SYNOPSIS
Endogenous Cushing syndrome (CS) in pediatrics is rare; it may be caused by tumors that produce corticotropin (ACTH) in the pituitary gland (this form of CS is called Cushing disease) or elsewhere (ectopic CS), tumors that produce corticotropin-releasing hormone (CRH) anywhere (mostly neuroendocrine tissues), and finally adrenocortical masses that produce cortisol, such as adrenocortical cancer (ACC) or adenomas, and bilateral adrenocortical hypeprlasia (BAHs). ACC is a very rare cause of CS in children but should be excluded first, especially among younger patients. CS in children is often caused by germline or somatic mutations in an expanding list of genes with implications for the prognosis of the patients and for their families. CS should be early recognized in children; otherwise, it can lead to significant morbidity and mortality. All patients with suspected CS should be referred to specialized clinical centers for work-up; these centers should have access to experienced endocrine and neurological surgeons.
Keywords: Pituitary gland, adrenal gland, cyclic AMP (cAMP), protein kinase A, PRKAR1A gene, AIP gene, ARMC5 gene, USP8 gene, GNAS gene
Introduction; epidemiology of Cushing syndrome and clinical presentation
Endogenous Cushing syndrome (CS) is a rare entity, especially in children (1, 2). The overall incidence of Cushing syndrome is approximately 2 to 5 new cases per million people per year; of these cases, only approximately 10% each year occur in children (1). As in adult patients, in children and adolescents with CS there is an overall female-to-male predominance, which decreases with younger age; there might even be a male-to-female predominance in infants and young toddlers (3).
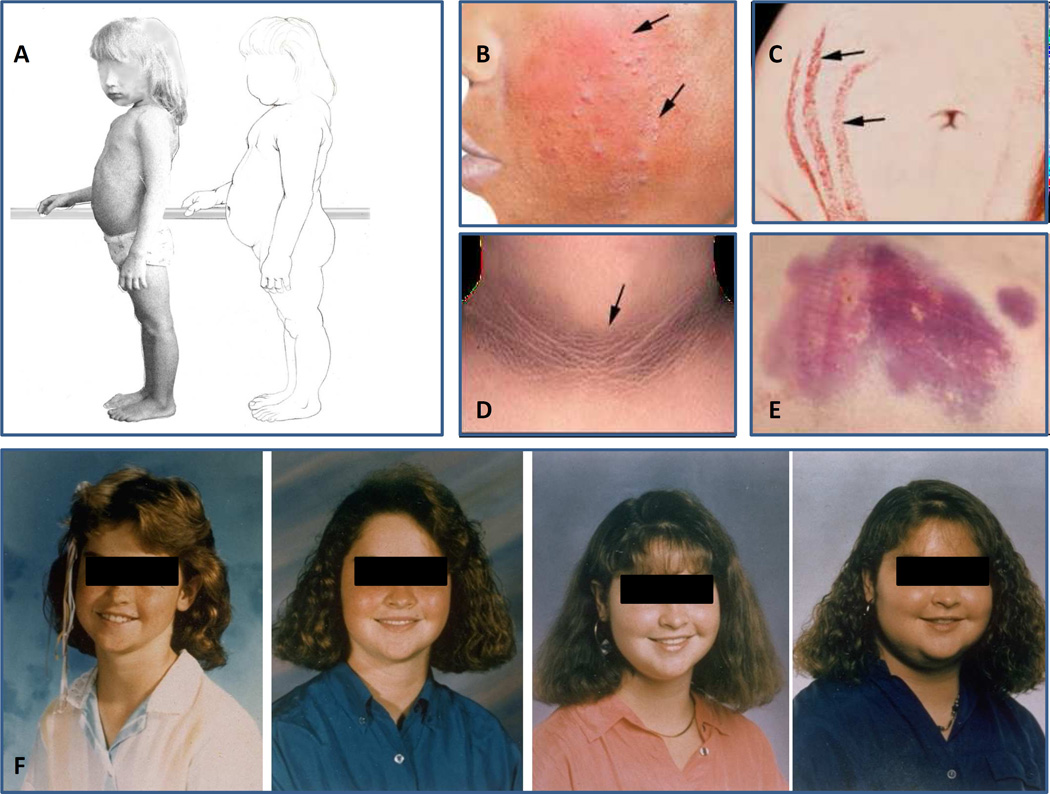
Like the exogenous forms, endogenous CS is due to chronic exposure to excess glucocorticoids. All patients with CS have a classic presentation (Figure 1): facial plethora (4), central body weight gain with limb thinning, glucose intolerance or diabetes (5) with extensive acanthosis nigricans, hypertension, osteoporosis and fractures (6), proximal muscle weakness, opportunistic infections (including fungal infections of the skin), easy bruising, and striae (7). However, by the time this presentation is fully developed, cure of the syndrome, especially of pituitary tumors, is less likely and comes with significant postoperative morbidity. Thus, the goal of the clinician should be to diagnose CS early (Figure 1F), well before the classic stigmata of CS, as shown in Figure 1 have developed. This is very important because even in individuals with hereditary types of CS, there is variable penetrance: while occasionally presenting with severe CS, cyclical or atypical CS may be insidious. It should be noted that morbidity and mortality are increased in CS, with a standard mortality ratio between 2 and 4 (2).
Figure 1.
A. Progression from a normal somatotype to that of Cushing syndrome in a young child: unlike in older children and adults thinning of the extremities is not as obvious; however, accumulation of abdominal fat and rounding of the face are obvious. B. Facial plethora with acne (arrows) in a patient with Cushing syndrome; C. Striae with bleeding (arrows); D. acanthosis nigricans in a patient with Cushing syndrome and severe insulin resistance and glucose intolerance; E. Skin bruising is frequent in older patients with Cushing syndrome but absent in toddlers and young children. F. The gradual facial changes of a pediatric patient with Cushing syndrome over 4 years.
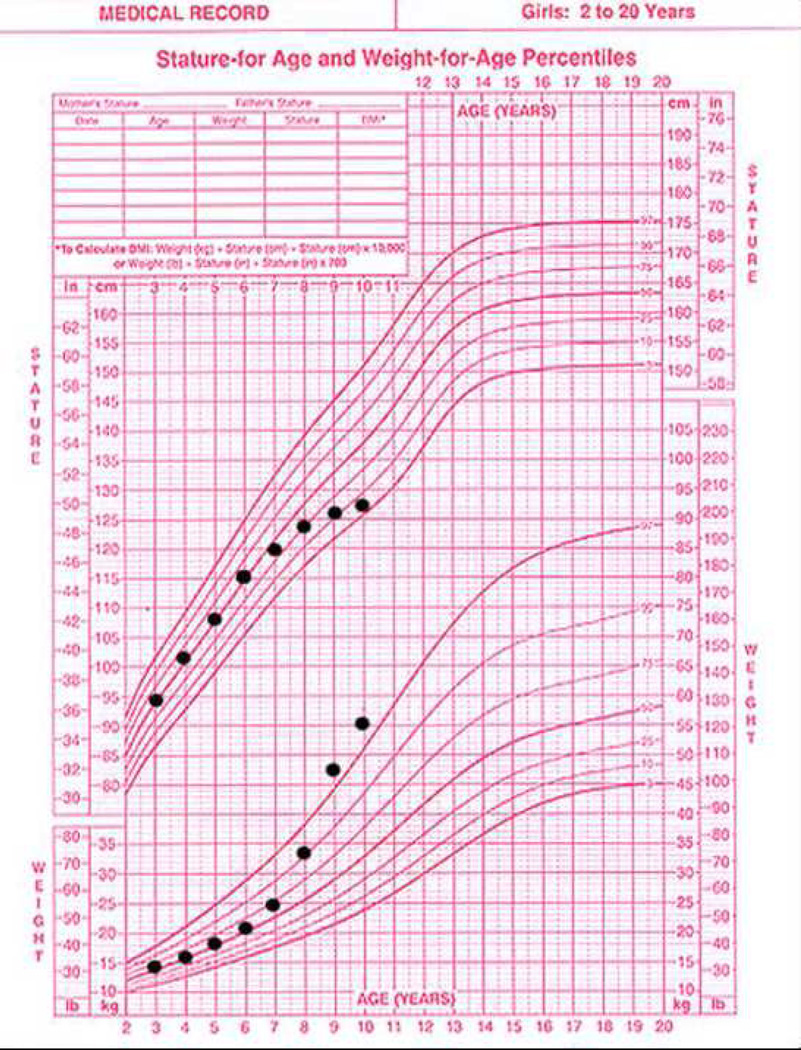
The clue to early diagnosis of CS in children is delayed growth that occurs simultaneously with increasing weight gain (8). This is obvious in the growth chart of the patients that are still growing (Figure 2) well before full clinical symptoms develop.
