Abstract
A standardized test for the serodiagnosis of cystic echinococcosis (CE) remains an important challenge because of the problems in specificity and sensitivity of the available commercial kits and lack of proper evaluation of antigen. Using appropriate sources of antigenic material is crucial in improvement of the serological methods such as enzyme-linked immunosorbent assay (ELISA). This study was conducted to evaluate the performance of protein named Echinococcus protoscolex calcium binding protein EPC1 for the detection of antibodies in sera from patients with CE. Expressed and purified recombinant protein EPC1 (rEPC1) was used as antigen in ELISA method. Characterization of the rEPC1 antigen was evaluated using the serum of 25 patients with both surgical and imaging confirmed CE and 25 healthy donors as negative controls. Also, a panel of sera including chronic toxoplasmosis (IgG positive), strongyloidosis, fascioliasis, toxocariasis, and kala azar were used and patients with related parasites were confirmed by medical laboratories or clinically by research centers using microscopy or specific ELISA. rEPC1 showed relatively promising performance in total IgG ELISA for the detection of antibodies in sera from the negative controls, and the cut off value 0.4 units of optical density at 490 nm was calculated for ELISA. In this study, sensitivity of 100%, specificity of 93.7, positive predictive value of 92.6%, and negative predictive value of 100% were calculated for rEPC1. On the other hand, commercial ELISA kit based on the native antigen B of Echinococcus granulosus had sensitivity of 96.2% and specificity of 96.8%. No significant difference was found for sensitivity or specificity between the rEPC1 and commercial kit. However, rEPC1 may be a valuable antigen for diagnosis of human CE.
Introduction
Echinococcus granulosus is considered one of the most significant parasitic infections in endemic areas throughout the world. This parasite is a causative agent of cystic hydatid disease, which can be transmitted between canines and numerous herbivorous livestock animals.1 It is regarded an important global parasitic disease of humans and animals, and is endemic in Iran, where a variety of animals act as intermediate hosts.2–4 Fasihi Harandi and others5 estimated the annual surgical incidences of cystic echinococcosis (CE) in Iran with a rate of 1.27/100,000 population from 2000 to 2009. The average annual cost of CE in Iran was estimated at US$232.3 million (95% confidence interval = US$103.1–397.8 million), including both direct and indirect costs.5 In this regard, diagnosis of the disease is still an important challenge due to the biology of the disease and lack of an authentic gold standard. Imaging techniques in humans such as magnetic resonance imaging or computed tomography cannot confirm the diagnosis of CE. In this regard, using World Health Organization international classification of ultrasound images of CE can be useful for diagnosis and staging of CE. Imaging techniques are usually sufficient for reliable diagnosis; however, they may sometimes be inconclusive.1 Ultrasound technique cannot normally detect pulmonary echinococcosis.6
Serological testing such as enzyme-linked immunosorbent assay (ELISA) can be used as complementary tests, which are usually based on the native antigen B or even hydatid cyst fluid prepared from metacestodes of E. granulosus. The main problems in the serodiagnosis of echinococcal diseases are the usually poor performance of the available tests and the difficulties associated with the standardization of antigenic preparations and techniques.
Nowadays, highly specific and sensitive antigens and antigenic constructs in serological tests are required and accurate interpretation of results is vital.6,7
Furthermore, the characteristics of the patient serums and the quality of the collected parasitic antigens can be effective on the efficiency of immunodiagnostic test that is used in human CE.8 However, such procedures induce a considerable number of cross-reactions. Several recombinant antigens and synthetic peptides (combining several defined antigens) have shown potential for CE serodiagnosis in humans.9–12 On the other hand, the identification of infection in dogs is also important. A recombinant antigen, EPC1, an 8.5-kDa antigen from E. granulosus has been shown to be efficient in diagnosis of human hydatidosis.11
Previously, we assess the sequence of EPC1 isolated from intermediate hosts, including sheep (G1 strain) and camel (G6 strain). The EPC1 sequence contains coding and noncoding regions and was compared between two predominant strains (G1 and G6) in Iran. Sequence polymorphism was not found in protein coding regions, suggesting that these regions may be useful for identification of protein expression as an antigen where the two strains are prevalent.13
This study was designed to assess the efficacy of rEPC1 antigen for the diagnosis of CE based on the strains and genotypes isolated from humans in Iran by ELISA. Therefore, sera of the most common human parasites were used in the assessment of rEPC1 cross-reactivity. Furthermore, our findings were compared with available commercial kit in Iran.
