Abstract
Nearly half of the world's population is at risk for malaria. Increasing drug resistance has intensified the need for novel therapeutics, including treatments with intrinsic transmission-blocking properties. In this study, we demonstrate that the isoprenoid abscisic acid (ABA) modulates signaling in the mammalian host to reduce parasitemia and the formation of transmissible gametocytes and in the mosquito host to reduce parasite infection. Oral ABA supplementation in a mouse model of malaria was well tolerated and led to reduced pathology and enhanced gene expression in the liver and spleen consistent with infection recovery. Oral ABA supplementation also increased mouse plasma ABA to levels that can signal in the mosquito midgut upon blood ingestion. Accordingly, we showed that supplementation of a Plasmodium falciparum-infected blood meal with ABA increased expression of mosquito nitric oxide synthase and reduced infection prevalence in a nitric oxide-dependent manner. Identification of the mechanisms whereby ABA reduces parasite growth in mammals and mosquitoes could shed light on the balance of immunity and metabolism across eukaryotes and provide a strong foundation for clinical translation.
Introduction
Roughly 3.2 billion people are at risk of malaria.1 Increased drug and insecticide resistance have precipitated the need for novel methods of control, in particular treatments with dual therapeutic and transmission-blocking properties. Ideal therapeutics would reduce parasite growth directly by targeting multiple life stages of the Plasmodium parasite or indirectly by enhancing defense responses of both mammalian and mosquito hosts.
The isoprenoid abscisic acid (ABA) was originally characterized as a plant hormone controlling leaf abscission, but has since been detected in a wide variety of eukaryotes including mammals, sponges, and the apicomplexan parasite Toxoplasma gondii.2–4 In these organisms, ABA functions in immune and metabolic processes, including desiccation tolerance in plants, heat stress response in sponges, and insulin release in humans.5–7 ABA also increases cell proliferation, regulating regeneration in hydroids and expansion of human mesenchymal stem cells.8,9 This conservation across eukaryotes led us to hypothesize that ABA might function in the interactions among mammals, mosquitoes, and Plasmodium to alter dynamics of malaria parasite infection and transmission.
In mammals, ABA can be pro- or anti-inflammatory depending on context. For example, treatment with ABA can activate human macrophages in vitro, stimulating phagocytosis and the release of reactive nitrogen and oxygen species (RNOS).2,10 In contrast, ABA supplementation in a mouse model of inflammatory bowel disease (IBD) reduced epithelial erosion and leukocyte infiltration.11 In this context, ABA has been proposed as a supplementary treatment of IBD due to its ability to limit harmful inflammation.12 Accordingly, we hypothesized that complications in malaria such as cerebral pathology and liver dysfunction that can be exacerbated by the host inflammatory response to high parasite levels could be mitigated by ABA supplementation.13,14
In insects, ABA ingested with nectar is detectable in honey bees and supplementation of hives enhanced would healing and immune cell activation in treated bees.15 Mosquitoes similarly ingest a variety of bioactive factors during host feeding. In particular, human insulin and transforming growth factor beta in ingested blood can signal in the mosquito midgut to alter parasite infection.16,17 Given that ABA has been detected in human blood in the nanomolar range, we hypothesized that mosquitoes could respond to ingested ABA.7,18 Further, ABA regulation of granulocyte synthesis of RNOS—compounds that are essential to the mosquito immune response to Plasmodium infection—suggested that ABA could regulate analogous mosquito responses to developing parasites.19
Based on these collective observations, we used a mouse model of Plasmodium yoelii 17XNL infection (hereafter P. yoelii) as well as cultured Plasmodium falciparum to investigate the ability of ABA to alter Plasmodium-associated pathology in the mammalian host, parasitemia and the formation of sexual stage gametocytes, as well as transmission of both parasite species to a competent mosquito host Anopheles stephensi.
Methods
Animals, infection, and histology.
Six to eight weeks old female CD1 mice (Jackson) were housed four to a cage and provided with unsupplemented water or water supplemented with 2.56 mM ABA (Sigma-Aldrich, St. Louis, MO). Water and supplemented water were changed daily. On day 3 of supplementation, mice were injected intraperitoneally with 5 × 106 P. yoelii 17XNL-infected red blood cells (RBCs). Mouse weight, water consumption per cage, and parasitemia were monitored daily. Parasitemia was determined from thin blood films stained with Giemsa. Parasitemia was measured as the percentage of infected RBCs divided by total RBCs, whereas gametocytemia was measured as the proportion of gametocyte-containing RBCs of total infected RBCs. Four mice were used per group in replicated studies. Daily parasitemias and gametocytemias were analyzed by unpaired t test.
Mice were euthanized on day 7 postinfection. Blood was collected by heart puncture, spun at 5,900 × g for 8 minutes and the plasma collected for ABA quantification. Livers and spleens were collected, retained in 10% formalin for 24 hours and then stored in 70% ethanol before sectioning and staining with hematoxylin and eosin. Liver sections were blindly scored on a scale from 0 to 3 for pigment levels, Kupffer cell density, portal tract inflammation, hemozoin deposition, extramedullary hematopoiesis, sinusoidal leukocyte density, sinusoidal adherence of leukocytes, and presence of microabscesses, using an adaptation of the procedure of Roux and others.20 At 400× and 1,000× (oil immersion) magnification, leukocytes midstream in sinusoidal lumens and not in approximation with the sinusoidal endothelium were classified as nonadherent. Using the same magnifications, nonmidstream leukocytes in tight approximation with the sinusoidal endothelium were classified as adherent.
Spleen sections were blindly scored on a scale from 0 to 3 for hyperplasia, extramedullary hematopoiesis, pigment, and levels of histiocytes and neutrophils. Pathology scores of control and treated mice were analyzed by unpaired t test.
Ethical statement.
Experiments with mice were carried out in strict accordance with the recommendations in the Guide for Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committees at the University of California, Davis under approved protocol 18948.
ABA extraction and quantification.
ABA was extracted from mouse plasma as described in Engelberth and others.21 Briefly, 50 μL of plasma was added to 200 μL extraction buffer (1-propanol : H2O : concentrated HCl at a 2:1:0.002 volume ratio) and vortexed. Five hundred microliter of ethyl acetate was added and the samples were vortexed again. Samples were spun at 10,000×g for 1 minute and the upper layer collected and dried under a steady stream of air. Dried samples were re-eluted in 100% methanol, and ABA levels were determined using the Phytodetek Abscisic Acid ELISA kit (Agdia, Elkhart, IN) according to the manufacturer's instructions. ABA levels in the plasma of control and ABA-supplemented mice (N = 4–5 per group) were analyzed by Mann–Whitney test.
ABA was extracted from four independent P. falciparum cultures as described with minor adjustments.22 In brief, infected RBCs (~200 mg) were collected, quickly weighed, and immediately frozen in liquid nitrogen. Frozen samples were finely ground in a mortar and transferred to a 4-mL screw top Supelco vial containing 1,200 μL of 2-propanol/H2O/HCl (2:1:0.002) and sonicated in a water bath for 10 minutes. Dichloromethane (2 mL) was added to each sample and resonicated for 10 minutes. The bottom dichloromethane/2-propanol layer was then transferred to a 4-mL glass vial, evaporated under a constant air stream and the resultant pellet was subsequently dissolved in 300μL of diethyl ether/methanol (9:1 vol/vol) followed by the addition of 9 μL of a 2.0 M solution of trimethylsilyldiazomethane in hexane to convert the carboxylic acids into methyl esters. During this step, ABA is converted to MeABA. The vials were capped, vortexed, and incubated at room temperature for 25 minutes. After incubation, 9 μL of 12% acetic acid in hexane was added to each sample and samples were incubated at room temperature for another 25 minutes to destroy all excess trimethylsilyldiazomethane.
