Abstract
French Polynesia is considered to be moderately endemic for chronic hepatitis B virus infection, with an estimated 3% of the population having hepatitis B surface antigen (HBsAg). From 1990 to 1992, a 3-dose hepatitis B vaccination series was introduced into the routine infant immunization schedule in French Polynesia, including a birth dose (BD). In 2014, a nationally representative 2-stage cluster survey was undertaken to evaluate the impact of the vaccination program on HBsAg prevalence among school children (∼6 years of age) in Cours Préparatoire (CP). Documented vaccination data were reviewed for all eligible children; children with consent were tested for HBsAg with a rapid point-of-care test. In total, 1,660 students were identified; 1,567 (94%) had vaccination data for review and 1,196 (72%) participated in the serosurvey. Three-dose vaccination coverage was 98%, while timely BD coverage, defined as a dose administered within 24 hours of life, was 89%. Receipt of the second and third doses was often delayed, with 75% and 55% receiving a second and third dose within 1 month of the recommended age, respectively. No children tested positive for HBsAg. French Polynesia's vaccination program has achieved high coverage and an HBsAg seroprevalence of 0% (0–0.5%) among CP school children, but timeliness of vaccination could be improved.
Introduction
Globally, an estimated 240 million people have chronic hepatitis B virus (HBV) infection and an estimated 600,000 die of the consequences of this infection yearly.1,2 The burden of disease is greatest among those living in the World Health Organization (WHO) Western Pacific Region (WPR), most of whom acquire the infection from perinatal or horizontal transmission occurring during the first 5 years of life.3,4 To help combat this public health problem, the member states of the WPR committed to controlling chronic HBV infection, defined as a hepatitis B surface antigen (HBsAg) seroprevalence < 1% among children aged ≥ 5 years by 2017.5
French Polynesia, a country in the WPR, has historically been considered endemic for hepatitis B; the WHO Regional Office of the Western Pacific has estimated that 3% of the population is HBsAg seropositive.6 However, the actual seroprevalence in French Polynesia is unknown, since a nationally representative serosurvey has never been conducted although two surveys have been done subnationally in Tahiti and the Austral Islands. In 1987, a small convenience sample survey of 50 persons on the island of Tahiti found an HBsAg prevalence of 2%, while a cluster survey of 957 individuals in the Austral Islands found a prevalence of 10.5%.7,8
To decrease the burden of chronic HBV infection, hepatitis B vaccine was introduced into the routine infant immunization schedule in French Polynesia in a phased manner starting in 1990, with nationwide implementation by 1992. Until 2014, the recommended schedule was to administer a birth dose (BD) to all infants within 24 hours, as well as doses at 1 and 6 months of age. In 2014, this schedule was changed to a BD within 24 hours of birth and subsequent doses at 2 and 10 months of age. In administrative records, 3-dose hepatitis B vaccination (HepB3) coverage has been ≥ 95% since 1999. In 2012, a census school-based survey found that HepB3 coverage was 98.3% among preschool children.9 In addition to provision of the standard BD within 24 hours, French Polynesia's perinatal hepatitis B prevention program includes screening pregnant women for HBsAg and providing hepatitis B immunoglobulin to neonates born to HBsAg-positive mothers.
To assess the progress of the national immunization program in reaching the 2017 WPR hepatitis B control goal, a serosurvey was undertaken to ascertain the HBsAg seroprevalence among children aged ∼6 years throughout French Polynesia years in Cours Préparatoire (CP), who were born after the national implementation of the hepatitis B vaccination program. A secondary objective was to assess hepatitis B vaccination coverage in this population.
Methods
From January to March 2014, we conducted a nationwide, cross-sectional, school-based, stratified 2-stage cluster survey among children in CP, equivalent to first grade in the American school system. School enrollment was estimated to be > 99%.
Setting.
