To the Editor:
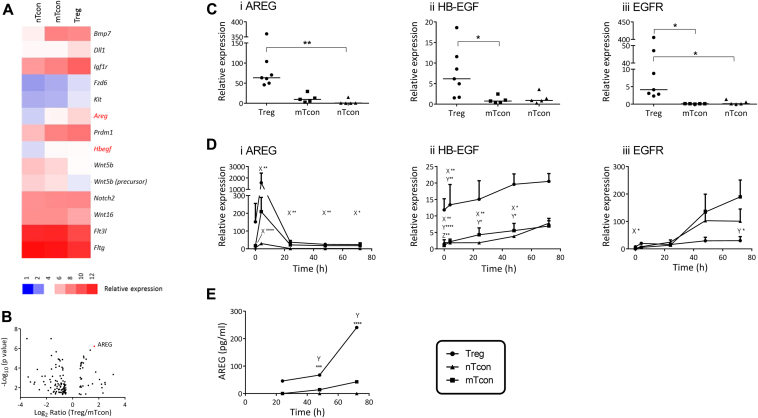
Amphiregulin is 1 of 7 structurally and functionally related growth factors that bind the epidermal growth factor receptor (EGFR); it is present in membrane-bound, intracellular and secreted forms. Interest in a role for amphiregulin in the immune system has been growing: various cells from both the innate and adaptive arms of the immune system express the ligand, including basophils,1 several subsets of human T cells, and murine TH2 cells.2 Populations of tissue-specific murine CD4+Foxp3+ regulatory T (Treg) cells in the colon and striated muscle are enriched in amphiregulin during inflammation; indeed, amphiregulin plays a role in repair functions of muscle Treg cells.3 Treg cells transferred to amphiregulin−/− RAG−/− mice show suboptimal ability to prevent colitis in an adoptive transfer model, a phenotype that is rescued by amphiregulin supplementation.4 To further understand the potential role of amphiregulin in Treg-cell function and its role in inflammation, the abundance and functional significance of amphiregulin expressed by Treg cells from the peripheral lymphoid tissues of mice was investigated (see the Methods section and Table E1 in this article's Online Repository at www.jacionline.org). Transcriptomic analysis revealed differential expression of amphiregulin between conventional T cells (Tcons) and Treg cells (Fig 1, A and B): amphiregulin mRNA was greater than 3-fold more abundant in Treg cells than in Tcons, confirmed by quantitative RT-PCR (Fig 1, A, B, and Ci). Heparin-binding epidermal growth factor-like growth factor (HB-EGF) also showed greater expression by Treg cells than by Tcons, as did EGFR itself (Fig 1, A-C); the remaining ligands fell below the limits of detection (data not shown).
Fig 1.
Treg cells show an amphiregulinhigh phenotype. Peripheral Treg cells and memory (m) and naive (n) Tcons selected from naive WT mice revealed greater amphiregulin and HB-EGF transcript abundance by microarray analysis (A: heat map; B: volcano plot) and quantitative RT-PCR assays (C; n ≥ 5), both ex vivo and following polyclonal stimulation (D, n = 3; E, n ≥ 2). AREG, Amphiregulin. Key: Statistical significance between X = Treg cells/nTcons, Y = Treg cells/mTcons, and Z = nTcons/mTcons (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Stimulation of the T-cell receptor (TCR) in human CD3+ T cells, CD4+ T cells,5 and murine TH2, but not TH1,6 cells, induces amphiregulin expression. In human CD4+ T cells, TCR engagement also augments HB-EGF expression.5 To determine whether a similar response occurs in murine Treg cells and Tcons (both naive and memory), we examined the kinetics of expression following TCR stimulation. A spike in amphiregulin expression was observed in all 3 populations at 3 hours (Fig 1, Di). Highest expression of amphiregulin mRNA at every time point was apparent in the Treg cells, followed by the mTcons and then nTcons. A significant difference between the regulatory and naive T cells was observed at every time point (Fig 1, Di).