The resulting methyl ester volatiles were captured on Super-Q (Alltech Inc., State College, PA) columns by vapor-phase extraction as described.23 The trapped metabolites were then eluted with 150 μL of dichloromethane and analyzed by gas chromatography–mass spectrometry using a Hewlett and Packard 6890 series gas chromatograph coupled to an Agilent Technologies, Santa Clara, CA 5973 network mass selective detector operated in electronic ionization mode. Of the sample, 1 μL was injected in splitless mode at 250°C and separated using an HP-5MS column (30 m × 0.25 mm, 0.25 μm film thickness) held at 40°C for 1 minute after injection, and then at increasing temperatures programmed to ramp at 15°C/minute to 250°C (10 minutes), with helium as the carrier gas (constant flow rate 1.0 mL/minute). Measurements were carried out in selected ion monitoring 190 m/z for MeABA and 194 m/z for MeDtABA (D6 deuterated standard).
Mosquito rearing and infections.
Anopheles stephensi Liston (Indian wild-type strain) were reared and maintained at 27°C and 75% humidity. For all feeding experiments, 3-to 5-day-old female mosquitoes were maintained on water pads for 24 hours and then allowed to blood feed for 30 minutes on control or ABA-supplemented P. yoelii 17XNL-infected mice. Cohorts of 50 mosquitoes were allowed to feed on each mouse at 5 days postinfection. Immediately after feeding nonengorged mosquitoes were removed. Ten days postfeeding, midguts were dissected, stained with 0.5% mercurochrome, and oocysts counted by microscopy.
For P. falciparum infections, An. stephensi were fed on P. falciparum cultures diluted 1:1 with uninfected RBCs and heat-inactivated human serum, containing a treatment or diluent control and provided in a Hemotek Insect Feeding System (Discovery Workshops, Accrington, UK). ABA stock was diluted with phosphate-buffered saline (PBS) and added into blood immediately before feeding for final concentrations of 10 and 100 nM. For feeds with Nω-Nitro-l-arginine methyl ester (l-NAME), mosquitoes were maintained on sugar cubes and water pads containing 1 mg/mL l-NAME for 72 hours before the feed and throughout the course of infection as previously described.24
Four infection replicates were combined when replicate controls were determined not to be significantly different from each other by analysis of variance. Infection prevalence data were analyzed by Fisher's exact test. Oocyst intensities were analyzed by Mann–Whitney test.
Plasmodium falciparum growth assays.
Plasmodium falciparum NF54 cultures were synchronized as previously described and aliquots plated in complete RPMI 1640 medium with HEPES, hypoxanthine, and 10% heat inactivated human serum.25 Parasites were treated with 10 nM ABA, 100 nM ABA, or a diluent control and incubated at 37°C for 48 or 96 hours before culture media were replaced with 10% formalin in RPMI 1640. RBCs were stained with 10 μg/mL propidium iodide (Sigma-Aldrich) in PBS at room temperature for 1 hour and infected cells counted with FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Four replicates were carried out, each using a separate parasite culture passage. Relative changes in growth were normalized to controls set at one and analyzed by one-sample t test.
Studies of the effects of ABA on gametocyte formation were performed with P. falciparum 3D7 engineered to express green fluorescent protein (GFP) under the control of alpha-tubulin II and Pfs25 promoters.26 Parasite cultures were synchronized using sorbitol as previously described.25 At 48 hours postsynchronization, 200 μL aliquots of parasite culture and complete RPMI 1640 medium were placed in a 96-well flat-bottomed plate and treated with 10 nM ABA, 100 nM ABA, or an equivalent volume of diluent control. Media and treatments were refreshed every 48 hours. At 4, 6, and 8 days after the start of treatment, separate aliquots were harvested, media were removed, and replaced with 10% formalin for 1 hour at room temperature. Formalin was removed and replaced with PBS before gametocyte levels were measured by flow cytometry using a FACSCalibur flow cytometer (BD Biosciences) with a GFP filter. Four replicates were carried out, each using a separate parasite culture passage. Relative changes in gametocyte numbers were normalized to controls set at one and analyzed by one-sample t test.
Gene expression.
For mosquito immune gene expression, 20 mosquitoes in each treatment group were dissected at 1, 2, 4, 6, 8, and 24 hours postfeeding. Midguts were sonicated and stored in TriZOL at −20°C for up to a week before RNA was extracted according to manufacturer's instructions. Complementary DNA (cDNA) synthesis was completed using QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA), and 200 ng of cDNA was used for each quantitative real-time reverse transcription polymerase chain reaction assay with SYBR Green (Applied Biosystems, Foster City, CA). Fold changes in expression levels were calculated using the comparative threshold cycle method.27 Data were normalized to the housekeeping gene ribosomal protein S7 and to the control treatment. Primers and cycling conditions for An. stephensi nitric oxide synthase (AsNOS), defensin, LRIM, TEP1, and APL1 were as previously described.28,29
For gene expression analysis of mouse liver and spleen, organs were flash frozen after necropsy and stored at −80°C before RNA was extracted in TriZOL and cDNA synthesized. Real-time polymerase chain reaction was performed using TaqMan® Gene Expression Assays for mouse NOS2, PPARγ, and β-actin (Applied Biosystems) with volumes and cycling conditions as previously described.30 Samples were analyzed in triplicate with 250 ng of cDNA per reaction to confirm uniformity of amplification. Data were normalized to β-actin and control messenger RNA (mRNA) levels. All expression data were analyzed by one-sample t test.
Results
ABA supplementation increased circulating ABA levels and decreased parasitemia, gametocytemia, and disease pathology in P. yoelii-infected mice.
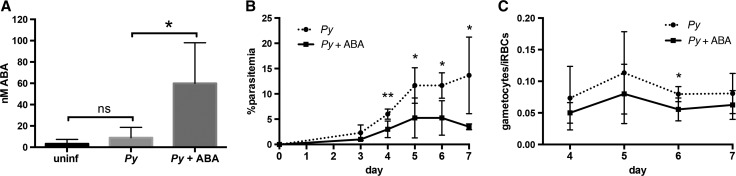
To determine the effect of ABA on parasite infection and disease pathology, we supplemented the water of female CD1 mice throughout the course of infection with 2.56 mM ABA, equivalent to 100 mg/kg and the lowest dose shown to ameliorate symptoms of influenza, IBD, and type II diabetes in mice.11,12,31–33 Infected ABA-supplemented mice consistently drank more water than controls when water consumption was normalized to account for differences in mouse weight (Supplemental Figure 1A). Among infected mice, daily weight change was not different over the course of infection, although the average weight of control mice began to drop on day 7 while that of ABA-supplemented mice did not (Supplemental Figure 1B). Plasmodium yoelii infection had no effect on ABA levels present in mouse plasma (Figure 1A ). In the absence of infection, oral ABA supplementation did not alter ABA levels (data not shown). However, in the context of P. yoelli infection ABA supplementation increased levels of ABA in plasma over 6-fold compared with nonsupplemented infected mice (Figure 1A), suggesting bioavailability of ABA may be determined by infection status. Within control and treatment groups, ABA levels were not correlated with parasitemia (data not shown).
Figure 1.
Oral supplementation of abscisic acid (ABA) increased plasma ABA levels and decreased parasitemia and gametocytemia in mice. (A) Concentrations of ABA in plasma of uninfected CD1 mice and on day 7 post-Plasmodium yoelii infection with and without ABA treatment. Four to five mice were used per group. Data were analyzed using the Mann–Whitney test. (B) Daily percent parasitemia of control and ABA-supplemented mice infected with P. yoelii. (C) Gametocytemia, measured as the proportion of gametocytes in infected red blood cells (RBCs). Data for (B) and (C) are shown as means of four mice per group. Daily parasitemia and gametocytemia were analyzed by unpaired t test. * P ≤ 0.05, ** P ≤ 0.01.