Materials and Methods
Recombinant protein EPC1.
rEPC1 was performed as previously reported.14 In brief, liver hydatid cysts were collected from slaughterhouses around the Tehran Province and transferred to parasitology laboratory of Tehran University, Iran. Protoscoleces (G1 strain) were aspirated from cysts, pooled, and washed in phosphate-buffered saline (PBS-1%). Total RNA was extracted from protoscoleces by using an RNeasy mini kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. Then cDNA was synthesized by using RevertAid Reverse Transcriptase (Fermentas, Vilnius, Lithuania). The following sequences were selected for using the EPC1: forward primer 5′ ATGAGTCTTCAGAAAACT and reverse primer 5′ TTAGAAGAGAGCCATTAA (gene accession no: JF964264.1). The EPC1 gene of E. granulosus was amplified by polymerase chain reaction (PCR) product containing a band of 228 bp, digested with BamHI and EcoRI and, the expression plasmid vector pET28a was double digested with the same restriction endonucleases as mentioned above. The EPC1-PCR products were cloned into the (between BamHI and EcoRI site) pET28a vector by using rapid DNA ligation kit (Roche, Mannheim, Germany).
To produce rEPC1 proteins, the cloned plasmid containing the EPC1 was transformed into Escherichia coli BL21 (DE3) strain. Then colony PCR was used to confirm the successful construction of the recombinant expression plasmid EPC1/pET28a. The recombinant plasmid extracted from transformed E. coli BL21 was digested by the restriction enzymes EcoRI and BamHI; also the sequencing result was used to confirm the desired construction of recombinant plasmid.
Expression and purification of rEPC1.
Positive clones of recombinant plasmid pET28a were transformed into E. coli BL21 (DE3) strain and incubated overnight with shaking in Luria–Bertani medium containing kanamycin 1 mg/mL until an optical density (OD650) of 0.5–0.6 was achieved. Expression of the recombinant protein was induced at 33°C for 6 hours in the presence of isopropyl-β-D-thiogalactoside at a final concentration of 0.5 mm. The recombinant plasmids led to the production of fusion proteins of 12.8 kDa. The recombinant His6-tagged EPC1 was purified by Ni-NTA agarose kit (Qiagen, Hilden, Germany).
Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis and Western blot analysis of recombinant antigen.
Expression and purification of His6-tagged protein were checked by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE [12%]) and Coomassie Blue R-250 staining.
Western blot analysis was performed with T7 tag antibody at a 1:10,000 dilution (Thermo Scientific) and HRP-conjugated mouse anti-T7 tag at a 1:5,000 dilution. Finally, the positive reaction was developed by using DAB (3, 3′-diaminobenzidine) (Sigma Aldrich) as substrate under visual observation within 5 minutes. Moreover, Western blot analysis was performed with sera of human CE at a 1:200 dilution. HRP-conjugated rabbit anti-human IgG (Sigma-Aldrich) was used at a 1:101,000 dilution.
Serum samples.
Sera were obtained from patients who were scheduled to undergo surgery in Velayat Hospital, Qazvin University of Medical Sciences, Iran, other sera were collected from different hospitals in Tehran. Sera from 25 healthy individuals were also included in this study as negative controls; the donors had no recent history of hydatidosis, and belonged to the age group of adolescents and children. Additionally, they were also screened for hydatidosis by commercial ELISA kit (Pishtaz, Tehran, Iran).
Thirty serum samples were obtained for assessing cross-reaction of rEPC1 with other human proven parasites including 10 samples from patients with toxoplasmosis (Toxoplasma gondii chronic infection with IgG positive), 3 samples from patients with strongyloidiasis (Strongyloides stercoralis infection), 6 samples from patients with fascioliasis, 6 samples from patients with Visceral leishmaniasis, and 5 samples from patients with toxocariasis that were serologically confirmed by medical laboratories or clinically by research centers. All serum samples were stored at −30°C until use. All the samples were examined with available commercial ELISA kit based on the native antigen B (Pishtaz) to confirm that they were negative for CE. In this study, specificity and sensitivity values of ELISA based on rEPC1 were compared with a standard kit for all similar samples.