Figure 2.
A growth chart diagnostic of patient with Cushing syndrome: there is no other pediatric disease where progressive acceleration of significant weight gain is accompanied by a decline of growth velocity down to complete stagnation of growth (while weight gain continues unabated). The challenge is to diagnose the disease in the early phases of these growth chart changes.
Causes of endogenous Cushing syndrome in children and adolescents
The most common cause of endogenous CS in children is corticotropin (ACTH) overproduction from the pituitary; this is called Cushing disease (CD) (1). It is usually caused by an ACTH-secreting pituitary microadenoma and, rarely, a macroadenoma. ACTH secretion occurs in a semiautonomous manner, maintaining some of the feedback of the hypothalamicpituitary-adrenal (HPA) axis. CD accounts for approximately 75% of all cases of Cushing syndrome in children over 7 years (3). In children under 7 years, CD is less frequent; adrenal causes of CS (adenoma, carcinoma or bilateral hyperplasia, see below) are the most common causes of the condition in infants and young toddlers (9). Ectopic corticotropin-releasing hormone (CRH) or ACTH production by a variety of tumors is very rare in young children; generally these tumors account for less than 1% of the cases of CS in adolescents (1, 10). Sources of ectopic ACTH include small cell carcinoma of the lungs, carcinoid tumors in the bronchus, pancreas or thymus, medullary carcinomas of the thyroid, pheochromocytomas and other lesions, mostly of neuroendocrine origin (10). Ectopic CRH-producing tumors are very challenging diagnostically: they typically co-secrete ACTH in addition to leading to ACTH overproduction by the pituitary (10). Thus, diagnostic tests that are usually used for the exclusion of ectopic sources of CS have frequently misleading results in the case of CRH-induced ACTH oversecretion.
Autonomous secretion of cortisol from the adrenal glands, or ACTH-independent CS, accounts for approximately 10–15% of the cases of CS (9). However, although adrenocortical tumors are rare in older children, in younger children they are more frequent. Adrenocortical neoplasms account for 0.6% of all childhood tumors; CS is a manifestation of approximately one third of all adrenal tumors. In young children most adrenal tumors presenting with CS (70%) are malignant; the remaining are adrenocortical adenomas (AA). The majority of patients with adrenocortical cancer (ACC) present under age 5, contributing thus to the first peak of the known bimodal distribution of this malignancy across the life span (1, 2). As in adults, there is a female-to-male predominance of both ACC and AA. ACC usually occurs unilaterally; however, in 2–10% of patients they occur bilaterally. All cases of AAs are unilateral, unless a bilateral process is suspected (see below). AAs are the most common cause of ACTH-independent CS in adolescents.
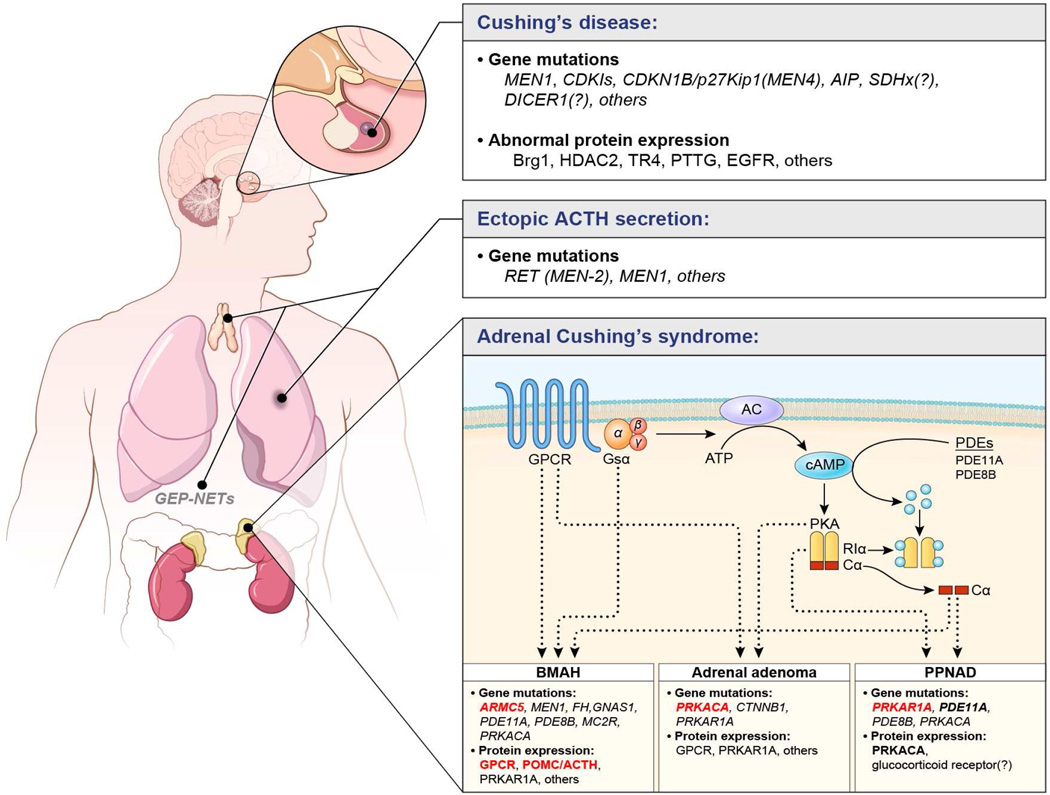
Bilateral adrenocortical hyperplasias (BAH) have been appreciated more recently as a cause of Cushing syndrome in both children and adults (9, 11). Their significance lies in the fact that these diseases are almost always caused by a genetic defect in several genes that have now been identified (Figure 3) (12). BAHs may be further classified by the size of the nodules on high resolution computed tomography (CT): diameter greater than 1 cm (macronodular) (13) or less than 1 cm (micronodular), and the presence or absence of pigmentation on pathological examination. Thus, the most common forms of BAH are: primary bilateral macronodular adrenal hyperplasia (PBMAH) also known as massive macronodular adrenal hyperplasia (MMAD) with nodules mostly (although not exclusively) larger than 1 cm (13), and primary pigmented nodular adrenocortical disease (PPNAD) and isolated micronodular adrenocortical disease (iMAD) (9, 11), with nodules smaller than 1 cm; the distinguishing feature between PPNAD and iMAD is the presence in the former and the absence in the latter of significant pigmentation upon histology (14). In both PPNAD and iMAD the adrenal glands contain multiple small bead-like nodules that are occasionally visible on CT (15).
Figure 3.
Summary of genetic and molecular mechanisms implicated in Cushing syndrome. Almost all have been identified in the last 15 years. The various genetic mutations or abnormal protein expression believed to play a role in the pathophysiology are indicated. Highlighted in red are frequent and confirmed genetic defects, whereas other characterized mechanisms are highlighted in bold. The remaining are less frequent genetic defects and some are shown with a question mark as they are not yet confirmed.
From Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015 Aug 29;386(9996):913-27, with permission.
PPNAD is a genetic disorder with the majority of cases associated with Carney complex (CNC), a syndrome of multiple endocrine gland abnormalities in addition to lentigines and myxomas, and predisposition to various cancers (16). The adrenal glands in PPNAD are most commonly normal or even small in size upon adrenal imaging; histologically, they are characterized by multiple pigmented nodules surrounded by an atrophic cortex (9, 11). A similar form of micronodular BAH, iMAD, is distinct genetically and histologically (14). Children and adolescents with PPNAD and iMAD frequently have periodic or cyclical or other forms of insidious CS (17).
PBMAH or MMAD is another rare disease, which leads to CS (12, 13). The adrenal glands are massively enlarged with nodules that are typical, yellow-to-brown cortisol-producing adenomas. Although most cases of PBMAH/MMAD are sporadic, the disease was recently found to be mostly familial (18); the disease starts in adolescence but may not become clinically apparent until late adulthood. In some patients with PBMAH/MMAD, cortisol levels appear to increase with food ingestion, a condition that is known as food-dependent CS (13, 19). These patients have an aberrant expression of the GIP receptor (GIPR) in the adrenal glands. In the majority of patients with PBMAH/MMAD, however, the disease does not appear to be GIPR-dependent; aberrant expression of other receptors might be responsible (19).
BAH associated with CS can also be seen in McCune Albright syndrome (MAS) and Beckwith-Widemann syndrome (BWS) (20, 21). CS in MAS is rare and usually presents in the infantile period (before 6 months of age); interestingly, a few children have had spontaneous resolution of their Cushing syndrome (20). There are only a few cases of BWS and CS that have been described, all in young toddlers (21). In both MAS and BWS, BAH is associated with the presence of cells with fetal-like properties in the adrenal cortex (20, 21).
Confirmation of endogenous Cushing syndrome and differential diagnosis
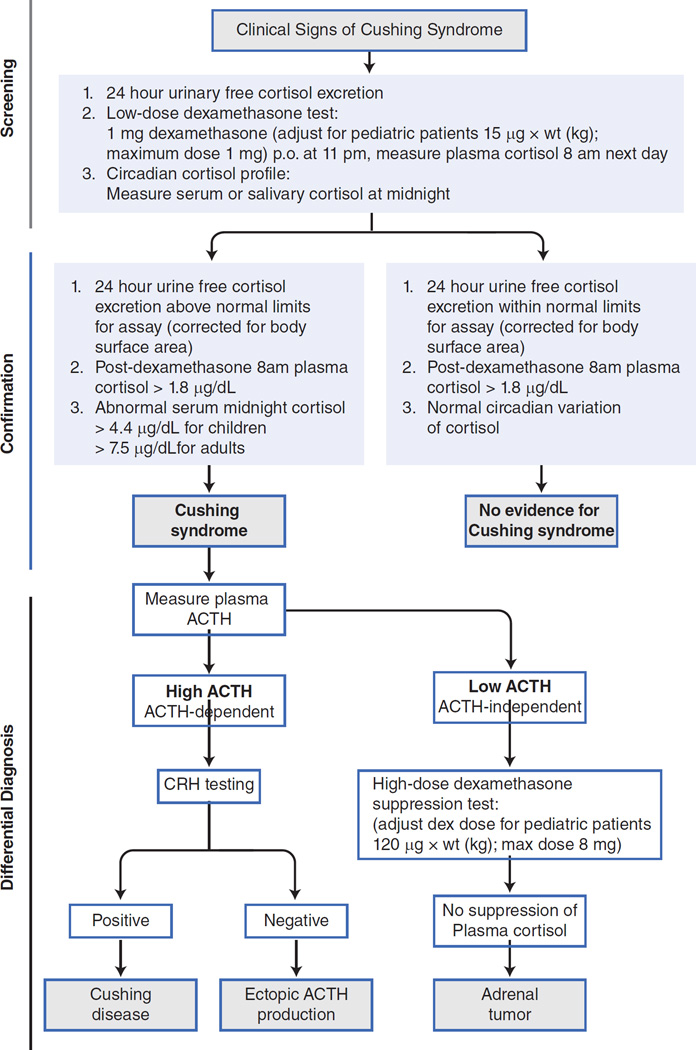
The appropriate therapeutic interventions in CS depend on accurate diagnosis and classification of the disease. The history and clinical evaluation, including growth charts, are important to make the initial diagnosis of Cushing syndrome. Upon suspicion of the syndrome, laboratory and imaging confirmations are necessary. These should be done in a certain order that is important to follow, in order to avoid over-testing, inappropriate imaging and confusing interpretations (Figure 4).
Figure 4.
The diagnostic algorithm that should be followed in the biochemical testing of a patient with suspected Cushing syndrome: for details, please refer to the text.
A. Documenting hypercortisolemia
The first step in the diagnosis of CS is to document hypercortisolism. This step is usually done in the outpatient setting (22). Because of the circadian nature of cortisol and ACTH, isolated cortisol and ACTH measurements are not of great value in diagnosis. One excellent screening test for hypercortisolism is a 24hour urinary free cortisol (UFC) excretion corrected for body surface area (1, 22). A 24-hour urine collection is often difficult for parents to do in children and may be done incorrectly, especially in the outpatient setting. Falsely high UFC may be obtained because of physical and emotional stress, chronic and severe obesity, pregnancy, chronic exercise, depression, alcoholism, anorexia, narcotic withdrawal, anxiety, malnutrition and excessive water intake (more than 5L/day). These conditions may lead to sufficiently high UFCs to cause what is known as pseudo-CS. On the other hand, falsely low UFC may be obtained mostly with inadequate collection.
Another baseline test for the establishment of the diagnosis of Cushing syndrome is a low dose dexamethasone suppression test. This test involves giving a 1mg of dexamethasone at 11pm (adjusted for weight for children<70 kg by dividing the dose by 70 and multiplying by the weight of the child) and measuring a serum cortisol level the following morning at 8AM. The problem with this test is that it has not been evaluated extensively in children; for adult patients, the cortisol cut-off level should be <1.8 ug/dl (50nmol/L). If it is greater than 1.8 ug/dl, further evaluation is necessary. This test has a low percentage of false normal suppression; however, at our institution, very rarely we obtain the 1 mg test for screening for CS in children. It should also be noted that the 1-mg overnight test (like the 24-hour UFCs), does not distinguish between hypercortisolism from CS and other hypercortisolemic states that include pseudo-CS.
If the response to both the 1mg dexamethasone overnight suppression test and the 24hour UFC are both normal, a diagnosis of CS may be excluded with the following caveat: 5–10% of patients may have intermittent or periodic cortisol hypersecretion and may not manifest abnormal results to either test. If periodic or intermittent CS is suspected, continuous follow up of the patients is recommended. Diurnal plasma cortisol variation, including midnight cortisol values, is a fairly good test for the establishment of the diagnosis of CS. In our institution, it has become the test of choice for the confirmation of endogenous hypercortisolemia and is routinely done in patients with confirmed elevated cortisol levels on the outside. There are several caveats for the interpretation of the test of which the most important ones are: 1) The venous catheter has to be placed at least two hours before the test; and 2) if the patient comes from another time zone, a 1-hour-per-day adjustment should be taken into account prior to obtaining the test. In general, serum cortisol levels are drawn at 11:30PM and 12:00MN and at 7:30AM and 8:00AM, while the patient is lying in bed and asleep; mid-night cortisol levels above 4.4 ug/dl in children (and 7.5 ug/dl in adults) are abnormal and confirm the diagnosis of CS, whereas an inverted diurnal rhythm is seen in PPNAD and occasionally in AAs and ACCs.