Parasitemia was significantly reduced in ABA-supplemented mice relative to controls starting on day 4 postinfection and continuing until necropsy on day 7 (Figure 1B). ABA-supplemented mice were also noticeably less lethargic at necropsy compared with controls. In addition to decreased parasitemia, ABA-supplemented mice exhibited reduced gametocytemia, which was significantly different relative to controls on day 6 of infection (Figure 1C). Despite this difference in gametocytemia, no differences in the ratios of male to female gametocytes were evident between control and ABA-treated mice (Supplemental Figure 2).
To define the histologic effects of ABA treatment and the ABA-dependent decrease in parasitemia in tissues relevant to infection, livers and spleens were scored and compared among supplemented and control mice in the presence and absence of parasite infection. Liver damage occurs during erythrocytic P. falciparum infection with foci of necrosis correlating with liver dysfunction.34,35 Liver damage has also been observed in mice infected with P. yoelii 17XNL, Plasmodium chabaudi, and Plasmodium berghei.36–38 In particular, apoptosis of hepatocytes has been shown to be induced by infection-associated oxidative stress and liver metabolic dysfunction can result from immunopathology during malaria.39,40 The spleen is crucial to clearance of Plasmodium-infected RBCs.41 Inflammation and hyperplasia, seen during Plasmodium infection can impair blood flow through the spleen and lead to organ failure.42
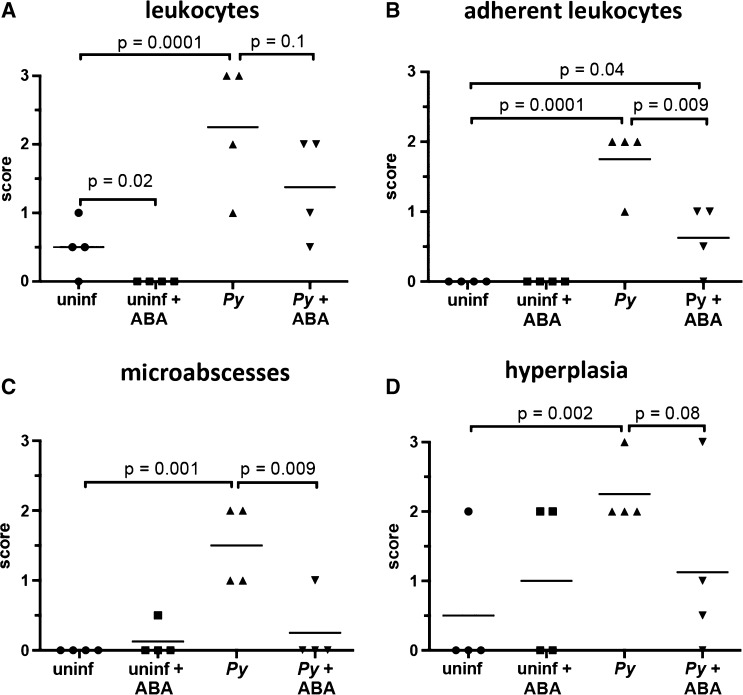
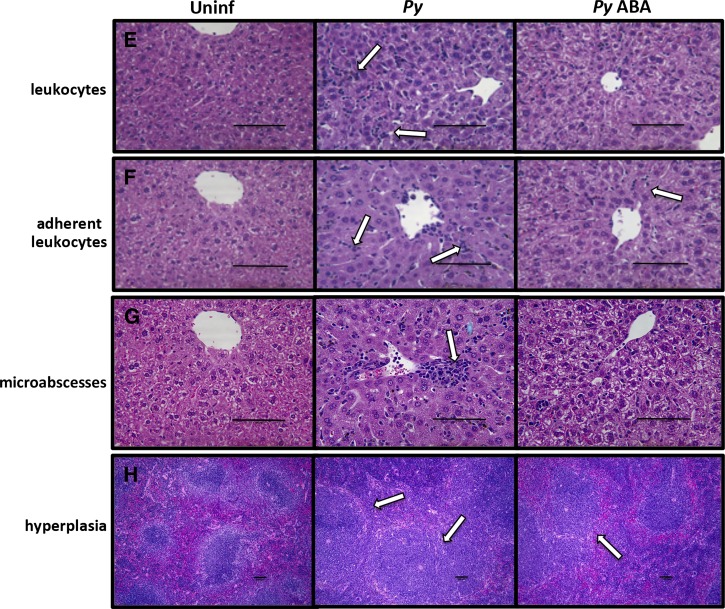
In the absence of infection, ABA treatment significantly decreased the number of sinusoidal leukocytes in the liver; this trend, although not significant, was also evident in P. yoelii-infected mice (Figure 2A and E ). In P. yoelii-infected mice, hemozoin deposition, pigment, portal tract inflammation, extramedullary hematopoeisis, and Kupffer cell density were increased relative to controls, but these levels were not altered by ABA treatment (Supplemental Figure 3A). However, the livers of ABA-treated infected mice had significantly fewer adherent leukocytes (Figure 2B and F) and significantly fewer microabscesses than control mice on day 7 postinfection (Figure 2C and G). Further, in ABA-treated mice, parasitemia was significantly inversely correlated with pathology scores for leukocytes and adherent leukocytes (Supplemental Figure 4A and B). In these cases, reduced pathology did not appear to simply follow from reduced parasitemia, but rather the beneficial effects of ABA appeared to be greater at higher parasitemia.
Figure 2.
Oral supplementation of abscisic acid (ABA) decreased tissue inflammatory responses of Plasmodium yoelii infected mice. (A) The livers of P. yoelii infected mice had significantly more nonadherent sinusoidal leukocytes than uninfected controls. ABA reduced the hepatic nonadherent leukocytes of uninfected mice, but failed to significantly reduce the hepatic nonadherent leukocytes of infected mice. (B) The livers of P. yoelii infected mice again had significantly more adherent sinusoidal leukocytes than uninfected controls. ABA had no effect on the hepatic adherent leukocytes of uninfected mice, but highly significantly reduced the hepatic adherent leukocytes of P. yoelii infected mice. (C) The livers of P. yoelii infected mice had significantly more microabscesses than uninfected controls. ABA had no effect on the hepatic microabscesses of uninfected mice, but highly significantly reduced the hepatic microabscesses of P. yoelii infected mice. (D) The spleens of P. yoelii infected mice had significantly more lymphoid hyperplasia of periarteriolar lymphoid sheaths than uninfected controls. ABA had no effect on the splenic lymphoid hyperplasia of uninfected mice, but showed a trend, though not statistically significant, toward reducing splenic lymphoid hyperplasia of P. yoelii infected mice. (E) Liver sections with arrows indicating sinusoidal nonadherent leukocytes. (F) Liver sections with arrows indicating adherent sinusoidal leukocytes. (G) Liver sections with a sinusoidal microabscess indicated by the arrow. (H) Spleen sections with lymphoidal hyperplasia of the periarteriolar lymphoid sheaths indicated by arrows. Bars = 100 μm; Py = P. yoelii-infected; Uninf = uninfected.