Indirect enzyme-linked immunosorbent assay.
Briefly, 96-well polystyrenes microtiter plates were coated with 1 μg/well of rEPC1 (in 100 μL volume) and diluted in 0.1 M carbonate/bicarbonate buffer (pH 9.6) for 13 hours at 4°C (Bora and others 2002) then rinsed with PBS Tween three times. Afterward, wells were blocked for 1 hour and 30 minutes at room temperature with a blocking solution (1% bovine serum albumin, 300 μL) in PBS-T. After three washes, 100 μL of diluted positive and negative sera was loaded into each well of the plates (at a dilution of 1:400 in PBS containing 0.1% Tween-20) and vibrated for 2 hours at room temperature. The plates were again washed three times in PBS-T and coated with 50 μ of antihuman conjugate at a dilution of 1:15,000 as the second antibody after incubating the plates for 1 hour on the vibrator in the dark. After rinsing, the plate was loaded with 100 μL TMB per well as substrate. The reaction was developed at room temperature and stopped with 50 μL/well of 1 N sulfuric acid (H2SO4), and the absorbance was measured at 450 nm. The OD was registered at 490 nm (OD490) after the addition of a stop solution (1 N H2SO4). The cut off value 0.4 units of OD at 490 nm was calculated for ELISA. It is worth noting that ELISA tests were performed in triplicate in the assays.
Statistics.
Sensitivity and specificity of the rEPC1 and commercial kit were calculated by using test results from confirmed CE patients by surgery as true positives. Blood donors and individuals infected by other parasites were taken as true negatives. Sensitivity and cross-reactive behavior were evaluated with 25 CE patients and 30 other parasitic sera. All statistical calculations were performed by using the software SPSS Version 16.0 for Windows (SPSS Inc., Chicago, IL).
Results
Recombinant EPC1 antigen.
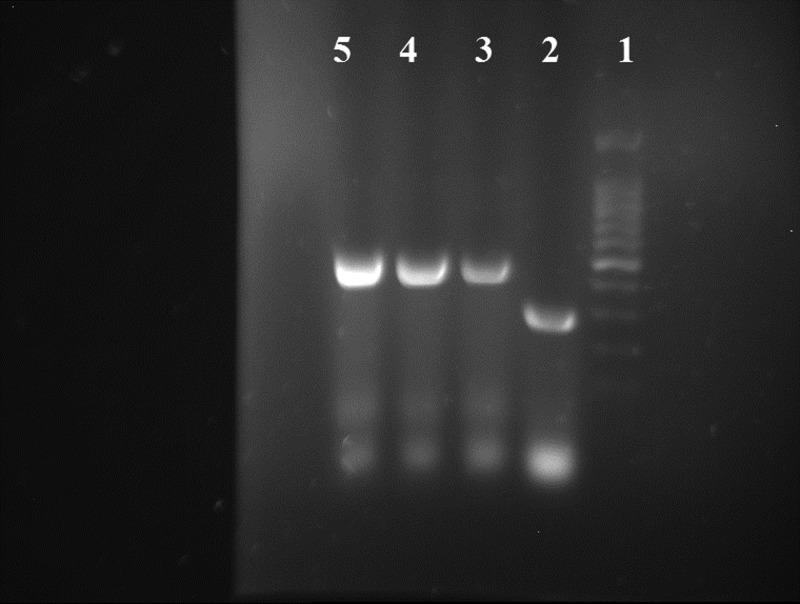
Three bands of EPC1 present in the PCR product and a band of pET28a (+) are shown in Figure 1 . Enzyme digestion of recombinant plasmid confirmed successful performance of ligation (Figure 2 ).
Figure 1.
Polymerase chain reaction (PCR) products (558 bp). Lane 1 = 100 bp DNA ladder; lane 2 = nonrecombinant plasmid (without EPC1sequence); lanes 3, 4, and 5 = recombinant plasmid containing EPC1 gene. The PCR was done by special primer of plasmid.
Figure 2.