B. Peudo-Cushing syndrome
If one of the tests is suggesting CS or, if there is any question about the diagnosis, tests that distinguish between pseudo-CS states and CS may be obtained. One such test is the combined dexamethasone-CRH test (23). In this test the patient is treated with low dose dexamethasone (0.5mg adjusted for weight for children<70 kg by dividing the dose by 70 and multiplying by the weight of the child) every 6 hours for 8 doses prior to the administration of CRH (ovine CRH – oCRH) the following morning. ACTH and cortisol levels are measured at baseline and every 15 minutes for one hour after the administration of oCRH. The patient with a pseudo-CS state will exhibit low or undetectable basal plasma cortisol and ACTH, and have a diminished or no response to oCRH stimulation. Patients with CS will have higher basal cortisol and ACTH levels and will also have a greater peak value with oCRH stimulation. The criterion used for the diagnosis of CS is a cortisol level of greater than 38 nmol/L (approximately 1.4 ug/dl) 15 minutes after oCRH administration (11); all other patients (<1.4 ug/dl) may suffer from a pseudo-CS state. These criteria may be modified for children with extreme obesity (23).
C. Differential diagnosis between various types of Cushing syndrome
Once the diagnosis of CS is confirmed there are several tests to distinguish ACTH-dependent disease from the ACTH-independent syndrome. A spot plasma ACTH may be measured; if this measurement is <5pmol/l it is indicative of ACTH-independent CS, although the sensitivity and specificity of a single ACTH measurement are not high because of the great variability in plasma ACTH levels and the instability of the molecule after the sample’s collection. Even if one assumes that the sample was collected and processed properly (collected on ice and spun down immediately in a refrigerated centrifuge for plasma separation; the sample should then be immediately processed or frozen), ACTH levels that are between 5 and 20 pmol/l are not informative in this era of high sensitivity assays; levels above 30 pmol/l are more suggestive of an ACTH-dependent condition, but again that is not a certainty until single ACTH levels are repeatedly over 50pmol/l (22).
The standard six-day low- and high dose dexamethasone suppression test (Liddle’s test) is used to differentiate CD from ectopic ACTH secretion and adrenal causes of Cushing syndrome (1). In the classic form of this test, after 2 days of baseline urine collection, 0.5 mg of dexamethasone (adjusted per weight for children <70kg by dividing the dose by 70 and multiplying by the weight of the child) every 6-hours are given per os starting at 6.00 am on day #3 (“low dose” phase of the test) for a total of 8 doses (2 days); this is continued with a 2 mg dose of dexamethasone per os (adjusted per weight for children <70kg by dividing the dose by 70 and multiplying by the weight of the child) on day #5 (“high dose” phase of the test) given every 6 hours for another 8 doses (final 2 days). UFC (and/or urinary 17-hydroxysteroids) excretion are measured at baseline, during, and 1 day after the end of the dexamethasone administration. Approximately 90% of patients with CD will have suppression of cortisol and 17-hydroxysteroid values, whereas less than 10% of patients with ectopic ACTH secretion will have suppression. UFC values should suppress to 90% of baseline value and 17-hydroxysteroid excretion should suppress to less than 50% of baseline value. The criteria are similar if one uses serum cortisol values obtained at 8 am of the morning after the last dose of dexamethasone, e.g. serum cortisol on day #7 should be 90% of baseline serum cortisol values (obtained at 8 am the day before dexamethasone administration). An increase of urinary free cortisol values of 50% or more over baseline during the Liddle’s test has been used in the differential diagnosis of PPNAD and other micro-nodular adrenocortical disease versus other causes of adrenal causes of CS.
The Liddle’s test has been modified to:
giving 2 mg every 6-hours (without the preceding low-dose phase);
administering dexamethasone intravenously over 5 hours at a rate of 1mg/hour; or,
giving a single high dose of dexamethasone (8mg, in children adjusted for weight<70kg) at 11pm and measuring the plasma cortisol level the following morning (24). This overnight, high dose dexamethasone test has sensitivity and specificity values similar to those of the classic Liddle’s test. A 50% suppression of serum cortisol levels from baseline is what differentiates CD (more than 50% suppression) from other causes of CS (adrenal or ectopic ACTH production) (less than 50% suppression). The problem is that these tests have not been validated in the pediatric population, or in conditions such as PPNAD – which tend to be more frequent in pediatrics.
An oCRH stimulation test may be obtained for the differentiation of CD from ectopic ACTH secretion (22). In this test, 85% of patients with CD respond to oCRH with increased plasma ACTH and cortisol production. 95% of patients with ectopic ACTH production do not respond to administration of oCRH. The criterion for diagnosis of CD is a mean increase of 20% above baseline for cortisol values at 30 and 45 minutes and an increase in the mean ACTH concentrations of at least 35% over basal value at 15 and 30 minutes after oCRH administration. When the oCRH and high dose dexamethasone (Liddle or overnight) tests are used together, diagnostic accuracy improves to 98%.
D. The use of imaging in the work-up of Cushing syndrome
Another important tool in the localization and characterization of CS is diagnostic imaging. The most important initial imaging when CD is suspected is pituitary magnetic resonance imaging (MRI). The MRI should be done in thin sections with high resolution and always with contrast (gadolinium) (25). The latter is important since only macroadenomas will be detectable without contrast; after contrast, an otherwise normal-looking pituitary MRI might show a hypoenhancing lesion, usually a microadenoma. More than 90% of ACTH-producing tumors are hypoenhancing, whereas only about 5% are hyperenhancing after contrast infusion. However, even with the use of contrast material, pituitary MRI may detect only up to approximately 30% of ACTH-producing pituitary tumors, although with the use of new modalities (e.g. SPGR-MRI) this percentage may be as high as 60% (25). High resolution adrenal CT is more preferable than MRI of the adrenal glands and is useful in both the distinction between CD and adrenal causes of CS, as well as for the detection of unilateral adrenal tumors (15). The distinction is harder in the presence of micronodular BAH (PPNAD or iMAD) or bilateral adrenal carcinoma (conditions, however, that are rare). Most patients with CD have ACTH-driven bilateral hyperplasia, and both adrenal glands will appear enlarged and nodular on CT or MRI.
Most cancers are unilateral and quite large by the time they are detected. ACCs are also heterogeneous in appearance and often show extensive mass effects. Adenomas, on the other hand, are usually small, unilateral masses that are less than 5cm in diameter and homogenous in appearance.
PBMAH/MMAD presents with massive enlargement of both adrenal glands, whereas PPNAD or iMAD, as mentioned above, is more difficult to diagnose radiologically because it is usually associated with normal or small-sized adrenal glands, despite the histologic presence of hyperplasia.
Ultrasound may not be used to image the adrenal glands for the diagnostic work up of CS, because its sensitivity and accuracy is much less than CT or MRI. A CT or MRI scan of the neck, chest, abdomen and pelvis may be used for the detection of an ectopic source of ACTH production (10). Labeled octreotide, and more recently dotatate scanning and venous sampling may also help in the localization of an ectopic CRH or ACTH source (1, 10). Since up to 50% of pituitary ACTH-secreting tumors and many of ectopic ACTH tumors can not be detected on routine imaging, and often laboratory diagnosis is not completely clear, catheterization studies must be used to confirm the source of ACTH secretion in ACTH-dependent CS (1, 2). Bilateral inferior petrosal sinus sampling (BIPSS) may also be used for the localization of a pituitary microadenoma (although not with great accuracy or sensitivity) (22, 26). BIPSS is an excellent test for the differential diagnosis between ACTH-dependent forms of CS with a diagnostic accuracy that approximates 100%, as long as it is performed in an experienced clinical center (22). BIPSS, however, may not lead to the correct diagnosis, if it is obtained when the patient is not sufficiently hypercortisolemic or, if venous drainage of the pituitary gland does not follow the expected, normal anatomy, or if a CRH-producing ectopically located tumor has caused pituitary corticotroph hyperplasia. In brief, sampling from each inferior petrosal sinuses is taken for measurement of ACTH concentration simultaneously with peripheral venous sampling. ACTH is measured at baseline and at 3, 5, and 10 minutes after oCRH administration. Patients with ectopic ACTH secretion have no gradient between either one of the two sinuses and the peripheral sample. On the other hand, patients with an ACTH-secreting pituitary adenoma have at least a 2-to-1 at baseline, and 3-to-1 central-to-peripheral gradient after stimulation with oCRH (22, 26).