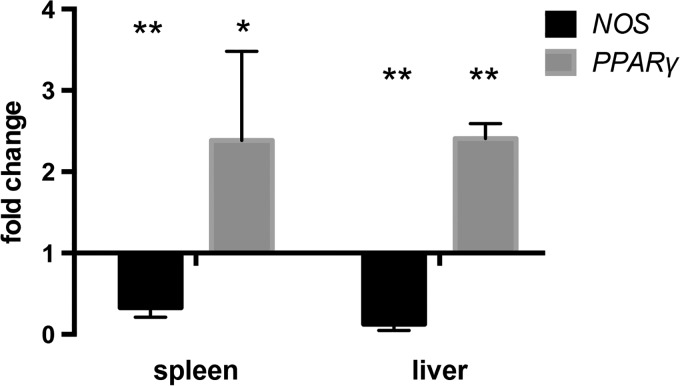
In the absence of parasite infection, spleen scores of ABA-supplemented and control mice were not significantly different (Figure 2D, Supplemental Figure 3B). In P. yoelii-infected mice, histiocyte density, pigment deposition, and extramedullary hematopoiesis in the spleen were increased relative to control mice, but these changes were not altered by ABA treatment (Supplemental Figure 3B). However, lymphoid hyperplasia of the spleen, which was increased in infected mice, was reduced by ABA treatment in three of four mice (Figure 2D and H). Consistent with this ABA-dependent reduction in inflammatory pathology, we observed decreased transcript levels of nitric oxide synthase (NOS) and increased transcript levels of the anti-inflammatory regulator peroxisome proliferator activated receptor gamma (PPARγ), which are regulated by ABA, in both the liver and spleen (Figure 3 ).11,12,30,31
Figure 3.
Abscisic acid (ABA) supplementation decreased nitric oxide synthase (NOS) and increased peroxisome proliferator activated receptor gamma (PPARγ) transcript expression in the spleen and liver of infected mice. Fold change in expression levels of NOS and PPARγ in spleens and livers of ABA-supplemented mice on day 7 post-Plasmodium yoelii infection. Quantitative reverse transcription polymerase chain reaction data are shown as fold change (2ΔΔCt) in gene expression relative to control-treated infected mice. Three mice were used per group. Data were analyzed by one-sample t test. * P = 0.08, ** P ≤ 0.005.
ABA supplementation of mice reduced P. yoelii transmission to mosquitoes.
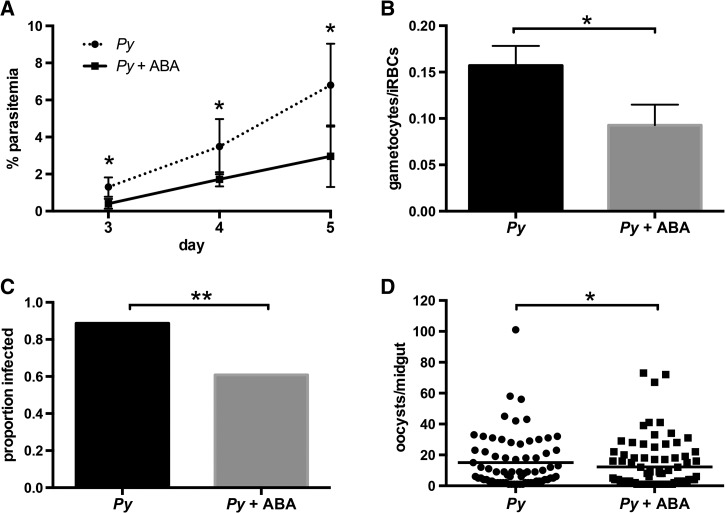
The effect of ABA on gametocytemia led us to investigate the effect of ABA supplementation on transmission of parasites from mice to mosquitoes. In these studies, ABA-supplemented mice again showed reduced parasitemia compared with controls starting on day 3 (Figure 4A ) and significantly reduced gametocytemia on day 5 postinfection (Figure 4B), near peak gametocytemia. On day 5, each mouse in the ABA-supplemented and control groups was exposed to 50 female An. stephensi. Ten days later, mosquito midguts were dissected and oocysts counted to measure mosquito infection prevalence and intensity. The prevalence of infected mosquitoes that fed on control mice was 88.8% (mean of 15 oocysts per midgut) compared with an infection prevalence of 60.9% of mosquitoes that fed on ABA-supplemented mice (mean of 12 oocysts per midgut) (Figure 4C and D). Although the difference in mean oocysts per midgut was significant, the effect was small. However, the 31.4% reduction in prevalence is notable and, given that a single oocyst can produce sufficient sporozoites for transmission to a human host, is arguably a more relevant measure for malaria control.
Figure 4.
Abscisic acid (ABA) supplementation of mice decreased parasite transmission to Anopheles stephensi. (A). Daily percent parasitemia of control and ABA-supplemented mice infected with Plasmodium yoelii. Three mice were used per group. (B) Gametocytemia on day 5 postinfection, measured as the proportion of gametocytes in infected red blood cells. (C) Mean proportions of mosquitoes that became infected after feeding on control or ABA-supplemented mice. Bars represent three to four combined cohorts per treatment. Each cohort consisted of 30–40 mosquitoes fed on a single mouse. Data were analyzed by Fisher's exact test. (D) Oocysts per midgut of infected mosquitoes that fed on control or ABA-supplemented mice. Medians are shown. Data were analyzed by the Mann–Whitney test. * P ≤ 0.05, ** P ≤ 0.001
ABA does not alter P. falciparum growth and gametocyte development in vitro.
The observed decreases in P. yoelii parasitemia and gametocytemia observed with ABA treatment (Figure 1B and C) could be due to a direct effect of ABA on parasite growth and development, an indirect effect on the parasite through alterations in host physiology and immunity, or a combination of both. Further, circulating ABA levels (in the absence of supplementation; Figure 1A) could derive from host or parasite synthesis or both. To address these possibilities, we used in vitro cultures of P. falciparum to minimize host-derived synthesis of ABA and to extend our observations beyond the mouse model.
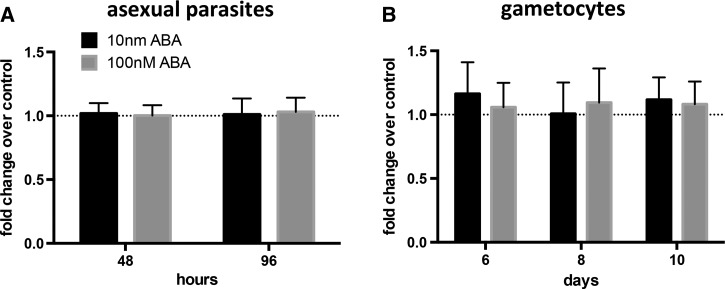
In four separate cultures, we were unable to detect ABA by either enzyme-linked immunosorbent assay or mass spectrometry in approximately 1.7 × 109 pooled RBCs per culture (mean of 8% parasitemia). Based on the potential for direct exposure of parasites to ABA after transport across the RBC plasma membrane and on circulating levels of ABA in unsupplemented and supplemented infected mice (Figure 1A), we treated cultures of P. falciparum with 10 nM ABA, 100 nM ABA, or an equivalent volume of diluent control to assess the effects of ABA on parasite development.43 At 48 and 96 hours after treatment, ABA had no effect on asexual growth relative to untreated control cultures (Figure 5A ). In addition, treatment with ABA had no effect relative to controls on gametocyte levels at 6, 8 or 10 days postculture synchronization, when gametocytogenesis is initiated (Figure 5B).44 These observations suggested that ABA-dependent decreases in parasitemia and gametocytemia in the mouse and decreased transmission to mosquitoes were not caused by direct effects of ABA on the parasites but rather the effects of ABA were likely due to indirect effects on mouse, and possibly mosquito, physiology.
Figure 5.