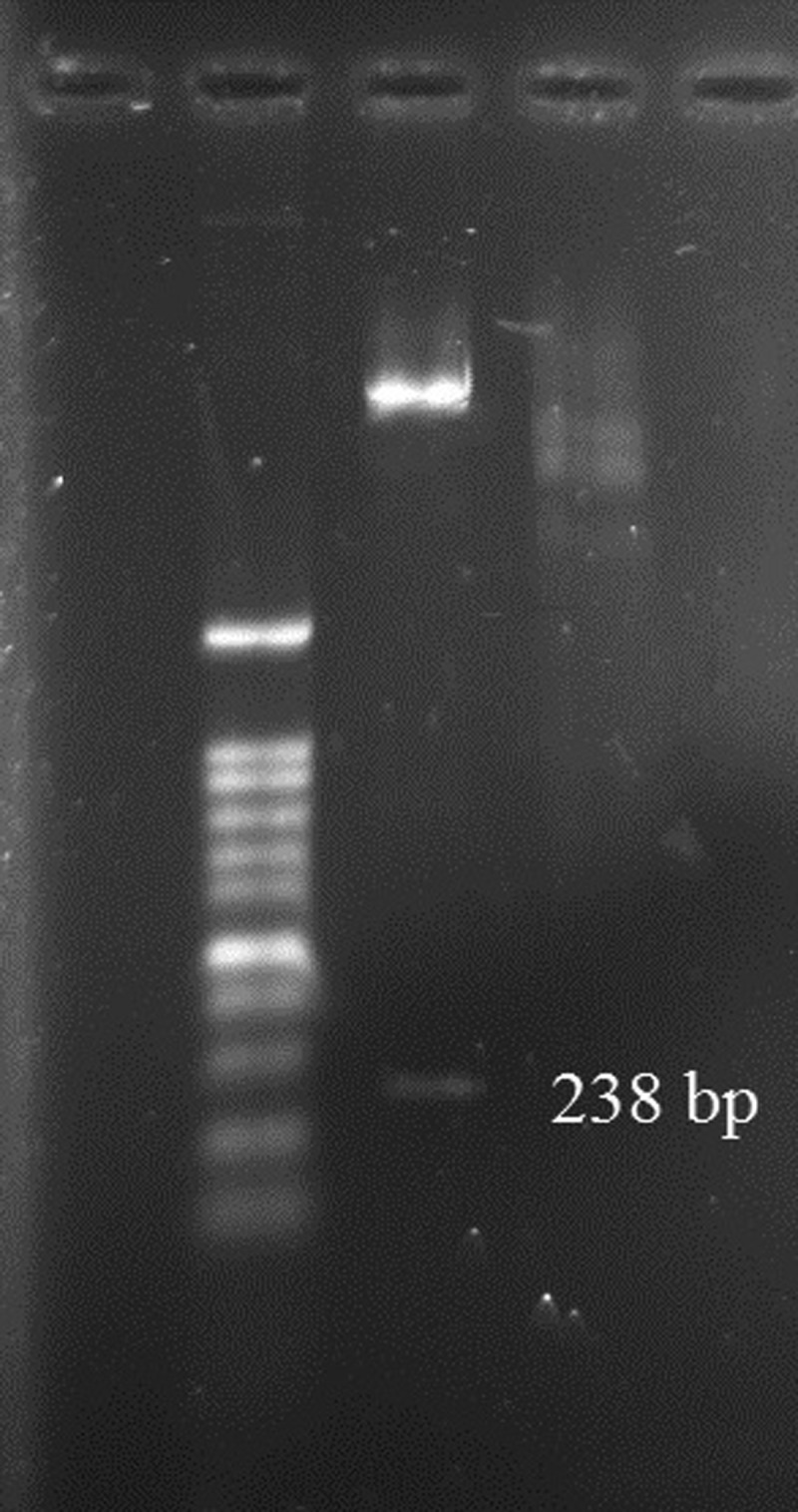
Digestion of recombinant plasmid pET28a/EPC1 by EcoRI and BamHI.
The SDS-PAGE showed that the His-tagged EPC1 protein was expressed in BL21 cells (12.8 kDa; Figure 3 ). In addition, the Western blot assay demonstrated a specific band of 12.8 kDa using T7 tag antibody (Figure 4 ).
Figure 3.