French Polynesia has a population of approximately 270,000 spread over approximately 67 islands. There are five major groups of islands: Leeward, Windward, Austral, Marquesas, and Tuamotu-Gambier. Of the population, 75% lives on the Windward Islands and 13% lives on the Leeward Islands.
Sample size and sampling.
In 2012, there were 4,742 students in CP in French Polynesia. Assuming a seroprevalence of 1% with a precision of ±0.5%, a design effect of 1.5, a 2-sided 95% confidence interval (CI), α = 0.05, and accounting for the finite population correction factor, a minimum sample size of 1,542 children was needed. Given 15% nonresponse because of refusals/absence, 1,814 children were targeted for enrollment.
A stratified single-stage cluster survey approach was used. To help ensure selection of some outer islands, where population sizes are smaller, two strata were defined. The inner island strata included all public and private schools with CP students in the Leeward and the Windward Islands. The outer island strata included all public and private schools with CP students in the Marquesas, Tuamotu-Gambier, and Austral Islands. For the inner island strata, the school was the primary sampling unit (PSU). From this stratum, 25 schools were chosen by probability proportional to size (PPS). For the outer island strata, all schools were pooled together by island, thus making the island the PSU. In this outer island stratum, five PSUs were chosen by PPS. All CP students in selected schools were targeted for enrollment.
Data collection.
Consent was requested from all parents/caregivers before participation in the serosurvey. To describe possible biases that might have resulted from refusal of children to participate in the serosurvey, data were collected on key demographic characteristics and routine vaccination coverage among all children in the schools using a standard form. These data were also collected from children who did not undergo testing for HBsAg. All vaccination data for the survey were obtained from public health vaccination records; no vaccination data were collected by parental recall.
Specimen collection and HBsAg testing.
Approximately 50 μL of blood was collected from each consented child by finger prick and tested in the field using the Alere Determine™ (Chiba, Japan) HBsAg point-of-care test strip (reported sensitivity = 95–100%; reported specificity = 96–100%).10–12 The test reports either a positive or negative result. If no control line appears, the test is considered invalid.
Data management/analysis.
Data were collected using a standardized data collection form, entered into an Excel spreadsheet (Microsoft Corp., Seattle, WA), and analyzed using survey methodology in SAS v9.3 (SAS Institute Inc., Cary, NC). Participants were defined as those who had consent for HBsAg testing; nonparticipants were those without consent for HBsAg testing. The BD was considered timely if administered within 24 hours of birth; the second dose was considered timely if administered between 30 and 60 days of life, and the third dose was considered timely if administered between 180 and 210 days of life. Complete and timely vaccination was defined as receiving at least three timely doses of hepatitis B vaccine as defined above, with ≥ 4 weeks between each dose. Estimates and Wilson 95% CI for vaccination coverage and population characteristics were calculated using Taylor series variance estimation methods and accounting for the survey design and sampling weights. Weights used for population characteristics, vaccination coverage, and seroprevalence estimates were adjusted for nonresponse, and weighted proportions are presented. Rao–Scott χ2 P values were calculated for comparisons of categorical variables, such as coverage among participants and nonparticipants. For seroprevalence, we used a logit-based interval to calculate the 95% CI.13 The finite population was not taken into account in the analysis.
Human subjects' rights and ethics.
Informed consent was obtained from parents/caregivers before testing. The study protocol was approved by the Ethics Committee of the French Polynesian Ministry of Health and the Ethics Review Committee at the WHO Regional Office for the Western Pacific. Centers for Disease Control and Prevention (CDC) determined the activity to be human subjects' research, but CDC involvement did not constitute direct engagement in human subjects' research; therefore, it did not require CDC Institutional Review Board review.