There was no significant difference in HB-EGF expression in Treg cells over the time course, remaining higher than both Tcon populations at each point except 72 hours (Fig 1, Dii). In both subsets of Tcons, EGFR mRNA expression displayed a sustained increase from the first to the final time point (Fig 1, Diii). To determine whether the differential expression of the mRNA encoding amphiregulin and HB-EGF reflected differences in protein abundance, secreted amphiregulin and HB-EGF were quantified in culture supernatants. Amphiregulin secreted from nTcons remained below the assay detection limit of 7.8 pg/mL. The concentration of amphiregulin in the Treg-cell supernatants exceeded that of mTcons from 48 hours onward (Fig 1, E). Concentrations of HB-EGF were below the lower limit of detection in all supernatant samples (data not shown).
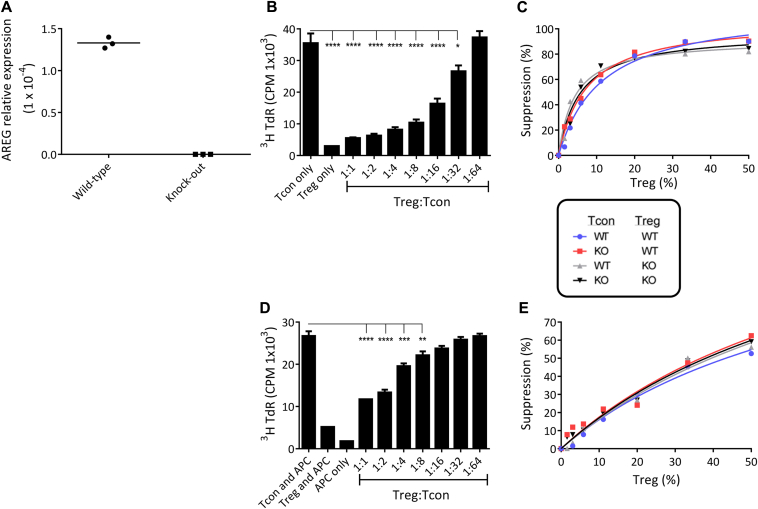
To establish whether the higher amphiregulin expression observed in Treg cells reflected a role in their suppressive function, bead-based assays using syngeneic and cross-over cocultures of Treg cells and Tcons from wild-type (WT) and amphiregulin knock-out (KO) mice were performed, following confirmation of their genotype (Fig 2, A). An inhibitory effect was apparent at all Treg-cell:Tcon ratios, with no difference between WT and KO cells; in particular, KO:KO cocultures, with neither an intrinsic nor an extrinsic source of amphiregulin in the cultures, showed potent suppression (Fig 2, B and C). Moreover, recombinant amphiregulin added to cultures of T cells in vitro impacted neither their proliferation nor their apoptosis, showing no evidence of an inhibitory role (data not shown). The suppression assays were repeated using WT accessory cells and soluble anti-CD3 mAb to activate cocultures of WT and KO Treg cells and Tcons, to establish whether Treg-cell suppression via antigen-presenting cells requires an intrinsic source of amphiregulin. Once again, there was no difference in suppression between any of the coculture permutations.
Fig 2.
Treg-cell–intrinsic amphiregulin is not required for suppressive function in vitro. A, Confirmation of genotype by quantitative RT-PCR. Cocultured Tcons and Treg cells were stimulated with (B and C) anti-CD3/CD28-coated Dynabeads or (D and E) soluble anti-CD3 with WT antigen-presenting cells (APCs). B and D, Counts per minute (CPM) in 1 representative experiment (*P = .011, **P = .0065, ***P = .0002, ****P < .0001); C and E, proportional suppression of CPM. A, n = 1; B and C, n = 3; D and E, n = 1. AREG, Amphiregulin.
A regulatory role for amphiregulin is not intuitive, as the ligand has a number of proinflammatory associations including its induction of the cytokines IL-1α and IL-1β, which positively feed back to promote amphiregulin secretion.7 However, other studies have found a role for the ligand in the function of various subsets of murine Treg cells.2 Augmentation of human Treg-cell–mediated suppression of CD4+ Tcons in vitro in response to recombinant amphiregulin was observed by Zaiss et al.4 In the same study, enhanced activity of murine Treg cells was also apparent in response to recombinant amphiregulin, but CD8+ T cells were used as the responder population in these experiments, with no information on the inhibitory effect on murine CD4+ Tcons. The ligand also enhances Treg-cell suppression of the antiviral effects of CD8+ T cells in vivo.8 Amphiregulin may therefore augment Treg-cells' suppressive activity in a CD8+ T-cell–specific manner, but whether amphiregulin itself represents a mechanism of suppression by Treg cells in certain cellular contexts remains unknown.
Our data demonstrate that despite being more abundantly expressed by Treg cells than by Tcons, Treg-cell–intrinsic amphiregulin is not required for suppressive function in vitro because functional redundancy was demonstrated in 2 well-established in vitro assays of suppression, suggesting that amphiregulin cannot be considered a core regulatory mechanism of these cells. Rather, we speculate that amphiregulin is involved in the tissue-reparative effects of Treg cells, in a manner that is independent of regulatory function. This viewpoint is supported by recently published work that complements our study2, 9 and extends our current insight into the complex, multifaceted roles of amphiregulin in health and disease.
Footnotes
This study was supported by the Mizutani Foundation for Glycoscience (P.S.R., O.A.G.), the Consortium for Functional Glycomics, National Institute of General Medical Sciences (T.G., G.H., L.S., S.R.H.), a Medical Research Council PhD Studentship (C.C., O.A.G.), a Biotechnology and Biological Sciences Research Council Collaborative Awards in Science and Engineering PhD Studentship (BB/H016376/1 to K.C., O.A.G.), and GlaxoSmithKine (S.W., J.M., K.A.).
Disclosure of potential conflict of interest: A. de Mestre, K. Carney, and O. A. Garden have received a grant from the Biotechnology and Biological Sciences Research Council (Collaborative Awards in Science and Engineering Award). J. Morley and K. Affleck are employed by and have received payment for the development of educational presentations from GlaxoSmithKline. The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
References
- 1.Qi Y., Operario D.J., Oberholzer C.M., Kobie J.J., Looney R.J., Georas S.N. Human basophils express amphiregulin in response to T cell-derived IL-3. J Allergy Clin Immunol. 2010;126:1260–1266.e4. doi: 10.1016/j.jaci.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaiss D.M., Gause W.C., Osborne L.C., Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–226. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burzyn D., Kuswanto W., Kolodin D., Shadrach J.L., Cerletti M., Jang Y. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaiss D.M., van Loosdregt J., Gorlani A., Bekker C.P., Grone A., Sibilia M. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi Y., Operario D.J., Georas S.N., Mosmann T.R. The acute environment, rather than T cell subset pre-commitment, regulates expression of the human T cell cytokine amphiregulin. PLoS One. 2012;7:e39072. doi: 10.1371/journal.pone.0039072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaiss D.M., Yang L., Shah P.R., Kobie J.J., Urban J.F., Mosmann T.R. Amphiregulin, a TH2 cytokine enhancing resistance to nematodes. Science. 2006;314:1746. doi: 10.1126/science.1133715. [DOI] [PubMed] [Google Scholar]
- 7.Streicher K.L., Willmarth N.E., Garcia J., Boerner J.L., Dewey T.G., Ethier S.P. Activation of a nuclear factor kappaB/interleukin-1 positive feedback loop by amphiregulin in human breast cancer cells. Mol Cancer Res. 2007;5:847–861. doi: 10.1158/1541-7786.MCR-06-0427. [DOI] [PubMed] [Google Scholar]
- 8.Dai K., Huang L., Chen J., Yang L., Gong Z. Amphiregulin promotes the immunosuppressive activity of intrahepatic CD4+ regulatory T cells to impair CD8+ T cell immunity against hepatitis B virus infection. Immunology. 2014;144:506–517. doi: 10.1111/imm.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arpaia N., Green J.A., Moltedo B., Arvey A., Hemmers S., Yuan S. A distinct function of regulatory T cells in tissue protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.