Abscisic acid (ABA) treatment had no direct effect on Plasmodium falciparum asexual growth or gametocyte formation. (A) Mean fold-changes in growth of asexual stage parasites as counted by flow cytometry 48 hours and 96 hours after treatment with 10 nM ABA, 100 nM ABA, or a diluent control; controls are set at one. Growth assays were performed with three different parasite culture passages. Data were analyzed by one-sample t tests. (B) Mean fold-changes in gametocyte density, counted using flow cytometry, at days 6, 8, and 10 postculture synchronization. Gametocyte assays were performed with continuous treatment with 10 nM ABA, 100 nM ABA or a diluent control using four different parasite culture passages; controls are set at one. Data were analyzed by one-sample t tests.
Supplementation of ABA in a P. falciparum-infected blood meal reduced transmission to An. stephensi by increasing nitric oxide production in the mosquito midgut.
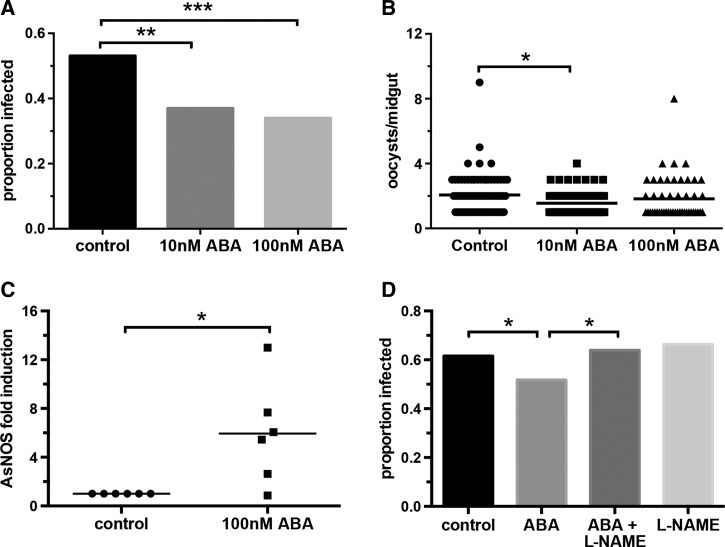
Although gametocytemia was reduced with ABA supplementation in infected mice (Figure 1C), it was unclear whether decreased transmission (Figure 4C and D) was due solely to this effect on gametocytemia or to a combination of reduced gametocytemia and effects of ingested ABA on An. stephensi. To isolate the effects of ABA on the mosquito host, we supplemented gametocyte cultures of P. falciparum with 10 nM ABA, 100 nM ABA, or an equivalent volume of diluent as a control immediately prior to feeding to An. stephensi. Mosquitoes were dissected at 10 days after feeding to record infection prevalence and intensity. Supplementation with 10 nM or 100 nM ABA reduced infection prevalence in An. stephensi by 30% and 36%, respectively, relative to control (Figure 6A ). Notably, these reductions were consistent with the reduction in the prevalence of infected mosquitoes fed on ABA-supplemented, P. yoelii-infected mice relative to controls (31.4%, Figure 4C). Though the reduction in infection prevalence was greater with 100 nM ABA, the difference between the two treatment concentrations was not statistically significant. Among P. falciparum-infected mosquitoes, mean oocysts per midgut decreased significantly from 2.1 in controls to 1.6 with 10 nM ABA (Figure 6B).
Figure 6.
Abscisic acid (ABA) supplementation of a Plasmodium falciparum-infected blood meal reduced infection prevalence in Anopheles stephensi by increasing nitric oxide production. (A) Mean proportions of mosquitoes that became infected upon feeding on P. falciparum-infected blood meals supplemented with 10 nM ABA, 100 nM ABA, or with an equivalent volume of diluent as a control. Bars represent four replicates with separate cohorts of mosquitoes. Data were analyzed by Fisher's exact test. (B) Oocysts per midgut of mosquitoes that were infected; medians are shown. Data were analyzed by the Kruskal–Wallis test followed by Dunn's multiple comparison test. (C) Fold change in An. stephensi nitric oxide synthase expression in the midgut of mosquitoes fed a P. falciparum-infected blood meal supplemented with 100 nM ABA. Each dot represents a replicate of 10 pooled midguts. Data are shown as fold change (2ΔΔCt) in gene expression relative to control-supplemented P. falciparum-fed mosquitoes. Data were analyzed by one-sample t test. (D) Mean proportions of mosquitoes that became infected upon feeding on P. falciparum-infected blood meals supplemented with 100 nM ABA, Nω-Nitro-l-arginine methyl ester (l-NAME), 100 nM ABA + l-NAME or an equivalent volume of diluent as a control. Bars represent four replicates with separate cohorts of mosquitoes. Data were analyzed by Fisher's exact test. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.005.
To discern how ABA might be reducing P. falciparum infection in An. stephensi, we examined transcript levels of the gene encoding the antimicrobial peptide defensin as well as those encoding the antiparasite effectors AsNOS and the complement-like proteins LRIM, APL1, and TEP1.27,45,46 Mosquitoes were provided with a P. falciparum-infected blood meal supplemented with 100 nM ABA or with an equivalent volume of diluent as control, then analyzed at 1–24 hours postfeeding. ABA treatment led to a small increase in defensin mRNA levels at 4 hours (Supplemental Figure 5A) and increased APL1 expression at 4 and 8 hours postfeeding to levels that approached statistical significance (Supplemental Figure 5C). However, the most notable response to supplementation with ABA was increased AsNOS expression. AsNOS transcript levels were induced up to 13-fold in the midguts of ABA-treated mosquitoes relative to controls between 4–6 hours postfeeding (Figure 6C). Given that nitric oxide (NO) is a critical parasite-killing factor in An. stephensi, we reasoned that ABA-dependent increased NO synthesis could contribute to observed differences in infection between ABA-treated and control mosquitoes. To test this hypothesis, we provided P. falciparum-infected blood meals to An. stephensi with 100 nM ABA, with ABA and 1 mg/mL of the NOS inhibitor l-NAME, the inhibitor alone, or with an equivalent volume of ABA and inhibitor diluents as a control.45–47 Addition of l-NAME to an ABA-supplemented infected blood meal rescued P. falciparum infection prevalence back to control levels, whereas the inhibitor alone had no effect on infection (Figure 6D). Complete inhibition of the effect of ABA on infection suggested that ABA-dependent NO synthesis reduced parasite survival in the mosquito midgut.
Discussion
We have shown that oral ABA supplementation reduced P. yoelii parasitemia and gametocytemia, the pathology associated with higher parasite loads, and parasite transmission to the mosquito An. stephensi. Hence, ABA supplementation limited growth and development of P. yoelii life stages in two different hosts: asexual growth and gametocyte formation in the mammalian host and survival to oocyst formation in the mosquito host. The lack of a direct effect on P. falciparum asexual growth or gametocyte formation in vitro suggests that ABA alters the biology of both the mammalian and insect hosts to limit infection and transmission.
The effect of ABA on inflammatory pathology in the mouse liver and spleen could derive from a direct, restorative effect of ABA on these tissues or from significantly reduced parasitemia. In a mouse model of influenza, ABA treatment decreased vascular infiltrates, but had no effect on viral load.32 Similarly, we saw a reduction in leukocyte infiltration into the liver with ABA treatment in the absence of infection, suggesting that ABA can directly affect some host responses independently of its effect on pathogen load. The negative correlation between liver leukocytes and parasitemia in ABA-treated mice also suggests that ABA can directly affect immune cell infiltration in a manner that is influenced by pathogen load and existing pathology. Indeed, previous studies have demonstrated that the effects of ABA can be context-dependent. In particular, ABA stimulates insulin release in a glucose concentration-dependent manner48 and can enhance or suppress nuclear factor-kappa B (NF-κB) activity depending on cell type.49 Here, we speculate that the reduction in pathology in our mouse model is likely due to a combination of the direct effects of ABA on host tissues and the reduction in parasitemia.