Clinical case presentation: case#1
A 10-year-girl presents with the growth chart shown in Figure 2. Her mother has noted decreased activity level and lack of interest in participation in fun activities along with increased appetite. Headaches started about a year ago. On physical examination, in addition to the growth parameters shown in the chart, she has a blood pressure of 135/80 mm Hg, heart rate of 110 beats/min, and respiratory rate of 20 breaths/min. She is overweight, and her face is plethoric, with acne on the forehead, saddle of the nose, and cheeks. She has lipomastia (Tanner stage 1), pubic hair (Tanner stage 3), and a normal clitoris. There is no vaginal discharge. The rest of her examination findings are normal. Her family physician had ordered a complete blood cell count, which was normal, and thyroid function tests, which revealed a TSH concentration of 0.45 mIU/L [(0.35 – 5.0 mIU/L)] and a free T4 concentration of 0.9 ng/dL [(0.8 – 1.7 ng/dL)].
Which one of the following is the best next step in this patient’s care?
Perform pituitary MRI immediately
No additional testing now; in 3 to 6 months, measure height and weight and assess thyroid function again
Immediately prescribe levothyroxine replacement therapy at a dosage of 2.5 mcg/kg per day to treat primary hypothyroidism; assess thyroid function again in 6 to 8 weeks to titrate therapy
Instruct the patient and her parents to collect a 24-hour urine specimen for measurement of urinary free cortisol
Order growth-hormone (GH) stimulation testing
Answer: D
The patient’s presentation and growth chart are typical of CS: the unabated weight increase with concurrent stagnation in height gain is the sine qua non of CS in prepubertal and peripubertal pediatric patients. In addition, these patients often present with acne, plethoric face, headaches, and hypertension like the patient in this vignette. Hirsutism is unusual in prepubertal children, but peripubertal children and adolescents often have increased hair growth on the face, trunk, and extremities. The inappropriately advanced pubic hair is another clue to the diagnosis and may even point to the cause of CS. This patient has not entered puberty as evidenced by her lack of breast development; yet, she has Tanner stage 3 pubic hair that developed because of excess adrenal androgens that are produced in the context of pituitary tumors producing ACTH or, more rarely, from an adrenocortical tumor producing both androgens and cortisol. Central, partially compensated hypothyroidism, like that of the patient in the vignette, is not unusual at presentation of patients with CS at any age. It is generally mild hypothyroidism and rarely requires treatment; euthyroidism is restored following cure of CS. Assuming that exogenous steroids have been excluded as the cause of the patient’s symptoms, the most prudent next step is to confirm endogenous hypercortisolemia, something that can be done easily with a relatively inexpensive and widely available outpatient test: the collection of 24-hour urine for the determination of endogenous urinary free cortisol. Generally, 2 to 3 consecutive 24-hour collections are needed, because over- or under-collection in the course of a single 24-hour collection often leads to false-positive or false-negative results, respectively. Once hypercortisolism is identified by urinary free cortisol, the next task is to confirm it by measuring midnight cortisol levels. In all causes of CS, the normal diurnal variation of ACTH and cortisol is lost, and cortisol levels in blood or saliva are high at midnight. To identify the cause of CS, ACTH must be measured next, followed by overnight dexamethasone testing. The most common cause of CS at this patient’s age would be a pituitary adenoma. However, it would be inappropriate to perform MRI before confirming the diagnosis biochemically and documenting ACTH levels that indicate an ACTH-dependent cause of CS. Pituitary MRI, an expensive test that often requires sedation in young children, is frequently normal in patients with ACTH-producing microadenomas. Alternatively, a false-positive MRI finding (indicating an “incidentaloma”) can misguide the investigation if imaging is obtained inappropriately early in the process of finding the cause of CS. It would also be inappropriate to wait, and reassess and retest thyroid function in 3–6 months or assess for short stature by ordering GH testing. The presentation of the patient is not compatible with mild central hypothyroidism or central precocious puberty, respectively.
Treatment for endogenous causes of Cushing syndrome
Pituitary adenomas causing CD are treated best by complete excision; successful transsphenoidal surgery (TSS) remains the cornerstone of therapy and the sole determinant of long-term cure (27). All patients with suspected CD should be referred to specialized clinical centers for their work-up; these centers should have access to experienced neurological surgeons. Recovery of the HPA axis typically occurs within 12 months post-operatively during which time replacement glucocorticoids are tapered down (28). Case#2 below presents an example of the complications that may occur in a patient with recurrent CD and discusses the available treatment options.
Treatment for benign primary adrenal causes of CS depends on the ability to lateralize a distinct adenoma versus having a bilateral familial hyperplasia. In the setting of unilateral cortisol-secreting adenomas, laparoscopic unilateral adrenalectomy is the procedure of choice. In individuals with PBMAH/MMAD, iMAD or PPNAD who have overt CS, bilateral adrenalectomy is warranted (17). If a unilateral adrenalectomy is performed in the case of bilateral disease, the physician must weigh the risks of having to perform a contralateral adrenalectomy later in the course of the disease and the potential for a unilateral procedure not yielding a cure, with the unavoidable risk of adrenal insufficiency should a bilateral adrenalectomy be performed (15, 17). With unilateral adrenalectomy, glucocorticoid replacement therapy is necessary until the hypothalamic –pituitary-adrenal axis recovers from suppression; after bilateral adrenalectomy, lifetime replacement with both glucocorticoid and mineralocorticoid is necessary. Genetic and molecular mechanisms responsible for excess cortisol secretion by primary adrenal lesions have been identified, as outlined in this review. As knowledge of the genetic etiologies of adrenal CS improves, targeted therapies may offer specific medical treatment in the future (29). In addition, knowledge of the genetic etiology of adrenal CS can help to guide the surgical approach – if only one adrenal gland is removed yet both adrenals are affected this may not lead to definitive cure, although debate on this issue remains among experts as a true randomized controlled trial has not been performed to answer this question (30).
Clinical case presentation: case#2
A 17-year-old female patient in whom CD was first diagnosed at age 12 years presents with obesity, hypertension, and extensive striae. She underwent TSS shortly after she first presented with CD at age 12.5 years. According to the available biopsy report, an ACTH-producing adenoma was found at surgery and she was cured. After taking glucocorticoid replacement for a year, she no longer took any medication. She completed puberty and reached a final height of 61 in (155 cm). At age 15 years, she presented to another center with recurrent CD. She underwent another TSS, which yet again confirmed an ACTH-producing adenoma on immunohistochemistry. The patient reports that because she was not cured, she received irradiation 1 year ago. The family moved to another country and medical follow-up has not been adequate. Currently, the patient is not on any medications, her last menstrual period was at least 6 months ago, and she lives with her parents. She is unable to go to school or engage in any activity. On physical examination, her weight is 297 lb (135 kg) and she has obvious signs of CS including a dorsocervical fat pad and striae on the abdomen and thighs. Her baseline laboratory test results, include a high serum ACTH and UFC excretion, low IGF1, and undetectable GH, LH and FSH, and a TSH of 0.3 mIU/L (reference range: 0.35–5.00 mIU/L) with a free T4 = 0.8 ng/dL (10.3 pmol/L) (reference range: 0.8–1.7 ng/dL [10.3–21.9 pmol/L]). MRI of the pituitary gland shows only postoperative changes and no obvious tumor.