Results
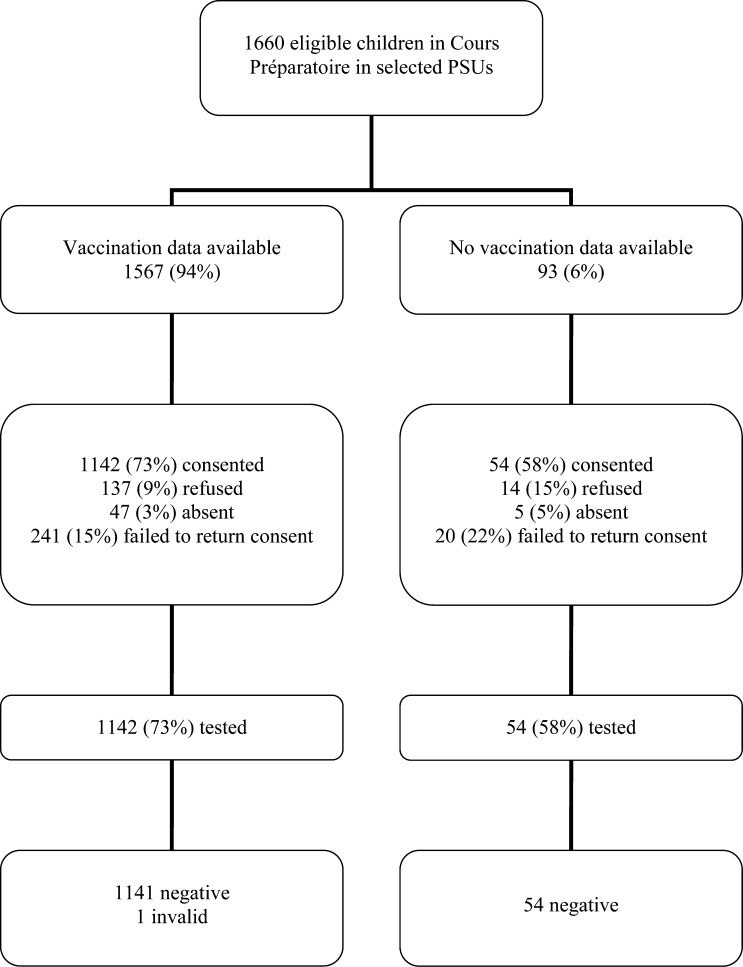
Among the 30 PSUs chosen, only 29 PSUs were visited because one PSU no longer had eligible children during the 2013–2014 school year. In total, 1,660 children were identified in the 29 PSUs. Of the 1,660 students, 1,567 (94%) had vaccination data available for review, and 1,196 (72%) had parental consent to participate and were tested for hepatitis B (Figure 1 ). Documented vaccination data or serological data on HBsAg were available of 1,621 (98%) students.
Figure 1.
Enrollment and results of the hepatitis B surface antigen serosurvey, French Polynesia, 2014.
Nonparticipants were from 28 of the 29 clusters, with a median nonparticipation percentage within clusters of 18% (range = 2–59%). Participants and nonparticipants of the serosurvey were compared (Table 1). Participants had a mean age and standard deviation of 6.5 ± 0.3 years compared with nonparticipants mean age of 6.5 ± 0.4 years. Nonparticipants were more likely to have been born outside French Polynesia (nonparticipants 10% versus participants 4%, P = 0.007) and were less likely to have vaccination data to review (nonparticipants 92% versus participants 96%, P = 0.05).
Table 1.
Characteristics of the enrolled Cours Préparatoire students by serosurvey participation status, French Polynesia, 2014
| Total |
Participants |
Nonparticipants |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | N | Weighted % | n | N | Weighted % | n | N | Weighted (%) | |
| Male* | 871 | 1,650 | 53 | 620 | 1,191 | 53 | 251 | 459 | 56 |
| Island group | |||||||||
| Inner island strata | |||||||||
| Leeward | 196 | 1,660 | 17 | 167 | 1,196 | 20 | 29 | 464 | 7 |
| Windward | 1,261 | 1,660 | 70 | 854 | 1,196 | 65 | 407 | 464 | 86 |
| Outer island strata | |||||||||
| Austral | 39 | 1,660 | 3 | 34 | 1,196 | 4 | 5 | 464 | 1 |
| Marquesas | 98 | 1,660 | 6 | 87 | 1,196 | 7 | 11 | 464 | 3 |
| Tuamotu-Gambier | 66 | 1,660 | 4 | 54 | 1,196 | 4 | 12 | 464 | 3 |
| Enrolled in private school | 476 | 1,660 | 19 | 284 | 1,196 | 16 | 192 | 464 | 30 |
| Born in French Polynesia* | 1,479 | 1,577 | 95 | 1,100 | 1,153 | 96 | 379 | 424 | 90 |
| Vaccination data reviewed | 1,567 | 1,660 | 95 | 1,142 | 1,196 | 96 | 425 | 464 | 92 |
Data missing from questionnaire.
Among eligible students with vaccination data, 98% (95% CI = 97–99%) received at least three doses of hepatitis B vaccine; coverage with ≥ 3 doses was higher among the participants (99%; 95% CI = 98–99%) compared with nonparticipants (96%; 95% CI = 93–97%) (χ2 P value = 0.0002) (Table 2). There were 14 children who received other childhood immunizations but failed to receive any dose of hepatitis B vaccine; eight of these children were born outside French Polynesia, although it is unknown when they immigrated to French Polynesia. Every PSU had ≥ 3 dose coverage of ≥ 92% among those children with available data.
Table 2.
Completeness and timeliness of hepatitis B vaccine receipt among eligible Cours Préparatoire students by serosurvey participation status, French Polynesia, 2013–2014
| Total |
Participants |
Nonparticipants |
χ2 P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | N | Weighted % | n | N | Weighted % | n | N | Weighted % | ||
| Number of doses of hepatitis B received | 0.13 | |||||||||
| 0 | 14 | 1,567 | 0.7 (0.4–1.3) | 6 | 1,142 | 0.3 (0.1–0.9) | 8 | 425 | 1.9 (0.9–3.7) | |
| 1 | 5 | 1,567 | 0.3 (0.1–0.9) | 2 | 1,142 | 0.1 (0.02–0.5) | 3 | 425 | 0.8 (0.2–3.8) | |
| 2 | 15 | 1,567 | 0.8 (0.5–1.4) | 7 | 1,142 | 0.6 (0.2–1.4) | 8 | 425 | 1.6 (0.7–3.2) | |
| 3 | 1,522 | 1,567 | 98 (96–98) | 1,119 | 1,142 | 98 (97–99) | 403 | 425 | 95 (93–97) | |
| 4 | 11 | 1,567 | 0.7 (0.4–1.2) | 8 | 1,142 | 0.7 (0.4–1.4) | 3 | 425 | 0.6 (0.2–1.8) | |
| Timing of BD | ||||||||||
| BD ≤ 24 hours* | 1,372 | 1,566 | 89 (86–92) | 1,025 | 1,141 | 91 (89–93) | 347 | 425 | 81 (74–87) | 0.002 |
| BD ≤7 days | 1,429 | 1,566 | 92 (89–94) | 1,062 | 1,141 | 94 (92–96) | 367 | 425 | 86 (80–91) | 0.002 |
| Timing and completeness of doses | ||||||||||
| BD ≤ 24 hours and ≥ 2 additional doses† | 1,366 | 1,566 | 89 (85–91) | 1,024 | 1,141 | 91 (88–93) | 342 | 425 | 81 (73–86) | 0.001 |
| Complete and timely three doses‡ | 658 | 1,566 | 42 (39–46) | 488 | 1,141 | 43 (39–48) | 170 | 425 | 39 (35–45) | 0.27 |
BD = birth dose. Bold values are considered statistically significant ≥ 0.05.
One child missing date of birth so unable to calculate timing of BD.
Two additional doses irrespective of timing of doses.