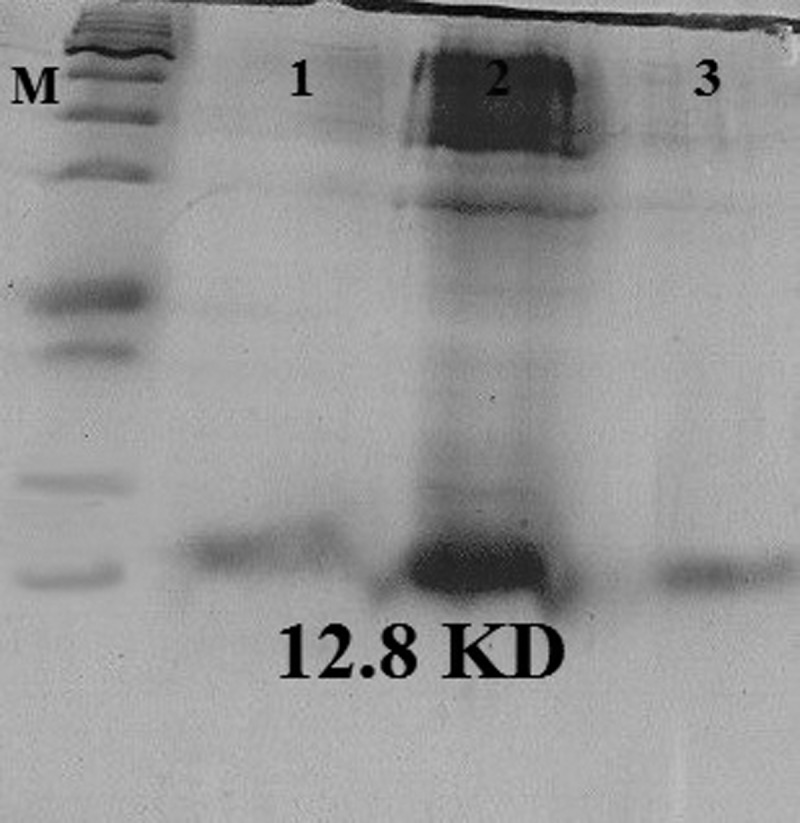
Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of the rEPC1. The gel was Coomassie Brilliant Blue stained, and the expected molecular mass of the His6-tagged rEPC1 protein was 12.8 kDa. M = protein marker; lane 2 = Escherichia coli BL21 (DE3) lysates with isopropyl-β-D-thiogalactoside 1 mM after 5 hours of induction; lanes 1 and 3 = rEPC1protein extracts.
Figure 4.
Western blot analysis of rEPC1. M = protein marker; lanes 1 and 2 = the protein rEPC1was immunoblotted with pooled T7 tag antibody.
Sensitivity and specificity of ELISA.
The sensitivity of rEPC1 was calculated to be 100% for the patients with CE and the control group, and the specificity was calculated for the serums without CE; however, it was done using the serum of other parasitic diseases (93.7%). Based on these findings, positive and negative predictive values and efficacy of rEPC1 were calculated as 92.6%, 100%, and 96%, respectively. Among the 25 CE serums used in this study, all of them react in total IgG ELISA with rEPC1; however, false-positive reactions were seen in two healthy serums and two sera pertaining to toxocariasis; also, the (1) toxocariasis serum reacted with commercial ELISA kit. Furthermore, one of the CE sera was negative with commercial ELISA kit (Pishtaz, Tehran, Iran). All samples were tested with a commercial kit and the results were compared with rEPC1, a sensitivity of 96.2% and specificity of 96.8% based on the native antigen obtained by commercial ELISA kit. The performance of rEPC1 was relatively good. However, the native antigen B from the commercial ELISA kit had better specificity than the rEPC1 protein while rEPC1 had better sensitivity. The difference in the performances of the two types of antigens was not significant when ELISA was used.