Which one of the following describes the most appropriate course of action for this patient now?
Initiate levothyroxine therapy and refer the patient to a radiation oncologist for more irradiation; follow-up with the patient after she completes the new course of radiation treatment
Initiate levothyroxine therapy and instruct the patient to follow-up in 6 months; explain that irradiation takes time to be effective for recurrent CD and that you are hopeful symptoms will improve with levothyroxine; postpone any decisions about CD until you see the patient back in 6 months
Initiate levothyroxine replacement, discuss estrogen replacement and testing for GH deficiency, and refer the patient for endocrine surgery for bilateral adrenalectomy
Initiate levothyroxine therapy; discuss estrogen replacement, testing for GH deficiency, and various therapeutic options for CD; and recommend medical adrenalectomy
Conclude that the patient has CS due to an ectopic ACTH-producing tumor. Initiate levothyroxine therapy, discuss estrogen replacement and testing for GH deficiency, and initiate a workup to localize this neuroendocrine tumor
Answer: D
This patient has persistent CS due to recurrent CD caused by an ACTH-producing pituitary adenoma. It is important to note that the biopsy identified a tumor during the first TSS and that the patient was cured of CD after the first surgery. Thus, the diagnosis of CD in this case is certain. This is further documented by the completion of puberty, the reasonable final height, and the resumption of menses. After approximately 3 years, however, the patient presented with recurrent CD and she underwent another TSS, which again documented an ACTH-producing adenoma. She then underwent irradiation. Recurrence of CD after a first successful TSS happens in approximately 10% to 20% of pediatric patients with CD, probably due to progression of remnants of the adenoma. Postoperative testing after the TSS, such measurement of serum cortisol and ACTH levels and oCRH testing, may determine which patients have a higher chance of recurrence. Repeated TSS has a lower chance of success (cure rates are typically 50% to 60% compared with rates of 80% to 90% after the first operation). If recurrent CD persists, irradiation may be offered by conventional fractionated radiotherapy or by stereotactic radiosurgery (gamma-knife or proton beam therapy), with no obvious advantage of either technique in terms of speed of cure. In either case, radiation therapy takes time to control high ACTH levels and recurrent CD; most studies show a 50% cure rate approximately 2 years after irradiation, which increases to 70% or more further out from treatment. Unfortunately, in up to two-thirds of patients, other pituitary hormone deficiencies develop, sometimes as late as 5 to 10 years after irradiation. One could argue that radiotherapy in this case was not a good choice shortly after the second TSS, given the patient’s age and the potential effect on reproductive options.
Dopaminergic and somatostinergic drugs can be used to decrease ACTH production from a recurrent adenoma. However, these drugs are not as effective in CD as they are in prolactinomas or GH-producing adenomas. Cabergoline (a dopamine agonist) and pasireotide (a somatostatin agonist) are two agents that are now available for patients such as the one in this vignette. Pasireotide in particular could have been used to delay irradiation in this young patient. Cabergoline may have been temporarily used after the second TSS, but it would have been unlikely to provide long-lasting remission. These medications may not be good options now because the patient has undergone irradiation already, and it is most likely too early to see the effects of irradiation.
Thus, the patient does not necessarily have newly recurrent CD, but rather failure of the second TSS to control CS combined with the fact that she is only 1 year out from irradiation. The best options, then and now, are to administer medications that block cortisol production by the adrenal gland (medical adrenalectomy conferred by adrenolytic agents) or block cortisol function by interfering with the action of the glucocorticoid receptor. The patient could have been treated with such a medication after the second TSS to delay irradiation to a later time when absolutely needed. The patient should now be treated with one or a combination of these medications until the effects of irradiation are seen. Oral adrenolytic agents include ketoconazole, metyrapone, and mitotane. Etomidate is another such drug that is given intravenously. Mitotane appears to be the most effective, although it is not widely used in United States because of serious adverse effects. Concentrations of the drug at 8 mg/L or higher are associated with resolution of hypercortisolemia in more than 71% of patients with recurrent CD. Mifepristone (RU486), an effective oral glucocorticoid receptor antagonist, is now approved for use in adult patients with recurrent CD. To increase effectiveness and decrease adverse effects, these medications are often used in combination in patients such as the one in this vignette. Thus, the correct course of action is to initiate levothyroxine therapy; discuss estrogen replacement, testing for GH deficiency, and various therapeutic options for Cushing disease; and recommend medical adrenalectomy. Surgical adrenalectomy is an option for this patient, but not before one ensures that all other efforts, medical therapies and irradiation, have failed. In such patients, Nelson syndrome—growth of an aggressive pituitary ACTH-producing adenoma—may develop after surgical adrenalectomy. In addition, up to 10% of patients with recurrent CD experience regrowth of the adrenal gland (from adrenocortical remnants driven by high ACTH levels) after surgical adrenalectomy, which may lead to recurrent CS.
The patient has recurrent CD and the disease should be treated. Although the symptoms of CD are exacerbated by the concurrent central hypothyroidism, treatment of the latter will not resolve the patient’s symptoms. Repeating the irradiation is not an option. Concluding that the patient has CS due to an ectopic ACTH-producing tumor is wrong because it ignores findings from two biopsies showing an ACTH-producing adenoma and the remission of CD after the first TSS. Although extraordinarily rare in the pediatric and young adult population, ectopic causes of ACTH-dependent CS should always be investigated in cases of recurrent disease. The data in this vignette are overwhelming in favor of CD.
Genetic causes of Cushing syndrome in pediatrics
Progress that has led to elucidation of the critical role played by the cyclic AMP-protein kinase A (cAMP/PKA) pathway in CS (Figure 3) has been made by initially studying rare familial disorders in which adrenal tumors could be observed. As mentioned above, MAS was found to be due to postzygotic activating mutations in the alpha subunit of the gene for the stimulatory guanine-nucleotide– binding protein (Gsα) which leads to activation of the cAMP/PKA pathway by leading to increased cAMP levels. Today somatic mutations of GNAS1 have been described in a small number of cortisol-producing AAs, and in PBMAH/MMAD, in addition to MAS (20, 29).
Inactivating germline mutations of the PRKAR1A gene coding for the regulatory 1A subunit of PKA were then found to be responsible for PPNAD (and CNC) (31). Inactivating PRKAR1A mutations lead to constitutive activation of the cAMP/PKA pathway by increasing the availability of the PKA catalytic subunit. PPNAD patients have a paradoxical response to dexamethasone with their cortisol levels increasing in response to glucocorticoids (32, 33). This phenomenon can be replicated in vitro and it thought to be due to increased glucocorticoid (GC) receptor expression in the adrenal tumors themselves whereby cortisol stimulates its own release by the adrenals (32). As mentioned above, this clinical finding is useful in the diagnostic evaluation of patients with CS to help elucidate if the disease is bilateral, especially caused by PPNAD, and to help guide treatment approaches.
Genetic defects in cAMP-binding phosphodiesterases (PDEs) (34) have been described in isolated PPNAD as well as in other forms of micronodular BAH, such as iMAD. cAMP-binding PDEs decrease cAMP levels after stimulation of the cAMP/PKA pathway; inactivating PDE mutations lead to accumulation of cAMP and dysregulated activation of the PKA pathway. Mutations in two different PDEs, PDE11A and PDE8B, are associated with iMAD (35, 36).
Recent discoveries have linked the cAMP/PKA pathway to an additional molecular explanation for isolated cortisol-secreting adenomas (AAs) (34). The catalytic subunit of PKA, PRKACA was found to be recurrently mutated: one hotspot mutation was the most frequent c.617A>G/p.Leu206Arg, a single amino acid change that leads to constitutive activation of PKA. Today, PRKACA-activating mutations have been identified in 42% of all AAs studied and additional mutations beyond the original one (p.Leu206Arg) -which to date remains the most frequent one- have been identified (2, 38). Patients harboring a PRKACA mutation were typically younger at the time of the diagnosis of CS and presented with smaller tumors. Activating PRKACA mutations cluster at the interface of the regulatory and catalytic subunits, interfere with the formation of a stable PKA holoenzyme, and, thus, result in constitutive activation of PKA and increased cortisol production and altered proliferation in these tumors. Interestingly, from a therapeutic standpoint, the constitutive activation of PKA can be suppressed in vitro by PRKACA inhibitors, thus leading to a potential future targeted treatment for these patients.
We have recently reported five patients with BAH and CS caused by genetic rearrangements in in the chromosome 19p13 locus, resulting in copy number gains encompassing the entire PRKACA gene (39, 40). Interestingly, in least one patient, an increased number of PRKACA gene copies was associated with a more severe phenotype. The PRKACA copy number variation may be inherited in an autosomal dominant manner or occur de novo (39). PRKACA amplification can lead to both micro- and macro-nodular BAH associated with CS that may have a milder or more severe clinical phenotype, depending on the gene dosage (40). However, the exact mechanism of how gain of gene copies leads to increased PKA activity has yet to be elucidated.
PBMAH/MMAD may rarely be found as a feature of multiple tumor syndromes including familial adenomatous polyposis (caused by APC gene mutations), multiple endocrine neoplasia type 1 (caused by menin gene mutations), or hereditary leiomyomatosis and renal cell carcinoma (caused by fumarate hydratase FH gene mutations) (13, 19).
Less than two years ago, integration of two different genomic approaches identified a new gene responsible for adrenocortical tumors – providing new evidence that PBMAH/MMAD is frequently a genetic disorder (41, 42). Genome wide screening of chromosomal alterations by single nucleotide polymorphism array was performed in patients with PBMAH/MMAD who had undergone adrenalectomy for CS, comparing tumor tissue to germline DNA. One gene, ARMC5, was found recurrently mutated on chromosome 16p11.2 (41). Inactivating ARMC5 mutations were detected in the tumors; in all cases, both alleles of ARMC5 carried mutations: one germline and the other somatic. This work identified that PBMAH/MMAD is frequently a genetic disorder, most often due to ARMC5-inactivating mutations, a putative tumor-suppressor gene. Subsequent studies have confirmed the high frequency of ARMC5 mutations in this disorder (43, 44).
Summary
Endogenous CS is rare; it may be caused by tumors that produce corticotropin (ACTH) in the pituitary gland (CD) or elsewhere (ectopic CS), tumors that produce CRH anywhere (mostly neuroendocrine tissues), and finally adrenocortical masses that produce cortisol, such as ACC or AAs, and BAHs. ACC is a very rare cause of CS in children but should be excluded first, especially among younger patients. CS in children is often caused by germline or somatic mutations in an expanding list of genes with implications for the prognosis of the patients and for their families. CS should be early recognized in children; otherwise, it can lead to significant morbidity and mortality. All patients with suspected CS should be referred to specialized clinical centers for their work-up; these centers should have access to experienced endocrine and neurological surgeons. Finally, the new genetics of CS may assist clinicians to provide appropriate counseling and in their decision-making regarding medical and/or surgical intervention. These findings may also lead to the development of individualized pharmacological treatment(s) for CS and other disease associated with PKA defects.
KEY POINTS.
Cushing syndrome in pediatrics is rare, but unless recognized early, it can lead to significant morbidity and even mortality.
Pituitary adenomas (Cushing disease) are treated best by complete excision; successful transsphenoidal surgery (TSS) remains the cornerstone of therapy and the sole determinant of long-term cure.
Bilateral adrenocortical hypeprlasias are relatively new and expanding causes of corticotropin-independent Cushing syndrome: they can be difficult to diagnose due to their rarity and often insidious or cyclical clinical presentation.
Adrenocortical cancer is a very rare cause of Cushing syndrome in children but should be excluded in any patient with corticotropin-independent Cushing syndrome, especially among younger patients with the condition.
Both pituitary adenomas and adrenocortical tumors, including bilateral adrenocortical hypeprlasias are often caused by germline or somatic mutations in an ever expanding list of genes with implications for the family of the patients and the prognosis of the patients.
Acknowledgments
The author wishes to acknowledge Dr. Keil and Dr. Lodish who have coauthored manuscripts on Cushing syndrome with Dr. Stratakis in the past; the present text followed the general outline of these manuscripts, in particular with regards to the description of the preferred way to test patients with Cushing syndrome for confirmation of their disease and differential diagnosis. Drs. Lodish, Keil and Stratakis run the NICHD, NIH clinical research protocols on Cushing syndrome to date.
DISCLOSURE STATEMENT
Funding for this work was provided exclusively by the Intramural Research Program, NICHD, NIH, Bethesda, MD20892, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Stratakis CA. Cushing syndrome in pediatrics. Endocrinol Metab Clin North Am. 2012;41(4):793–803. doi: 10.1016/j.ecl.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lodish M, Stratakis CA. Protein kinase A signaling and Cushing Syndrome. Nat Rev Endocrinol. 2016 doi: 10.1038/nrendo.2016.24. [DOI] [PubMed] [Google Scholar]
- 3.Libuit LG, Karageorgiadis AS, Sinaii N, Nguyen May NM, Keil MF, Lodish MB, Stratakis CA. A gender-dependent analysis of Cushing's disease in childhood: pre- and postoperative follow-up. Clin Endocrinol (Oxf) 2015 Jul;83(1):72–77. doi: 10.1111/cen.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshari A, Ardeshirpour Y, Lodish MB, Gourgari E, Sinaii N, Keil M, Belyavskaya E, Lyssikatos C, Chowdhry FA, Chernomordik V, Anderson AA, Mazzuchi TA, Gandjbakhche A, Stratakis CA. Facial Plethora: Modern Technology for Quantifying an Ancient Clinical Sign and Its Use in Cushing Syndrome. J Clin Endocrinol Metab. 2015 Oct;100(10):3928–3933. doi: 10.1210/jc.2015-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keil MF, Graf J, Gokarn N, Stratakis CA. Anthropometric measures and fasting insulin levels in children before and after cure of Cushing syndrome. Clin Nutr. 2012;31(3):359–363. doi: 10.1016/j.clnu.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodish MB, Hsiao HP, Serbis A, Sinaii N, Rothenbuhler A, Keil MF, Boikos SA, Reynolds JC, Stratakis CA. Effects of Cushing disease on bone mineral density in a pediatric population. J Pediatr. 2010 Jun;156(6):1001–1005. doi: 10.1016/j.jpeds.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stratakis CA, Mastorakos G, Mitsiades NS, Mitsiades CS, Chrousos GP. Skin manifestations of Cushing disease in children and adolescents before and after the resolution of hypercortisolemia. Pediatr Dermatol. 1998;15(4):253–258. doi: 10.1046/j.1525-1470.1998.1998015253.x. [DOI] [PubMed] [Google Scholar]