Complete and timely vaccination is defined as receiving at least three doses of hepatitis B, with the timing of the first dose ≤ 24 hours, the second dose between days 30–60 of life, and the third dose between days 180–210 of life, with ≥ 4 weeks between each dose.
For those with vaccination data, 1,372 (89%; 95% CI = 86–92%) received a timely BD; timely BD coverage was higher among participants (91%) than among nonparticipants (81%) (Table 2). An additional 57 (3%) children received the BD between 2 and 7 days of life; reasons for the delay in BD receipt are unknown. By PSU, timely BD coverage ranged from 68% to 100%; three PSUs had a timely BD coverage of < 80%.
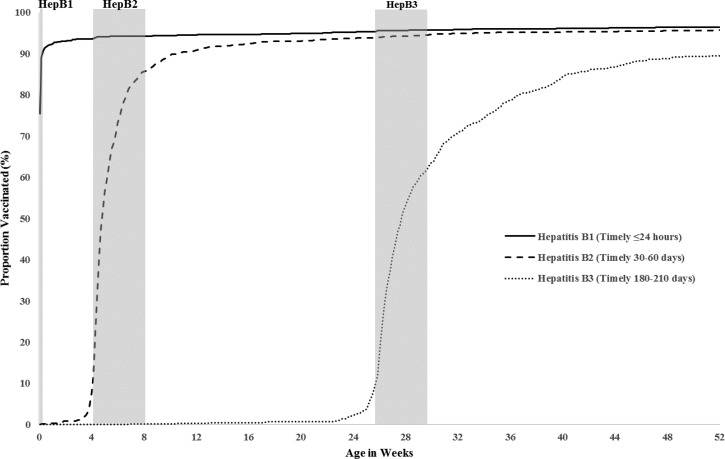
Of 1,547 CP students who received the second dose of hepatitis B vaccine, 1,149 (75%; 95% CI = 72–79%) received it in a timely manner. Hepatitis B vaccine was administered early (< 30 days of life) to 182 (12%) children; another 216 (13%) received it after 60 days of life (Figure 2 ). Of these 216 children with delayed vaccination, 131 (59%) missed the dose at 1 month of age and were not caught up at 2 months of age when they received the first dose of pentavalent vaccine (diphtheria, tetanus, pertussis–Haemophilus influenzae type b–inactivated polio vaccine); the median delay between receipt of the first dose of pentavalent vaccine and the second dose of hepatitis B vaccine was 19 weeks (interquartile range [IQR] = 5–99 weeks).
Figure 2.
Vaccination timeliness: cumulative proportion of Cours Préparatoire students vaccinated with each hepatitis B dose by age (in weeks) compared with the EPI Schedule, French Polynesia, 2013–2014. The vertical reference bars indicate age at vaccination recommended by the national program. The first dose was considered timely if it was given within 24 hours of birth; the second dose, if it was given between days 30 and 60 of life; and the third dose, if it was given between days 180 and 210 of life.
Of 1,534 CP students who received the third dose of hepatitis B vaccine, 863 (55%; 95% CI = 52–59%) received the dose in a timely manner. Hepatitis B vaccine was administered early (< 180 days of life) to 122 (9%) CP students, whereas 549 (36%) received the dose after 210 days of life (Figure 2). There was no significant difference in timeliness between participants and nonparticipants (data not shown). The median interval between doses 1 and 2 was 33 days (IQR = 30–41 days); the median interval between doses 2 and 3 was 158 days (IQR = 149–189 days).
Receipt of a timely BD plus two or more doses, irrespective of timing of these subsequent two doses, was seen in 1,366 (89%; 95% CI = 85–91%) of the students; again, coverage was higher among participants in the serosurvey. Only 658 (42%; 95% CI = 39–46%) students received the complete series in a timely manner. (Table 2).
Among the 1,196 children tested for HBsAg, 1,195 were HBsAg negative (Figure 1). One child had an invalid result and refused a retest. Therefore, without the invalid result, the prevalence was 0% (95% CI = 0–0.5%).
Discussion
In this evaluation, we found no French Polynesian CP students who were positive for HBsAg; this is in contrast to the 3% adult population estimate. This decline is the result of the strong hepatitis B vaccination program that French Polynesia has been advancing for the past two decades. Vaccination coverage with at least three doses of hepatitis B vaccine is 98%; this is consistent with a 2012 national vaccination survey that found 98.4% of preschool children completed the hepatitis B vaccine series.9 Furthermore, all selected PSUs had a 3-dose vaccination coverage of > 90%, in line with the “Reach Every Community” strategy outlined in the Global Vaccine Action Plan.14
There are several reasons that French Polynesia has been able to attain such remarkable progress in hepatitis B control. First, French Polynesia has a strong government commitment to routine immunization, with adequate financial and human resources. Second, French Polynesia has a relatively small birth cohort of approximately 4,500 infants born yearly. This small cohort is spread over 67 islands, but there is a system in place to ensure that every child has access to vaccination services. Almost all islands have an immunization focal point, and the Ministry of Health makes routine immunization services accessible mostly through clinics, but also through outreach services, such as in Tuamotu-Gambier, where some children reside on a few atolls. Almost 100% of children are born in health facilities, simplifying access to and provision of BD.15 Finally, public acceptance of all vaccines is high.
Achieving high 3-dose coverage is vital to achieving hepatitis B control; however, timeliness is also critical to help protect infants as early as possible. Timeliness of vaccine administration is a problem in many countries for different vaccines and can lead to immunity gaps among those at highest risk for the disease.16–23 In this survey, we found that timeliness of receipt of all three doses could be improved. Timely BD coverage was < 80% among CP students in three of 29 schools. Other countries have similar challenges, such as the United States, where only 72.4% of children receive the BD within 3 days of life in 2014.24 Timing of BD is critical to preventing perinatal transmission. Evaluating the problems and finding solutions to improve timeliness of BD should be a priority for the hepatitis B prevention program.25–27 Timeliness of administration of the second and third doses also needs improvement, since delays might leave children unprotected from horizontal transmission. For the French Polynesia program, one simple solution is to stress the importance of reviewing vaccination records at every visit and providing catch-up doses as needed. For example, in this cohort, 139 children could have received a timely second dose of hepatitis B vaccine if the clinician had provided the second dose at the time of the 2-month visit, at the same time as the first doses of pentavalent and pneumococcal vaccines. Children in French Polynesia rarely receive more than two vaccines at one visit; therefore, it is assumed that health-care providers chose to delay hepatitis B vaccination further rather than delay other vaccines.
In the analysis, we did not account for the finite population of French Polynesia because of the complexity of the analysis, given high vaccination coverage and no children with HBsAg positivity. The finite population analysis would have accounted for the high sampling rate, which would reduce variance. Since we did not account for this, we are presenting more conservative confidence limits for vaccination coverage. In addition, we presented a logit-based confidence limit for the seroprevalence, as methodology for doing this with zero cases in a finite population has not been well defined; this was the most conservative CI of various methodologies attempted.
This study was subject to a few limitations. First, we obtained a 76% participation rate in the seroprevalence portion of this study. Participants and nonparticipants were not similar on key characteristics, which could have introduced bias into our results. However, if we were to use 3-dose vaccination as a surrogate for immune protection (because the 3-dose series protects > 95%), we could account for the serologic status or vaccination status for 98% of the selected CP students.25 We did see significant differences in hepatitis B vaccination between participants and nonparticipants, which could translate to a higher burden in nonparticipants who were less likely to receive a timely BD. Vaccination data were not available for everyone, and some data quality issues existed in the vaccination data. We did not identify reasons for failure to receive the complete series or reasons why vaccines were delayed, though anecdotal evidence suggested that if a child was sick on the day of vaccination, the vaccination was delayed for about a month. This study did not account for children not enrolled in school, but school enrollment was > 99%, so this bias is minimal. Finally, we could not account for the contribution to the zero seroprevalence rate in the study population provided by the provision of hepatitis B immunoglobulin to newborns born to HBsAg-positive mothers, as maternal HBsAg status was not documented in the vaccination records.
French Polynesia has successfully met the WPR hepatitis B control goal of < 1% HBsAg seroprevalence among children at least 5 years of age. With a continued highly effective vaccination program, French Polynesia should be able to eliminate chronic HBV infections in future generations.
ACKNOWLEDGMENTS
We would like to thank Karen Hennessey, Jayaprakash Valiakolleri, and Eric Wiesen of WHO for their assistance in procuring supplies and providing funding. We want to extend our sincerest thanks to the Ministry of Education and school teachers of French Polynesia for their efficient help to the school heath service, as well as the pharmacy of the directory of health and all public health staff who coordinated the survey logistics in the field. Finally, we would like to thank the participants and their families, without whom this survey would not have been possible.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Financial support: Funding for this survey was provided by the WHO.
Authors' addresses: Minal K. Patel and Kathleen Wannemuehler, Global Immunization Division, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: hgo9@cdc.gov and kpw9@cdc.gov. Evelyne Le Calvez and Jean-Marc Ségalin, Bureau des Programmes de Pathologies Infectieuses, Direction de la Santé, Papeete, French Polynesia, E-mails: evelyne.lecalvez@sante.gov.pf and jean-marc.segalin@sante.gov.pf.
References
- 1.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–1339. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 3.Clements CJ, Baoping Y, Crouch A, Hipgrave D, Mansoor O, Nelson CB, Treleaven S, van Konkelenberg R, Wiersma S. Progress in the control of hepatitis B infection in the Western Pacific Region. Vaccine. 2006;24:1975–1982. doi: 10.1016/j.vaccine.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 4.Hennessey K, Mendoza-Aldana J, Bayutas B, Lorenzo-Mariano KM, Diorditsa S. Hepatitis B control in the World Health Organization's Western Pacific Region: targets, strategies, status. Vaccine. 2013;31(Suppl 9):J85–J92. doi: 10.1016/j.vaccine.2012.10.082. [DOI] [PubMed] [Google Scholar]
- 5.Regional Committee of the Western Pacific . Manila, Philippines: WHO Regional Office for the Western Pacific; 2013. Hepatitis B Control through Vaccination: Setting the Target. WPR/RC64.R5. [Google Scholar]
- 6.World Health Organization . Western Pacific Regional Plan for Hepatitis B Control through Immunization. Manila, Philippines: WHO Regional Office for the Western Pacific; 2007. [Google Scholar]
- 7.Boutin JP, Sainte Marie FF, Cartel JL, Cardines R, Girard M, Roux J. Prevalence of hepatitis B virus infection in the Austral archipelago, French Polynesia: identification of transmission patterns for the formulation of immunization strategies. Trans R Soc Trop Med Hyg. 1990;84:283–287. doi: 10.1016/0035-9203(90)90288-p. [DOI] [PubMed] [Google Scholar]
- 8.Brindle RJ, Eglin RP, Parsons AJ, Hill AV, Selkon JB. HTLV-1, HIV-1, hepatitis B and hepatitis delta in the Pacific and south-east Asia: a serological survey. Epidemiol Infect. 1988;100:153–156. doi: 10.1017/s095026880006564x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renou L, Ségalin J-M. Evaluation de la Couverture Vaccinale des élèves scolarisés en Polynésie française, année scolaire 2011–2012. Tahiti, Polynésie Française: Direction de la Santé; 2013. [Google Scholar]
- 10.Lien TX, Tien NT, Chanpong GF, Cuc CT, Yen VT, Soderquist R, Laras K, Corwin A. Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen, and syphilis in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg. 2000;62:301–309. doi: 10.4269/ajtmh.2000.62.301. [DOI] [PubMed] [Google Scholar]
- 11.Alere . Alere Determine HBsAg Package Insert. Chiba, Japan: Alere; 2011. http://www.alere.com/en/home/product-details/determine-hbsag.html Available at. [Google Scholar]
- 12.World Health Organization . Geneva, Switzerland: World Health Organization; 2001. Hepatitis B Surface Antigen Assays: Operational Characteristics (Phase 1). Report 1. [Google Scholar]
- 13.Rubin D, Schenker N. Logit-based interval estimation for binomial data using the Jeffrey's prior. Sociol Methodol. 1987;17:131–144. [Google Scholar]
- 14.World Health Organization . Global Vaccine Action Plan, 2011–2020. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 15.WHO Regional Office of the Western Pacific . Western Pacific Country Health Information Profiles: 2011 Revision. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 16.Wallace AS, Sobel H, Ryman TK, Mantaring JB, 3rd, Silvestre M, Thorley M, Ducusin J, Nyunt US. Timing of hepatitis B vaccination and impact of non-simultaneous vaccination with DTP vaccine following introduction of a hepatitis B birth dose in the Philippines. J Public Health Policy. 2012;33:368–381. doi: 10.1057/jphp.2012.18. [DOI] [PubMed] [Google Scholar]
- 17.Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373:1543–1549. doi: 10.1016/S0140-6736(09)60317-2. [DOI] [PubMed] [Google Scholar]
- 18.Hull BP, McIntyre PB. Timeliness of childhood immunisation in Australia. Vaccine. 2006;24:4403–4408. doi: 10.1016/j.vaccine.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Wang H, Zheng J, Zhu X, Xia W, Hipgrave DB. Coverage of and influences on timely administration of hepatitis B vaccine birth dose in remote rural areas of the People's Republic of China. Am J Trop Med Hyg. 2009;81:869–874. doi: 10.4269/ajtmh.2009.09-0238. [DOI] [PubMed] [Google Scholar]
- 20.Bielicki JA, Achermann R, Berger C. Timing of measles immunization and effective population vaccine coverage. Pediatrics. 2012;130:e600–e606. doi: 10.1542/peds.2012-0132. [DOI] [PubMed] [Google Scholar]
- 21.Luman ET, Barker LE, Shaw KM, McCauley MM, Buehler JW, Pickering LK. Timeliness of childhood vaccinations in the United States: days undervaccinated and number of vaccines delayed. JAMA. 2005;293:1204–1211. doi: 10.1001/jama.293.10.1204. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez SR, Parrott JS, Lauderdale DS, Daum RS. On-time immunization rates among children who enter Chicago public schools. Pediatrics. 2004;114:e741–e747. doi: 10.1542/peds.2004-1053. [DOI] [PubMed] [Google Scholar]
- 23.Heininger U, Zuberbuhler M. Immunization rates and timely administration in pre-school and school-aged children. Eur J Pediatr. 2006;165:124–129. doi: 10.1007/s00431-005-0014-y. [DOI] [PubMed] [Google Scholar]
- 24.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, state, and selected local area vaccination coverage among children aged 19–35 months—United States, 2014. MMWR. 2015;64:889–896. doi: 10.15585/mmwr.mm6433a1. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization Hepatitis B vaccines: WHO position paper—recommendations. Vaccine. 2010;28:589–590. doi: 10.1016/j.vaccine.2009.10.110. [DOI] [PubMed] [Google Scholar]
- 26.Ruff TA, Gertig DM, Otto BF, Gust ID, Sutanto A, Soewarso TI, Kandun N, Marschner IC, Maynard JE. Lombok Hepatitis B Model Immunization Project: toward universal infant hepatitis B immunization in Indonesia. J Infect Dis. 1995;171:290–296. doi: 10.1093/infdis/171.2.290. [DOI] [PubMed] [Google Scholar]
- 27.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ. Advisory Committee on Immunization Practices A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1–31. [PubMed] [Google Scholar]