Discussion
Diagnosis of the CE is still an important challenge because of the biology of the disease and lack of a gold standard. Diagnosis is usually based on the native antigen B or even hydatid cyst fluid prepared from metacestodes of E. granulosus all over the world. It has been suggested that CE serology may be improved by using recombinant proteins. Several recombinant antigens and synthetic peptides have been used for CE serodiagnosis in humans.9–12,15–18 A highly antigenic protein, named EPC1, was characterized and isolated from a protoscolex (larval) stage and is encoded by the EPC1 gene.13,17
Our previous study was aimed to assess the sequence of EPC1 isolated from different intermediate hosts of E. granulosus, including sheep (G1 strain) and camel (G6 strain). The EPC1 sequence contains coding and noncoding regions, which were compared between two predominant strains (G1 and G6) in Iran. Sequence polymorphism was not found in protein coding regions, suggesting that these regions may be useful sequences of protein expression as an antigen.13
EPC1 protein displays high sequence, similar to a molecule from Taenia solium, the causative agent responsible for neurocysticercosis. However, Li and others11 reported that the serological cross-reactivity was limited (about 9%), indicating that EPC1 has B cell epitopes not cross-reactive with antibodies in neurocysticercosis sera. Despite these interpretations, neurocysticercosis is not a hygiene problem in Iran. Moreover, Li and others11 reported an overall sensitivity of 92.2% and an overall specificity of 95.6% for rEPC1-GST fusion protein that was an unprecedented result, and therefore, the most promising. In this study, overall sensitivity of 100% and overall specificity of 93.7 were observed. The present results indicate that the ELISA based on rEPC1 were slightly more sensitive than previous studies. It has been indicated that the rEPC1-GST antigen for the detection of specific IgG in ELISA show 92.2% sensitivity.17 On the other hand, specificity of rEPC1 in this study can be interpreted as relatively acceptable; however, the number of used sera must be higher to detect the reliable specificity. Thus, this higher sensitivity cannot be attributed to specific variables. Using synthetic peptide with specific epitopes of EPC1 may lead to an increase in specificity.
From these studies, the specificity reported by Li and others11 is relatively similar to the specificity found here for the rEPC1 antigen. Sensitivity of 100% and an overall specificity of 93.7%, in comparison with commercial ELISA kit (sensitivity of 96.2% and specificity of 96.8 based on the native antigen), indicates that native antigen B from the commercial ELISA kit had better specificity compared with the rEPC1 protein, whereas rEPC1 had better sensitivity.
Kalantari and others8 in a study in Iran reported that commercial ELISA kit had better efficacy (100%) in comparison with the recombinant antigen B (92.3%), and found that it provided a sensitivity of 91.7% and specificity of 97.2% in ELISA when tested against a panel of sera.
In a study in Iran, a recombinant 12-kDa antigen B subunit from E. granulosus has shown a sensitivity and specificity of 96.0% and 97.0%, respectively; the corresponding results for the ELISA based on native antigen B (98.6%, and 100%) were slightly better.19 In this study, there were no negative reactions among the 25 sera from the CE patients with rEPC1; however, false-positive reactions were seen in two healthy serums and two serums pertaining to toxocariasis, whereas one serum of toxocariasis reacted with commercial ELISA kit. Using IgG subclasses is one of the methods to improve CE serologic testing associated with the problem of specificity due to cross-reactivity with sera from patients with other parasites. Because of the association-specific response to antigens of E. granulosus, it can reduce cross-reactivity, whereas IgG4 recognizes antigen B or other immunomodulatory molecules; therefore, it would be a choice for serodiagnostic tests of complementary value associated with a total IgG assay.12,20–24 rEPC1 has a relatively promising performance in total IgG ELISA for the detection of antibodies in sera from patients with CE.
The limitation of this study is the number of used sera to detect the specificity of the test. We hope to improve the serum panel in future by collecting more sera.
Although the present results indicate that ELISA based on the native antigen B are at least as good as rEPC1, the preparation of the recombinant antigen in large scale is easier and more affordable than native antigen B. If it is adapted to produce a reliable and accurate method and high purification, it could be useful for the detection of CE in humans. Nevertheless, the current strategies for immunodiagnosis of CE require reappraisal. Studies must be conducted to standardize the techniques and antigenic preparations (large quantity, high purification), characterize new antigens, produce highly purified recombinant and synthetic peptide, and detect distinct Ig classes.24–27
Valiente-Gabioud and others26 reported that an increase in the number of repetitive units of a specific Trypanosoma cruzi antigen could result in an enhanced antigenic response. Hernández-González and others25 improved serodiagnosis of CE using repetitive units of antigen B. Currently, using synthetic peptides instead of native or recombinant antigens has been proposed for the diagnosis of infectious diseases, and is a perfect tool for analyzing the antigenicity of biological antigens.25 Complicated structures of recombinant and native epitopes cause the cross-reactivity of epitopes with other paratopes. So far, transcriptomics approach, proteomics approach, and genomics approach have been used for designing synthetic peptides.27 Using synthetic peptide with specific epitopes may lead to an increase in specificity.
Footnotes
Financial support: This study was supported by Iran National Science Foundation.
Authors' addresses: Saeid Fathi, Fatemeh Jalousian, Seyed Hossein Hosseini, and Somayeh Kordafshari, Department of Parasitology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran, E-mails: saeidfathi@ut.ac.ir, jalousian_f@ut.ac.ir, hhoseini@ut.ac.ir, and somayeh.kordafshari@gmail.com. Hossein Parsa, Department of Surgery, Velayat Hospital, Qazvin University of Medical Sciences, Qazvin, Iran, E-mail: Hoparsaar2004@yahoo.com.
References
- 1.WHO . In: WHO/OIE Manual on Echinococcosis in Humans and Animals, a Public Health Problem of Global Concern. Eckert J, Gemmel MA, Meslin FX, Pawlowski ZS, editors. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 2.Eslami A, Hosseini SH. Echinococcus granulosus infection of farm dogs of Iran. Parasitol Res. 1998;84:205–207. doi: 10.1007/s004360050383. [DOI] [PubMed] [Google Scholar]
- 3.Rokni MB. Echinococcosis/hydatidosis in Iran. Iran J Parasitol. 2009;4:1–16. [Google Scholar]
- 4.Umhang G, Richomme C, Boucher JM, Hormaz V, Boue F. Prevalence survey and first molecular characterization of Echinococcus granulosus in France. Parasitol Res. 2013;112:1809–1812. doi: 10.1007/s00436-012-3245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fasihi Harandi M, Budke CM, Rostami S. The monetary burden of cystic echinococcosis in Iran. PLoS Negl Trop Dis. 2012;6:e1915. doi: 10.1371/journal.pntd.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torgerson PR, Deplazes P. Echinococcosis: diagnosis and diagnostic interpretation in population studies. Trends Parasitol. 2009;25:164–170. doi: 10.1016/j.pt.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Carmena D, Benito A, Eraso E. Antigens for the immunodiagnosis of Echinococcus granulosus infection: an update. Acta Trop. 2006;98:74–86. doi: 10.1016/j.actatropica.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Kalantari E, Bandehpour M, Pazoki R, Taghipoor-Lailabadi N, Khazan H, Mosaffa N, Nazaripouya MR, Kazemi B. Application of recombinant Echinococcus granulosus antigen B to ELISA kits for diagnosing hydatidosis. Parasitol Res. 2010;106:847–851. doi: 10.1007/s00436-010-1726-0. [DOI] [PubMed] [Google Scholar]
- 9.Barnes TS, Li J, Coleman GT, McManus DP. Development and evaluation of immunoblot-based serodiagnostic tests for hydatid infection in macropodids. J Wildl Dis. 2008;44:1036–1040. doi: 10.7589/0090-3558-44.4.1036. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Gonzalez A, Muro A, Barrera I, Ramos G, Orduna A, Siles-Lucas M. Usefulness of four different Echinococcus granulosus recombinant antigens for serodiagnosis of unilocular hydatid disease (UHD) and postsurgical follow-up of patients treated for UHD. Clin Vaccine Immunol. 2008;15:147–153. doi: 10.1128/CVI.00363-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Zhang WB, Wilson M, Ito A, McManus DP. A novel recombinant antigen for immunodiagnosis of human cystic echinococcosis. J Infect Dis. 2003;188:1951–1960. doi: 10.1086/379976. [DOI] [PubMed] [Google Scholar]
- 12.Virginio VG, Hernandez A, Rott MB, Monteiro KM, Zandonai AF, Nieto A, Zaha A, Ferreira HB. A set of recombinant antigens from Echinococcus granulosus with potential for use in the immunodiagnosis of human cystic hydatid disease. Clin Exe Imonol. 2003;132:309–315. doi: 10.1046/j.1365-2249.2003.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etebar F, Jalousian F, Hosseini SH, Kordafshari S, Najafi A. Immunoproteomics approach for EPC1 antigenic epitope prediction of G1 and G6 strains of Echinococcus granulosus. Parasitol Res. 2013;112:3129–3135. doi: 10.1007/s00436-013-3489-x. [DOI] [PubMed] [Google Scholar]