- 8.Lodish MB, Gourgari E, Sinaii N, Hill S, Libuit L, Mastroyannis S, Keil M, Batista DL, Stratakis CA. Skeletal maturation in children with Cushing syndrome is not consistently delayed: the role of corticotropin, obesity, and steroid hormones, and the effect of surgical cure. J Pediatr. 2014 Apr;164(4):801–806. doi: 10.1016/j.jpeds.2013.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stratakis CA. Cushing syndrome caused by adrenocortical tumors and hyperplasias (corticotropin-independent Cushing syndrome) Endocr Dev. 2008;13:117–132. doi: 10.1159/000134829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karageorgiadis AS, Papadakis GZ, Biro J, Keil MF, Lyssikatos C, Quezado MM, Merino M, Schrump DS, Kebebew E, Patronas NJ, Hunter MK, Alwazeer MR, Karaviti LP, Balazs AE, Lodish MB, Stratakis CA. Ectopic adrenocorticotropic hormone and corticotropin-releasing hormone co-secreting tumors in children and adolescents causing cushing syndrome: a diagnostic dilemma and how to solve it. J Clin Endocrinol Metab. 2015 Jan;100(1):141–148. doi: 10.1210/jc.2014-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stratakis CA, Boikos SA. Genetics of adrenal tumors associated with Cushing's syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab. 2007;3(11):748–757. doi: 10.1038/ncpendmet0648. [DOI] [PubMed] [Google Scholar]
- 12.Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015 Aug 29;386(9996):913–927. doi: 10.1016/S0140-6736(14)61375-1. [DOI] [PubMed] [Google Scholar]
- 13.De Venanzi A, Alencar GA, Bourdeau I, Fragoso MC, Lacroix A. Primary bilateral macronodular adrenal hyperplasia. Curr Opin Endocrinol Diabetes Obes. 2014 Jun;21(3):177–184. doi: 10.1097/MED.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 14.Gunther DF, Bourdeau I, Matyakhina L, Cassarino D, Kleiner DE, Griffin K, Courkoutsakis N, Abu-Asab M, Tsokos M, Keil M, Aidan Carney J, Stratakis CA. Cyclical Cushing syndrome presenting in infancy: An early form of primary pigmented nodular adrenocortical disease, or a new entity? J Clin Endocrinol Metab. 2004;89:3173–3182. doi: 10.1210/jc.2003-032247. [DOI] [PubMed] [Google Scholar]
- 15.Powell AC, Stratakis CA, Patronas NJ, Steinberg SM, Batista D, Alexander HR, Pingpank JF, Keil M, Bartlett DL, Libutti SK. Operative management of Cushing syndrome secondary to micronodular adrenal hyperplasia. Surgery. 2008 Jun;143(6):750–758. doi: 10.1016/j.surg.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correa R, Salpea P, Stratakis CA. Carney complex: an update. Eur J Endocrinol. 2015 Oct;173(4):M85–M97. doi: 10.1530/EJE-15-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarlis NJ, Chrousos GP, Doppman JL, Carney JA, Stratakis CA. Clinical case seminar: Primary Pigmented Nodular Adrenocortical Disease (PPNAD): re-evaluation of a patient with Carney complex 27 years after unilateral adrenalectomy. J Clin Endocrinol Metab. 1997;82(4):1274–1278. doi: 10.1210/jcem.82.4.3857. [DOI] [PubMed] [Google Scholar]
- 18.Assié G, Libé R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, Barreau O, Lefévre L, Sibony M, Guignat L, Rodriguez S, Perlemoine K, René-Corail F, Letourneur F, Trabulsi B, Poussier A, Chabbert-Buffet N, Borson-Chazot F, Groussin L, Bertagna X, Stratakis CA, Ragazzon B, Bertherat J. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing's syndrome. N Engl J Med. 2013;369(22):2105–2114. doi: 10.1056/NEJMoa1304603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, Robinson-White AJ, Nesterova M, Lacroix A, Stratakis CA. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab. 2009;94(8):2930–2937. doi: 10.1210/jc.2009-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carney JA, Young WF, Stratakis CA. Primary bimorphic adrenocortical disease: cause of hypercortisolism in McCune-Albright syndrome. Am J Surg Pathol. 2011;35(9):1311–1326. doi: 10.1097/PAS.0b013e31821ec4ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carney JA, Ho J, Kitsuda K, Young WF, Jr, Stratakis CA. Massive neonatal adrenal enlargement due to cytomegaly, persistence of the transient cortex, and hyperplasia of the permanent cortex: findings in Cushing syndrome associated with hemihypertrophy. Am J Surg Pathol. 2012;36(10):1452–1463. doi: 10.1097/PAS.0b013e31825d538b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children referred for the investigation of Cushing syndrome. Pediatrics. 2007;120(3):e575–e586. doi: 10.1542/peds.2006-2402. [DOI] [PubMed] [Google Scholar]
- 23.Batista DL, Courcoutsakis N, Riar J, Keil MF, Stratakis CA, Stratakis CA. Severe obesity confounds the interpretation of low dose dexamethasone test combined with the administration of ovine corticotrophin releasing hormone in childhood Cushing syndrome. J Clin Endocrinol Metab. 2008;93(11):4323–4330. doi: 10.1210/jc.2008-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dichek HL1, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler GB., Jr A comparison of the standard high dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1994 Feb;78(2):418–422. doi: 10.1210/jcem.78.2.8106630. [DOI] [PubMed] [Google Scholar]
- 25.Batista D, Courkoutsakis NA, Oldfield EH, Griffin KJ, Keil M, Patronas NJ, Stratakis CA. Detection of adrenocorticotropin-secreting pituitary adenomas by magnetic resonance imaging in children and adolescents with Cushing disease. J Clin Endocrinol Metab. 2005;90(9):5134–5140. doi: 10.1210/jc.2004-1778. [DOI] [PubMed] [Google Scholar]
- 26.Batista D, Gennari M, Riar J, Chang R, Keil MF, Oldfield EH, Stratakis CA. An assessment of petrosal sinus sampling for localization of pituitary microadenomas in children with Cushing disease. J Clin Endocrinol Metab. 2006;91:221–224. doi: 10.1210/jc.2005-1096. [DOI] [PubMed] [Google Scholar]
- 27.Batista DL, Oldfield EH, Keil MF, Stratakis CA. Postoperative testing to predict recurrent Cushing disease in children. J Clin Endocrinol Metab. 2009;94:2757–2765. doi: 10.1210/jc.2009-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodish M, Dunn SV, Sinaii N, Keil MF, Stratakis CA. Recovery of the hypothalamic-pituitary-adrenal axis in children and adolescents after surgical cure of Cushing's disease. J Clin Endocrinol Metab. 2012;97(5):1483–1491. doi: 10.1210/jc.2011-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stratakis CA. cAMP/PKA signaling defects in tumors: genetics and tissue-specific pluripotential cell-derived lesions in human and mouse. Mol Cell Endocrinol. 2013;371(1–2):208–220. doi: 10.1016/j.mce.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zilbermint M, Stratakis CA. Protein kinase A defects and cortisol-producing adrenal tumors. Curr Opin Endocrinol Diabetes Obes. 2015 Jun;22(3):157–162. doi: 10.1097/MED.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the carney complex. Nat Genet. 2000;26(1):89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 32.Bourdeau I, Lacroix A, Schurch W, Caron P, Antakly T, Stratakis CA. Primary pigmented nodular adrenocortical disease (PPNAD): Paradoxical responses of cortisol secretion to dexamethasone occur in vitro and are associated with increased expression of the glucocorticoid receptor. J Clin Endocrinol Metab. 2003;63:5308–5319. doi: 10.1210/jc.2002-022001. [DOI] [PubMed] [Google Scholar]
- 33.Stratakis CA, Sarlis NJ, Kirschner LS, Carney JA, Doppman JL, Chrousos GP, Papanicolaou DA. Paradoxical response to dexamethasone assists with the diagnosis of primary pigmented nodular adrenocortical disease (PPNAD) Ann Intern Med. 1999;131(8):585–591. doi: 10.7326/0003-4819-131-8-199910190-00006. [DOI] [PubMed] [Google Scholar]
- 34.Azevedo MF, Faucz FR, Bimpaki E, Horvath A, Levy I, de Alexandre RB, Ahmad F, Manganiello V, Stratakis CA. Clinical and molecular genetics of the phosphodiesterases (PDEs) