at the site of trauma plus lymphocutaneous sporotrichosis with fungal spreading along the lymphatic channels. Atypical sporotrichosis is a challenge to diagnosis because it can mimic many other dermatological diseases. We report an unusual, itraconazole-resistant cutaneous lesion of sporotrichosis in a 66-year-old Brazilian man. Histopathological examination of the skin revealed vascular and fibroblastic proliferation with chronic granulomatous infiltrate composed of multinucleated giant cells. Sporothrix were isolated from the skin lesion, and phylogenetic analyses confirmed it to be sporotrichosis due to S. globosa, a widespread pathogen. Immunoblotting analysis showed several IgG-reactive molecules in autochthonous preparations of the whole cellular proteins (160, 80, 60, 55, 46, 38, 35, and 30 kDa) and exoantigen (35 and 33 kDa). The patient was first unsuccessfully treated with daily itraconazole, and then successfully treated with potassium iodide.
Introduction
Sporotrichosis is a mycosis found worldwide, which is usually acquired by the traumatic inoculation of Sporothrix propagules into cutaneous or subcutaneous tissue.1,2 Cutaneous lymphatic disease is the most common clinical manifestation in clinical practice.3 Sporotrichosis can be distinguished from other skin diseases, such as leishmaniasis, tuberculosis, sarcoidosis, paracoccidioidomycosis, and chromoblastomycosis.4 Developments in molecular phylogeny have clarified species boundaries and suggest that there are several cryptic species of clinical interest, including Sporothrix brasiliensis, Sporothrix schenckii s.s., Sporothrix globosa, and Sporothrix luriei.5–7 The distribution of the species may vary, with S. brasiliensis considered to be geographically restricted,8,9 and other species, like S. schenckii and S. globosa, being more widespread.9–12 The differential virulence,13,14 transmissibility,9 and antifungal susceptibility characteristics15,16 highlight the importance of accurate diagnosis for predicting the clinical outcome.
We report an atypical clinical presentation of itraconazole-resistant sporotrichosis due to S. globosa, which mimicked sarcoidosis in a Brazilian man. In contrast to the sapronoses of epidemic proportions due to this pathogen in northeastern China, the prevalence of S. globosa is low in Brazil, highlighting the importance of this description.
Case Report
A 66-year-old man from Minas Gerais, Brazil, was referred to the Department of Dermatology at the Federal University of São Paulo. The man was a farmer, and he presented with a scaly erythematous lesion that mimicked a sarcoid lesion with a well-demarcated ulcer and no signs of suppuration in the middle of his left arm (Figure 1A ). There were no subcutaneous nodules or fistulization.
Figure 1.
Atypical sporotrichosis due to Sporothrix globosa. (A) Plaque sporotrichosis lesion with a well-demarcated lesion. The patient was unsuccessfully treated with itraconazole (200 mg/day) for 6 months. (B) The cured atrophic sporotrichosis lesion after 6 months of treatment with oral potassium iodide.
The patient reported that the lesion had developed over the last 2 years. He was involved in rural activities and described trauma involving hay 3 years before this medical visit. He had no history of recent travel to areas with endemic sporotrichosis in Brazil, including Rio de Janeiro or Rio Grande do Sul, and denied contact with felines with sporotrichosis. The patient was taking the following medications: captopril (50 mg/day), propanolol (40 mg/day), enalapril (25 mg/day), and lorazepan (1 mg/day) for treatment of chronic cardiology problems. He reported that he had used the following antifungal drugs: ketoconazole (2% cream) on the lesion and itraconazole (200 mg/day) irregularly over the last 2 years without resolution. He was advised to discontinue the antifungal treatment and to return 30 days later for a skin biopsy, so that histopathological and microbiological examination could be performed. Blood samples were collected to perform serological tests for hepatitis B and C, toxoplasmosis, and human immunodeficiency virus (HIV). Samples were positive for hepatitis B but negative for toxoplasmosis and HIV. A serum sample was sent to the laboratory for Western blotting analysis to test for subcutaneous mycosis.
Direct microscopic examination of the tissue fragments after KOH clarification was negative for fungal structures, and the appearance of a skin biopsy specimen from the site of the lesion was not consistent with classical sporotrichosis. Hematoxylin and eosin staining revealed vascular and fibroblastic proliferation in addition to chronic granulomatous infiltrate composed of multinucleated giant cells. No fungal elements were evident by periodic acid–Schiff (PAS) and Grocott stains. After 4–5 days of incubation, Sabouraud dextrose agar (SDA) plates seeded with skin scrapings and crusts from the lesion showed membranous colonies that were initially cream colored, but that became dark brown during incubation. Macroscopically, the colonies resembled Sporothrix spp.
Treatment with itraconazole (200 mg/day for 6 months) did not resolve the lesion and was discontinued. Treatment with a saturated solution of potassium iodide (KI) began with an initial dose of 1 g/day that was increased weekly by 1 g up to a maximum of 4 g/day until the end of the treatment. The lesion regressed substantially over the course of 2 months, and the protocol was continued for four additional months until complete remission of the lesion (Figure 1B).
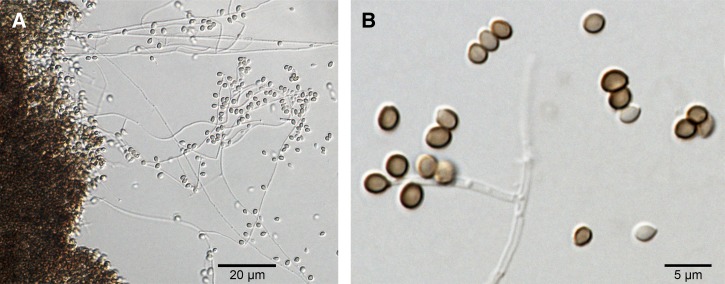
To characterize the phenotype of the infectious organism, colonies were grown on SDA, potato dextrose agar (PDA), corn meal agar (CMA), and brain heart infusion agar (BHI) as described previously.17 Carbohydrate assimilation tests were performed, and the following sugars were tested as described elsewhere: glucose, sucrose, raffinose, and ribitol.5 The cultures on SDA, PDA, and CMA at 25°C were glabrous and cream colored at first; after 10–14 days, the cultures developed dark pigmentation in the mycelia. Microscopic examination showed hyaline and septate hyphae with globose to subglobose hyaline and melanized conidia in sympodial conidiophores (Figure 2 ). The BHI agar cultures at 37°C showed the growth of moist, glabrous, yeast-like colonies with small globose or subglobose yeast cells. Carbohydrate assimilation testing revealed that the isolate could not use raffinose or ribitol as the only source of carbon but could assimilate sucrose and glucose. Taken together, the morphological data revealed the isolate to be S. globosa.
Figure 2.
Microscopic morphology of Sporothrix globosa after 21 days of culture at 30°C in potato dextrose agar (CBS 132925 = Ss236). Hyaline, thin, septated hyphae and numerous globose, dark-brown sessile conidia are visible. (A) 20× and (B) 40× magnifications.
DNA was extracted and purified from a fungal colony using the FastDNA Kit protocol (MP Biomedicals, Vista, CA). The calmodulin (CAL) locus region was amplified directly from the genomic DNA by polymerase chain reaction,18 producing an 800-bp amplicon corresponding to exons three through five. The amplified products were gel purified, sequenced directly in two reactions with forward and reverse primers, and the sequences were determined using an ABI 3730 DNA Analyzer 48-well capillary sequencer (Applied Biosystems Inc., Foster City, CA).18 The sequences were assembled into single sequences via the CAP3 algorithm (BioEdit software), aligned with MAFFT v.7,19 and edited manually to avoid mispaired bases. The consensus sequence was deposited online at GenBank (GenBank accession number KC693877).
Comparison with other Sporothrix sequences in GenBank revealed a 100% match with the CAL sequence of a Spanish isolate of S. globosa (FMR 8595; CBS 130104), which was consistent with the phenotypic characterization. Moreover, phylogenetic analyses placed our isolate with the S. globosa type strain (CBS 120340) in the Sporothrix clade IIIa, a group with widespread occurrence. Figure 3 shows the neighbor-joining tree based on the calmodulin nucleotide sequences. The isolate was deposited in the CBS-KNAW collection under the code CBS 132925 (Ss236).
Figure 3.
Neighbor-joining phylogenetic tree using the Kimura-2 parameter distance matrix. The tree was based on partial calmodulin-encoding gene sequences from Sporothrix species. GenBank accession numbers and geographic origin of strains are shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches (neighbor-joining/maximum likelihood). All positions containing gaps and missing data were eliminated.