The reduction in liver and spleen pathology corresponded to increased PPARγ expression in both tissues on day 7 of infection (Figure 3). ABA induces PPARγ expression and this is required for its beneficial effects in the context of widely different conditions: decreased pathology in models of IBD, enhanced insulin sensitivity, and extended mouse lifespan during influenza infection.11,32,50 PPARγ is notable in the context of malaria because of its ability to limit inflammation-based pathology while also increasing pathogen clearance. In particular, enhanced PPARγ activity has been shown to increase CD36-mediated phagocytosis of P. falciparum-infected RBCs and the PPARγ agonist rosiglitazone was shown to enhance parasite clearance and reduce inflammation biomarkers in a human trial.51–53 The reduction in leukocyte adherence in the liver with ABA treatment (Figure 2B) corresponds with increased PPARγ transcript levels (Figure 3), which supports the use of PPARγ agonists as adjunctive treatments for severe malaria.54 By extension, ABA supplementation, through these beneficial effects on PPARγ, could be used to ameliorate symptoms of severe or complicated malaria.
The reduction in liver and spleen pathology also corresponded to decreased NOS expression in both tissues on day 7 of infection (Figure 3). The impact of NOS expression in malaria is tissue specific and time specific. For example, early NOS expression in the spleen, but not in the liver, was shown to correlate with resistance to blood-stage P. chabaudi.55 In addition, during lethal P. berghei infection, inducible NOS mRNA levels in the liver decreased as parasitemia rose but then increased later in infection when mice were close to death.56 Here, failure to clear parasites was associated with increased inducible NOS expression in the liver late during infection. In the context of the observed reduction in tissue pathology and increased PPARγ transcript levels, it is likely that decreased NOS expression in the liver and spleen of ABA-supplemented mice on day 7 of infection reflects recovery from inflammation.
In contrast to later resolution of inflammation in the mouse, ingestion of ABA by the mosquito induced high levels of AsNOS expression within 4–6 hours after infection, a response that likely targets newly formed ookinetes in the midgut lumen.57 Coincident with this response were induced levels of defensin and APL1 transcripts, suggesting that ABA signaling regulates NF-κB-dependent gene expression in a manner analogous to that observed in mammalian cells.58–60 The impact of immune genes other than AsNOS, however, remains to be determined. Given the significant reduction in parasitemia in ABA-treated mice by 3–4 days postinfection (Figures 1B and 4A), ABA may function to promote early inflammatory responses in both hosts to reduce parasite development and then feedback to promote restoration from inflammation as observed in the mouse.
The development of drugs to target multiple parasite life stages has been identified as a crucial goal for future antimalarials.61 We have shown that dietary supplementation with ABA reduced infection, pathology, and transmission in malaria. ABA is a natural part of the human diet, present for example in honey and spinach.62,63 Magnone and others found that the consumption of fruits with high ABA content increased plasma ABA levels and reduced glycemia in rats and humans compared with glucose-fed controls.64 No harmful effects have been reported to date for ABA supplementation, although how ABA supplementation interacts with other treatments and to what extent the effects of ABA can be enhanced will be the focus of future studies. Importantly, identification of the mechanisms whereby ABA alters mosquito physiology and transmission as well as inflammation and health of the mammalian host could shed light on the balance of immunity and metabolism across eukaryotes and provide a strong foundation for clinical and public health translation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kong Cheung (Department of Medical Microbiology and Immunology, University of California, Davis) for assistance with mosquito infection studies and Plasmodium falciparum growth assays. We also thank Rashaun Potts (Department of Medical Microbiology and Immunology, University of California, Davis) for assistance with mouse infections.
Footnotes
Financial support: This work was supported by the University of California, Davis College of Biological Sciences Dean's Kingdom Crossing Project, funded by the Stanley and Jacqueline Schilling Endowment. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Disclosure: Shirley Luckhart and Elizabeth K. K. Glennon are authors on a provisional patent.
Authors' addresses: Elizabeth K. K. Glennon and Shirley Luckhart, Department of Medical Microbiology and Immunology, University of California, Davis, Davis, CA, E-mails: ekglennon@ucdavis.edu and sluckhart@ucdavis.edu. L. Garry Adams, Department of Veterinary Pathobiology, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX, E-mail: gadams@cvm.tamu.edu. Derrick R. Hicks, Department of Plant Pathology, University of California, Davis, Davis, CA, E-mail: drhicks1@uw.edu. Katayoon Dehesh, Department of Plant Biology, University of California, Davis, Davis, CA, E-mail: kdehesh@ucdavis.edu.
References
- 1.World Health Organization World Malaria Report 2015. 2015 http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ Available at. Accessed December 1, 2015. [Google Scholar]
- 2.Bruzzone S, Moreschi I, Usai C, Guida L, Salis A, Scarfi S, Millo E, De Flora A, Zocchi E. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc Natl Acad Sci USA. 2007;104:5759–5764. doi: 10.1073/pnas.0609379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zocchi E, Carpaneto A, Cerrano C, Bavestrello G, Giovine M, Bruzzone S, Guida L, Franco L, Usai C. The temperature-signaling cascade in sponges involves a heat-gated cation channel, abscisic acid, and cyclic ADP-ribose. Proc Natl Acad Sci USA. 2001;98:14859–14864. doi: 10.1073/pnas.261448698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehrotra R, Bhalothia P, Bansal P, Basantani MK, Bharti V, Mehrotra S. Abscisic acid and abiotic stress tolerance—different tiers of regulation. J Plant Physiol. 2014;171:486–496. doi: 10.1016/j.jplph.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Zocchi E, Basile G, Cerrano C, Bavestrello G, Giovine M, Bruzzone S, Guida L, Carpaneto A, Magrassi R, Usai C. ABA- and cADPR-mediated effects on respiration and filtration downstream of the temperature-signaling cascade in sponges. J Cell Sci. 2003;116:629–636. doi: 10.1242/jcs.00277. [DOI] [PubMed] [Google Scholar]
- 7.Bruzzone S, Ameri P, Briatore L, Mannino E, Basile G, Andraghetti G, Grozio A, Magnone M, Guida L, Scarfi S, Salis A, Damonte G, Sturla L, Nencioni A, Fenoglio D, Fiory F, Miele C, Beguinot F, Ruvolo V, Bormioli M, Colombo G, Maggi D, Murialdo G, Cordera R, De Flora A, Zocchi E. The plant hormone abscisic acid increases in human plasma after hyperglycemia and stimulates glucose consumption by adipocyte myoblasts. FASEB J. 2011;26:1251–1260. doi: 10.1096/fj.11-190140. [DOI] [PubMed] [Google Scholar]
- 8.Puce S, Basile G, Bavestrello G, Bruzzone S, Cerrano C, Giovine M, Arillo A, Zocchi E. Abscisic acid signaling through cyclic ADP-ribose in hydroid regeneration. J Biol Chem. 2004;279:39783–39788. doi: 10.1074/jbc.M405348200. [DOI] [PubMed] [Google Scholar]
- 9.Scarfi S, Ferraris C, Fruscione F, Fresia C, Guida L, Bruzzone S, Usai C, Parodi A, Millo E, Salis A, Burastero G, De Flora A. Cyclic ADP-ribose-mediated expansion and stimulation of human mesenchymal stem cells by the plant hormone abscisic acid. Stem Cells. 2008;26:2855–2864. doi: 10.1634/stemcells.2008-0488. [DOI] [PubMed] [Google Scholar]
- 10.Bodrato N, Franco L, Fresia C, Guida L, Usai C, Salis A, Moreschi I, Ferraris C, Verderio C, Basile G, Bruzzone S, Scarfi S, De Flora A, Zocchi E. Abscisic acid activates the murine microglial cell line N9 through the second messenger cyclic ADP-ribose. J Biol Chem. 2009;284:14777–14787. doi: 10.1074/jbc.M802604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guri AJ, Evans NP, Hontecillas R, Bassaganya-Riera J. T cell PPARγ is required for the anti-inflammatory efficacy of abscisic acid against experimental IBD. J Nutr Biochem. 2011;22:812–819. doi: 10.1016/j.jnutbio.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guri AJ, Hontecillas R, Bassaganya-Riera J. Abscisic acid ameliorates experimental IBD by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration. Clin Nutr. 2010;29:824–831. doi: 10.1016/j.clnu.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rupani AB, Amarapurkar AD. Hepatic changes in fatal malaria: an emerging problem. Ann Trop Med Parasitol. 2009;103:119–127. doi: 10.1179/136485909X385054. [DOI] [PubMed] [Google Scholar]
- 14.Schofield L. Intravascular infiltrates and organ-specific inflammation in malaria pathogenesis. Immunol Cell Biol. 2007;85:130–137. doi: 10.1038/sj.icb.7100040. [DOI] [PubMed] [Google Scholar]
- 15.Negri P, Maggi MD, Ramirez L, de Feudis L, Szwarski N, Quintana S, Eguaras MJ, Lamattina L. Abscisic acid enhances the immune response in Apis mellifera and contributes to the colony fitness. Apidologie (Celle) 2015;46:542–557. [Google Scholar]
- 16.Luckhart S, Crampton AL, Zamora R, Lieber MJ, Dos Santos PC, Peterson TM, Emmith N, Lim J, Wink DA, Vodovotz Y. Mammalian transforming growth factor beta 1 activated after ingestion by Anopheles stephensi modulates immunity. Infect Immun. 2003;71:3000–3009. doi: 10.1128/IAI.71.6.3000-3009.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pakpour N, Corby-Harris V, Green GP, Smithers HM, Cheung KW, Riehle MA, Luckhart S. Ingested human insulin inhibits the mosquito NF-κB-dependent immune response to Plasmodium falciparum. Infect Immun. 2012;80:2141–2149. doi: 10.1128/IAI.00024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruzzone S, Basile G, Mannino E, Sturla L, Magnone M, Grozio A, Salis A, Fresia C, Vigliarolo T, Guida L, De Flora A, Tossi V, Cassia R, Lamattina L, Zocchi E. Autocrine abscisic acid mediates the UV-B-induced inflammatory response in human granulocytes and keratinocytes. J Cell Physiol. 2012;227:2502–2510. doi: 10.1002/jcp.22987. [DOI] [PubMed] [Google Scholar]
- 19.Marois E. The multifaceted mosquito anti-Plasmodium response. Curr Opin Microbiol. 2011;14:429–435. doi: 10.1016/j.mib.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Roux CM, Butler BP, Chau JY, Paixao TA, Cheung KW, Santos TL, Luckhart S, Tsolis RM. Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to Nontyphoidal Salmonella enterica serovar Typhimurium infection in mice. Infect Immun. 2010;78:1520–1527. doi: 10.1128/IAI.00887-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelberth MJ, Engelberth J. Monitoring plant hormones during stress responses. J Vis Exp. 2009;28:1127. doi: 10.3791/1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmelz EA, Alborn HT, Engelberth J, Tumlinson JH. Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol. 2003;133:295–306. doi: 10.1104/pp.103.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelberth J, Schmelz EA, Alborn HT, Cardoza YJ, Huang J, Tumlinson JH. Simultaneous quantification of jasmonic acid and salicylic acid in plants by vapor-phase extraction and gas chromatography-chemical ionization-mass spectrometry. Anal Biochem. 2003;312:242–250. doi: 10.1016/s0003-2697(02)00466-9. [DOI] [PubMed] [Google Scholar]
- 24.Drexler AL, Pietri JE, Pakpour N, Hauck E, Wang B, Glennon EK, Georgis M, Riehle MA, Luckhart S. Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi. PLoS Pathog. 2014;10:e1004231. doi: 10.1371/journal.ppat.1004231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 26.Miao J, Fan Q, Parker D, Li X, Li J, Cui L. Puf mediates translation repression of transmission-blocking vaccine candidates in malaria parasites. PLoS Pathog. 2013;9:e1003268. doi: 10.1371/journal.ppat.1003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crampton A, Luckhart S. The role of As60A, a TGF-beta homolog, in Anopheles stephensi innate immunity and defense against Plasmodium infection. Infect Genet Evol. 2001;1:131–141. doi: 10.1016/s1567-1348(01)00017-x. [DOI] [PubMed] [Google Scholar]
- 28.Dong Y, Das S, Cirimotich C, Souza-Neto JA, McLean KJ, Dimopoulos G. Engineered Anopheles immunity to Plasmodium infection. PLoS Pathog. 2011;7:e1002458. doi: 10.1371/journal.ppat.1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauck ES, Antonova-Koch Y, Drexler A, Pietri J, Pakpour N, Liu D, Blacutt J, Riehle MA, Luckhart S. Overexpression of phosphatase and tensin homolog improves fitness and decreases Plasmodium falciparum development in Anopheles stephensi. Microbes Infect. 2013;15:775–787. doi: 10.1016/j.micinf.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chau JY, Tiffany CM, Nimishakavi S, Lawrence JA, Pakpour N, Mooney JP, Lokken KL, Caughey GH, Tsolis RM, Luckhart S. Malaria-associated L-arginine deficiency induces mast cell-associated disruption to intestinal barrier defenses against nontyphoidal Salmonella bacteremia. Infect Immun. 2013;81:3515–3526. doi: 10.1128/IAI.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr. 2007;26:107–116. doi: 10.1016/j.clnu.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Hontecillas R, Roberts PC, Carbo A, Vives C, Horne WT, Genis S, Velayudhan B, Bassaganya-Riera J. Dietary abscisic acid ameliorates influenza-virus-associated disease and pulmonary immunopathology through a PPARγ-dependent mechanism. J Nutr Biochem. 2013;24:1019–1027. doi: 10.1016/j.jnutbio.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hontecillas R, Bassaganya-Riera J. Expression of PPAR γin intestinal epithelial cells is dispensable for the prevention of colitis by dietary abscisic acid. ESPEN J. 2012;7:e189–e195. doi: 10.1016/j.clnme.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guha M, Maity P, Choubey V, Mitra K, Reiter RJ, Bandyopadhyay U. Melatonin inhibits free radical-mediated mitochondrial-dependent hepatocyte apoptosis and liver damage induced during malarial infection. J Pineal Res. 2007;43:372–381. doi: 10.1111/j.1600-079X.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava A, Khanduri A, Lakhtakia S, Pandey R, Choudhuri G. Falciparum malaria with acute liver failure. Trop Gastroenterol. 1996;17:172–174. [PubMed] [Google Scholar]
- 36.Fu Y, Ding Y, Zhou TL, Ou QY, Xu WY. Comparative histopathology of mice infected with the 17XL and 17XNL strains of Plasmodium yoelii. J Parasitol. 2012;98:310–315. doi: 10.1645/GE-2825.1. [DOI] [PubMed] [Google Scholar]
- 37.Brugat T, Cunnningham D, Sodenkamp J, Coomes S, Wilson M, Spence PJ, Jarra W, Thompson J, Scudamore C, Langhorne J. Sequestration and histopathology in Plasmodium chabaudi malaria are influenced by the immune response in an organ-specific manner. Cell Microbiol. 2014;16:687–700. doi: 10.1111/cmi.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríquez-Acosta A, Finol HJ, Pulido-Méndez M, Márquez A, Andrade G, González N, Aguilar I, Girón ME, Pinto A. Liver ultrastructural pathology in mice infected with Plasmodium berghei. J Submicrosc Cytol Pathol. 1998;30:299–307. [PubMed] [Google Scholar]
- 39.Guha M, Kumar S, Choubey V, Maity P, Bandyopadhyay U. Apoptosis in liver during malaria: role of oxidative stress and implication of mitochondrial pathway. FASEB J. 2006;20:1224–1226. doi: 10.1096/fj.05-5338fje. [DOI] [PubMed] [Google Scholar]
- 40.Wunderlich F, Al-Quraishy S, Dkhil MA. Liver-inherent immune system: its role in blood-stage malaria. Front Microbiol. 2014;5:559. doi: 10.3389/fmicb.2014.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engwerda CR, Beattie L, Amante FH. The importance of the spleen in malaria. Trends Parasitol. 2005;21:75–80. doi: 10.1016/j.pt.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Del Portillo HA, Ferrer M, Brugat T, Martin-Jaular L, Langhorne J, Lacerda MVG. The role of the spleen in malaria. Cell Microbiol. 2012;14:343–355. doi: 10.1111/j.1462-5822.2011.01741.x. [DOI] [PubMed] [Google Scholar]
- 43.Vigliarolo T, Guida L, Millo E, Fresia C, Turco E, De Flora A, Zocchi E. Abscisic acid transport in human erythrocytes. J Biol Chem. 2015;290:13042–13052. doi: 10.1074/jbc.M114.629501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamour SD, Straschil U, Saric J, Delves MJ. Changes in metabolic phenotypes of Plasmodium falciparum in vitro cultures during gametocyte development. Malar J. 2014;13:468. doi: 10.1186/1475-2875-13-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clayton AM, Dong Y, Dimopoulos G. The Anopheles innate immune system in the defense against malaria infection. J Innate Immun. 2014;6:169–181. doi: 10.1159/000353602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson TML, Gow AJ, Luckhart S. Nitric oxide metabolites induced in Anopheles stephensi control malaria parasite infection. Free Radic Biol Med. 2007;42:132–142. doi: 10.1016/j.freeradbiomed.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruzzone S, Bodrato N, Usai C, Guida L, Moreschi I, Nano R, Antonioli B, Fruscione F, Magnone M, Scarfi S, De Flora A, Zocchi E. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic ADP ribose as second messenger. J Biol Chem. 2008;283:32188–32197. doi: 10.1074/jbc.M802603200. [DOI] [PubMed] [Google Scholar]
- 49.Bassaganya-Riera J, Guri AJ, Lu P, Climent M, Carbo A, Sobral BW, Horne WT, Lewis SN, Bevan DR, Hontecillas R. Abscisic acid regulates inflammation via ligand-binding domain-independent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2011;286:2504–2516. doi: 10.1074/jbc.M110.160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guri AJ, Hontecillas R, Ferrer G, Casagran O, Wankhade U, Noble AM, Eizirik DL, Ortis F, Cnop M, Liu D, Si H, Bassaganya-Riera J. Loss of PPAR gamma in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissue. J Nutr Biochem. 2008;19:219–228. doi: 10.1016/j.jnutbio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Serghides L, Kain KC. Peroxisome proliferator-activated receptor gamma-retinoid x receptor agonists increase CD36-dependent phagocytosis of Plasmodium falciparum-parasitized erythrocytes and decrease malaria-induced TNF-α secretion by monocytes/macrophages. J Immunol. 2001;166:6742–6748. doi: 10.4049/jimmunol.166.11.6742. [DOI] [PubMed] [Google Scholar]
- 52.Patel SN, Serghides L, Smith TG, Febbraio M, Silverstein RL, Kurtz TW, Pravenec M, Kain KC. CD36 mediates the phagocytosis of Plasmodium falciparum-infected erythrocytes by rodent macrophages. J Infect Dis. 2004;189:204–213. doi: 10.1086/380764. [DOI] [PubMed] [Google Scholar]
- 53.Ak Boggild, Krudsood S, Patel SN, Serghides L, Tangpukdee N, Katz K, Wilairatana P, Liles WC, Looareesuwan S, Kain KC. Use of peroxisome proliferator-activated receptor gamma agonists as adjunctive treatments for Plasmodium falciparum malaria: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2009;49:841–849. doi: 10.1086/605431. [DOI] [PubMed] [Google Scholar]
- 54.Serghides L, McDonald CR, Lu Z, Friedel M, Cui C, Ho KT, Mount HT, Sled JG, Kain KC. PPARγ agonists improve survival and neurocognitive outcomes in experimental cerebral malaria and induce neuroprotective pathways in human malaria. PLoS Pathog. 2014;10:e1003980. doi: 10.1371/journal.ppat.1003980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobs P, Radzioch D, Stevenson MM. Nitric oxide expression in the spleen, but not in the liver, correlates with resistance to blood-stage malaria in mice. J Immunol. 1995;155:5306–5313. [PubMed] [Google Scholar]
- 56.Nahrevanian H, Dascombe MJ. Expression of inducible nitric oxide synthase (iNOS) mRNA in target organs of lethal and non-lethal strains of murine malaria. Parasite Immunol. 2002;24:471–478. doi: 10.1046/j.1365-3024.2002.00490.x. [DOI] [PubMed] [Google Scholar]
- 57.Meis JF, Ponnudurai T. Ultrastructural studies on the interaction of Plasmodium falciparum ookinetes with the midgut epithelium of Anopheles stephensi mosquitoes. Parasitol Res. 1987;73:500–506. doi: 10.1007/BF00535323. [DOI] [PubMed] [Google Scholar]
- 58.Eggleston P, Lu W, Zhao Y. Genomic organization and immune regulation of the defensin gene from the mosquito, Anopheles gambiae. Insect Mol Biol. 2000;9:481–490. doi: 10.1046/j.1365-2583.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 59.Mitri C, Jacques JC, Thiery I, Riehle MM, Xu J, Bischoff E, Morlais I, Nsango SE, Vernick KD, Bourgouin C. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog. 2009;5:e1000576. doi: 10.1371/journal.ppat.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magnone M, Bruzzone S, Guida L, Damonte G, Millo E, Scarfi S, Usai C, Sturla L, Palombo D, De Flora A, Zocchi E. Abscisic acid released by human monocytes activates monocytes and vascular smooth muscle cell responses involved in atherogenesis. J Biol Chem. 2009;284:17808–17818. doi: 10.1074/jbc.M809546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith PW, Diagana TT, Yeung BK. Progressing the global antimalarial portfolio: finding drugs which target multiple Plasmodium life stages. Parasitology. 2014;141:66–76. doi: 10.1017/S0031182013000747. [DOI] [PubMed] [Google Scholar]
- 62.Gheldof N, Wang X, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. 2002;50:5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- 63.Zeevart JAD. (+)-Abscisic acid content of spinach in relation to photoperiod and water stress. Plant Physiol. 1971;48:86–90. doi: 10.1104/pp.48.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magnone M, Ameri P, Salis A, Andraghetti G, Emionite L, Murialdo G, De Flora A, Zocchi E. Microgram amounts of abscisic acid in fruit extracts improve glucose tolerance and reduce insulinemia in rats and in humans. FASEB J. 2015;29:4783–4793. doi: 10.1096/fj.15-277731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.