- 14.Kordafshari S, Hosseini SH, Jalousian F, Rajabibazl M, Jones M, Etebar F. Evaluation of dot immunogold filtration assay (DIGFA) by recombinant protein EPC1 for anti-Echinococcus granulosus IgG antibody. Iran J Parasitol. 2015;10:30–38. [PMC free article] [PubMed] [Google Scholar]
- 15.Ben Nouir N, Gianinazzi C, Gorcii M, Muller N, Nouri A, Babba H, Gottstein B. Isolation and molecular characterization of recombinant Echinococcus granulousus P29 protein (recP29) and its assessment for the post-surgical serological follow-up of human cystic echinococcosis in young patients. Trans R Soc Trop Med Hyg. 2009;103:355–364. doi: 10.1016/j.trstmh.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Chen X, Lu X, Feng X, Wen H. The production and comparative evaluation of native and recombinant antigens for the fast serodiagnosis of cystic echinococcosis with dot immunogold filtration assay. Parasite Immunol. 2015;37:10–15. doi: 10.1111/pim.12151. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Zhang WB, Donald P, McManus DP. Recombinant antigens for immunodiagnosis of cystic echinococcosis. Biol Proc. 2004;6:67–77. doi: 10.1251/bpo74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, McManus DP. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Immunol Med Microbiol. 2006;47:24–41. doi: 10.1111/j.1574-695X.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 19.Abdi J, Kazemi B, Mohebali M, Bandehpour M, Rahimi MT, Rokni MB. Gene cloning, expression and serological evaluation of EgAgB12 kDa from Echinococcus granulosus. Ann Trop Med Parasitol. 2010a;104:399–407. doi: 10.1179/136485910X12743554760261. [DOI] [PubMed] [Google Scholar]
- 20.Ioppolo S, Notargiacomo S, Profumo E, Franchi C, Ortona E, Rigano R, Siracusano A. Immunological responses to antigen B from Echinococcus granulosus cyst fluid in hydatid patients. Parasite Immunol. 1996;18:571–578. doi: 10.1046/j.1365-3024.1996.d01-31.x. [DOI] [PubMed] [Google Scholar]
- 21.Grimm F, Maly FE, Lu J, Llano R. Analysis of specific immunoglobulin G subclass antibodies for serological diagnosis of echinococcosis by a standard enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1998;5:613–616. doi: 10.1128/cdli.5.5.613-616.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McVie A, Ersfeld K, Rogan MT, Craig PS. Expression and immunological characterisation of Echinococcis granulosus recombinant antigen B for IgG4 subclass detection in human cystic echinococcosis. Acta Trop. 1997;67:19–35. doi: 10.1016/s0001-706x(97)00056-9. [DOI] [PubMed] [Google Scholar]
- 23.Wen H, Craig PS. Immunoglobulin G subclass responses in human cystic and alveolar echinococcosis. Am J Trop Med Hyg. 1994;51:741–748. doi: 10.4269/ajtmh.1994.51.741. [DOI] [PubMed] [Google Scholar]
- 24.Ortona E, Margutti P, Delunardo F, Riganò R, Profumo E, Buttari B, Teggi A, Siracusano A. Recombinant antigens of Echinococcus granulosus recognized by IgE and IgG4 of sera from patients with cystic echinococcosis. Parasitologia. 2004;46:435–436. [PubMed] [Google Scholar]
- 25.Hernández-González A, Santivañez S, García HH, Rodríguez S, Muño S, Ramos G, Orduña A, Siles-Lucas M. Improved serodiagnosis of cystic echinococcosis using the new recombinant 2B2t antigen. PLoS Negl Trop Dis. 2012;6:e1714. doi: 10.1371/journal.pntd.0001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valiente-Gabioud AA, Veaute C, Perrig M, Galan-Romano FS, Sferco SJ, Marcipar IS. Effect of repetitiveness on the immunogenicity and antigenicity of Trypanosoma cruzi FRA protein. Exp Parasitol. 2011;127:672–679. doi: 10.1016/j.exppara.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Abdi J, Kazemi B, Haniloo A, Mohebali M, Mahmoudi M, Rezaei S, Bandehpour M, Maghen L, Rokni MB. Serological evaluation of EgAgB16 kDa, a recombinant antigen from Echinococcus granulosus for diagnosis of human hydatidosis. Iran J Parasitol. 2010;5:1–10. [PMC free article] [PubMed] [Google Scholar]