Susceptibility testing was performed using the method recommended in the Clinical and Laboratory Standards Institute (CLSI) document M38-A2. Culture conditions and inoculum preparation are described elsewhere.20 We evaluated the minimum inhibitory concentration (MIC) for azoles, polyene, and echinocandin. The in vitro antifungal activity tests showed moderate-to-high MIC values for amphotericin B (2 μg/mL), fluconazole (> 64 μg/mL), itraconazole (4 μg/mL), voriconazole (> 16 μg/mL), caspofungin (> 16 μg/mL), and posaconazole (2 μg/mL).
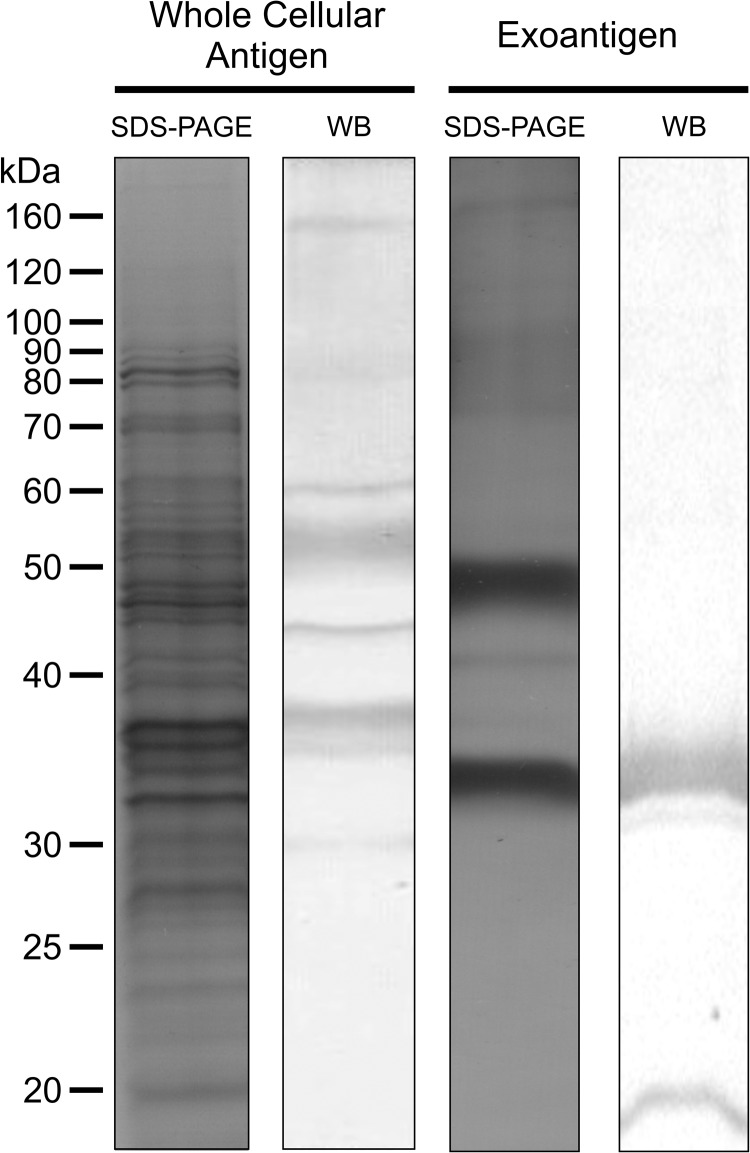
Total proteins from the yeast phase of S. globosa (autochthonous isolate Ss236) were isolated,21 and the exoantigen fraction was obtained from filtrated mycelia cultured on Sabouraud.22 Proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting.23 The membranes were probed using patient sera diluted 1:500 as the primary antibody and horseradish peroxidase-conjugated goat antihuman IgG (1:1000 dilution) as the secondary antibody,21 and the signal was detected using 3,3′-diaminobenzidine tetrahydrochloride. The serological profile showed greater complexity when the whole cellular antigen was used, revealing eight immunogenic molecules (160, 80, 60, 55, 46, 38, 35, and 30 kDa). Notably, only two molecules (35 kDa and 33 kDa) were recognized in exoantigen preparations (Figure 4 ). The results of the serological analysis were in accordance with information in the literature.13,21,23,24
Figure 4.
Immunoblot assay using Sporothrix globosa (CBS 132925 = Ss236) whole cellular yeast proteins and the mycelium exoantigen. Fungal proteins were resolved on 10% SDS-PAGE, electrotransferred to polyvinylidene difluoride (PVDF) membranes, and incubated with the patient's serum (dilution 1:500). Several IgG-reactive molecules in the whole cellular proteins (160, 80, 60, 55, 46, 38, 35, and 30 kDa) and exoantigen (35 kDa and 33 kDa) were detected using patient's serum due to antibodies against S. globosa. Lanes labeled SDS-PAGE show all proteins as visualized using silver staining, and lanes labeled WB are membranes probed using patient serum.
Discussion
Sporotrichosis is a subcutaneous mycosis that has been recognized as a public health problem in recent years in South America, especially in Brazil. Previously this mycosis was attributed to a unique species, S. schenckii s.l. Morphological, physiological, and molecular characterization show that S. schenckii is a complex that includes other new species.5 The major source of sporotrichosis infections appears to be potting and garden soil, which are often used in horticultural occupations.25,26 Our patient was engaged in rural activities that put him at risk of traumatic infection during handling of contaminated materials.11,25,27
The strain of S. globosa isolated in this study had phenotypic similarities to clinical S. globosa strains reported previously,11,12,28 although it was able to grow at 37°C, in contrast with the findings of Marimon and others.5 Although S. globosa is thought to have several clinical manifestations, such as fixed cutaneous, lymphocutaneous, and disseminated cutaneous manifestations,2,11 clinical reports involving S. globosa mostly have few clinical details.9,17 Sporothrix globosa species are considered to be low virulent or even avirulent by some researchers,13,14 and the large sapronoses occurring in China reflects localized manifestations of the disease.2,11,29
Serology using S. globosa proteome showed that a 60-kDa protein (gp60) was one of eight molecules recognized by the patient's IgG. We recently proposed that the recognition of a 60 kDa molecule by the sera of mice infected with Sporothix spp. yeast is related to the virulence traits of the causative strain,13 and Rodrigues and others21,23 reported a convergent humoral response to isoforms and glycoforms of gp60–70 (3-carboxymuconate cyclase) in human and feline sporotrichosis.23 Moreover, this molecule may be related to Sporothrix spp. cell wall proteins,30 which are considered potential protein biomarkers for the diagnosis of sporotrichosis, as demonstrated here.21,23
The patient's clinical response to therapy with itraconazole (200 mg/day) was satisfactory at first; however, after 30 days on itraconazole, there was no further improvement. The patient showed important improvement in his clinical condition when itraconazole was replaced with a KI solution, which is quite effective for treating cutaneous sporotrichosis.31,32 The in vitro tests revealed high MICs to itraconazole (4 μg/mL), which have also been reported by some studies15,20,33,34 and which correlated with itraconazole treatment failure. Successful KI treatment has been reported in a highly endemic area of S. brasiliensis,31,32,35 so KI could be an important alternative for treating itraconazole-resistant sporotrichosis due to S. globosa.
This description of S. globosa in Brazil contributes to our understanding of the global epidemiology of this species. Because of itraconazole resistance, a cure was achieved after implementation of therapy with KI. Accurate identification of the species and the correct therapy led to an effective cure.
Disclaimer: The authors alone are responsible for the content and the writing of the paper.
Footnotes
Financial support: Anderson M. Rodrigues (FAPESP 2015/19746-8) and Geisa F. Fernandes (FAPESP 2011/01628-8) are fellows and received financial support from the São Paulo Research Foundation (FAPESP). This work was supported in part by grants from the São Paulo Research Foundation (FAPESP 2009/54024-2) and from the National Council for Scientific and Technological Development (CNPq 472169/2012-2). The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Authors' addresses: Olga Fischman Gompertz, Anderson M. Rodrigues, Geisa F. Fernandes, Henri D. L. Bentubo, and Zoilo Pires de Camargo, Department of Microbiology, Immunology, and Parasitology, Federal University of São Paulo, São Paulo, Brazil, E-mails: olga.gompertz@unifesp.br, amrodrigues.amr@gmail.com, geisafernandes@yahoo.com.br, hbentubo@yahoo.com.br, and zpcamargo1@gmail.com. Valéria Petri, Department of Dermatology, Federal University of São Paulo, São Paulo, Brazil, E-mail: valeriapetrivp@gmail.com.
References
- 1.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53:3–14. doi: 10.1093/mmy/myu062. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Hagen F, Stielow B, Rodrigues AM, Samerpitak K, Zhou X, Feng P, Yang L, Chen M, Deng S, Li S, Liao W, Li R, Li F, Meis JF, Guarro J, Teixeira M, Al-Zahrani HS, Camargo ZP, Zhang L, de Hoog GS. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14000 human and animal case reports. Persoonia. 2015;35:1–20. doi: 10.3767/003158515X687416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonifaz A, Vázquez-González D. Diagnosis and treatment of lymphocutaneous sporotrichosis: what are the options? Curr Fungal Infect Rep. 2013;7:252–259. [Google Scholar]
- 4.Pappas PG, Tellez I, Deep AE, Nolasco D, Holgado W, Bustamante B. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65–70. doi: 10.1086/313607. [DOI] [PubMed] [Google Scholar]
- 5.Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, Guarro J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198–3206. doi: 10.1128/JCM.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marimon R, Gené J, Cano J, Guarro J. Sporothrix luriei: a rare fungus from clinical origin. Med Mycol. 2008;46:621–625. doi: 10.1080/13693780801992837. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X, Rodrigues AM, Feng P, Hoog GS. Global ITS diversity in the Sporothrix schenckii complex. Fungal Divers. 2014;66:153–165. [Google Scholar]
- 8.Rodrigues AM, de Melo Teixeira M, de Hoog GS, Schubach TMP, Pereira SA, Fernandes GF, Bezerra LML, Felipe MS, de Camargo ZP. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis. 2013;7:e2281. doi: 10.1371/journal.pntd.0002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues AM, de Hoog GS, Zhang Y, Camargo ZP. Emerging sporotrichosis is driven by clonal and recombinant Sporothrix species. Emerg Microbes Infect. 2014;3:e32. doi: 10.1038/emi.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madrid H, Cano J, Gene J, Bonifaz A, Toriello C, Guarro J. Sporothrix globosa, a pathogenic fungus with widespread geographical distribution. Rev Iberoam Micol. 2009;26:218–222. doi: 10.1016/j.riam.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Wan Z, Zhang Z, Li F, Li R, Liu X. Phenotypic and molecular identification of Sporothrix isolates of clinical origin in northeast China. Mycopathologia. 2013;176:67–74. doi: 10.1007/s11046-013-9668-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camacho E, León-Navarro I, Rodríguez-Brito S, Mendoza M, Niño-Vega GA. Molecular epidemiology of human sporotrichosis in Venezuela reveals high frequency of Sporothrix globosa. BMC Infect Dis. 2015;15:94. doi: 10.1186/s12879-015-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes GF, dos Santos PO, Rodrigues AM, Sasaki AA, Burger E, de Camargo ZP. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence. 2013;4:241–249. doi: 10.4161/viru.23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arrillaga-Moncrieff I, Capilla J, Mayayo E, Marimon R, Mariné M, Gené J, Cano J, Guarro J. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651–655. doi: 10.1111/j.1469-0691.2009.02824.x. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues AM, de Hoog GS, de Cassia Pires D, Brihante RSN, da Costa Sidrim JJ, Gadelha MF, Colombo AL, de Camargo ZP. Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect Dis. 2014;14:219. doi: 10.1186/1471-2334-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borba-Santos LP, Rodrigues AM, Gagini TB, Fernandes GF, Castro R, de Camargo ZP, Nucci M, Lopes-Bezerra LM, Ishida K, Rozental S. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles, and terbinafine. Med Mycol. 2015;53:178–188. doi: 10.1093/mmy/myu056. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues AM, de Hoog S, de Camargo ZP. Emergence of pathogenicity in the Sporothrix schenckii complex. Med Mycol. 2013;51:405–412. doi: 10.3109/13693786.2012.719648. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues AM, Najafzadeh MJ, de Hoog GS, de Camargo ZP. Rapid identification of emerging human-pathogenic Sporothrix species with rolling circle amplification. Front Microbiol. 2015;6:1385. doi: 10.3389/fmicb.2015.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marimon R, Serena C, Gené J, Cano J, Guarro J. In vitro antifungal susceptibilities of five species of Sporothrix. Antimicrob Agents Chemother. 2008;52:732–734. doi: 10.1128/AAC.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues AM, Kubitschek-Barreira PH, Fernandes GF, de Almeida SR, Lopes-Bezerra LM, de Camargo ZP. Immunoproteomic analysis reveals a convergent humoral response signature in the Sporothrix schenckii complex. J Proteomics. 2015;115:8–22. doi: 10.1016/j.jprot.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes GF, Amaral CCD, Sasaki A, Godoy PM, De Camargo ZP. Heterogeneity of proteins expressed by Brazilian Sporothrix schenckii isolates. Med Mycol. 2009;47:855–861. doi: 10.3109/13693780802713216. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues AM, Fernandes GF, Araujo LM, Della Terra PP, Dos Santos PO, Pereira SA, Schubach TM, Burger E, Lopes-Bezerra LM, de Camargo ZP. Proteomics-based characterization of the humoral immune response in sporotrichosis: toward discovery of potential diagnostic and vaccine antigens. PLoS Negl Trop Dis. 2015;9:e0004016. doi: 10.1371/journal.pntd.0004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott EN, Muchmore HG. Immunoblot analysis of antibody responses to Sporothrix schenckii. J Clin Microbiol. 1989;27:300–304. doi: 10.1128/jcm.27.2.300-304.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues AM, Bagagli E, de Camargo ZP, Bosco SMG. Sporothrix schenckii sensu stricto isolated from soil in an armadillo's burrow. Mycopathologia. 2014;177:199–206. doi: 10.1007/s11046-014-9734-8. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues AM, Cruz Choappa R, Fernandes GF, De Hoog GS, Camargo ZP. Sporothrix chilensis sp. nov. (Ascomycota: Ophiostomatales), a soil-borne agent of human sporotrichosis with mild-pathogenic potential to mammals. Fungal Biol. 2016;120:246–264. doi: 10.1016/j.funbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Vismer HF, Hull PR. Prevalence, epidemiology and geographical distribution of Sporothrix schenckii infections in Gauteng, South Africa. Mycopathologia. 1997;137:137–143. doi: 10.1023/a:1006830131173. [DOI] [PubMed] [Google Scholar]
- 28.de Oliveira MM, de Almeida-Paes R, de Medeiros Muniz M, de Lima Barros MB, Galhardo MC, Zancope-Oliveira RM. Sporotrichosis caused by Sporothrix globosa in Rio De Janeiro, Brazil: case report. Mycopathologia. 2010;169:359–363. doi: 10.1007/s11046-010-9276-7. [DOI] [PubMed] [Google Scholar]
- 29.Song Y, Li SS, Zhong SX, Liu YY, Yao L, Huo SS. Report of 457 sporotrichosis cases from Jilin province, northeast China, a serious endemic region. J Eur Acad Dermatol Venereol. 2013;27:313–318. doi: 10.1111/j.1468-3083.2011.04389.x. [DOI] [PubMed] [Google Scholar]
- 30.Castro RA, Kubitschek-Barreira PH, Teixeira PAC, Sanches GF, Teixeira MM, Quintella LP, Almeida SR, Costa RO, Camargo ZP, Felipe MSS, de Souza W, Lopes-Bezerra LM. Differences in cell morphometry, cell wall topography and Gp70 expression correlate with the virulence of Sporothrix brasiliensis clinical isolates. PLoS One. 2013;8:e75656. doi: 10.1371/journal.pone.0075656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, Orofino-Costa R. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29:719–724. doi: 10.1111/jdv.12667. [DOI] [PubMed] [Google Scholar]
- 32.Orofino-Costa R, de Macedo PM, Bernardes-Engemann AR. Hyperendemia of sporotrichosis in the Brazilian southeast: learning from clinics and therapeutics. Curr Fungal Infect Rep. 2015;9:220–228. [Google Scholar]
- 33.Ottonelli Stopiglia CD, Magagnin CM, Castrillon MR, Mendes SD, Heidrich D, Valente P, Scroferneker ML. Antifungal susceptibilities and identification of species of the Sporothrix schenckii complex isolated in Brazil. Med Mycol. 2014;52:56–64. doi: 10.3109/13693786.2013.818726. [DOI] [PubMed] [Google Scholar]
- 34.Silveira CP, Torres-Rodríguez JM, Alvarado-Ramírez E, Murciano-Gonzalo F, Dolande M, Panizo M, Reviakina V. MICs and minimum fungicidal concentrations of amphotericin B, itraconazole, posaconazole and terbinafine in Sporothrix schenckii. J Med Microbiol. 2009;58:1607–1610. doi: 10.1099/jmm.0.007609-0. [DOI] [PubMed] [Google Scholar]
- 35.de Macedo PM, Sztajnbok DC, Camargo ZP, Rodrigues AM, Lopes-Bezerra LM, Bernardes-Engemann AR, Orofino-Costa R. Dacryocystitis due to Sporothrix brasiliensis: a case report of a successful clinical and serological outcome with low-dose potassium iodide treatment and oculoplastic surgery. Br J Dermatol. 2015;172:1116–1119. doi: 10.1111/bjd.13378. [DOI] [PubMed] [Google Scholar]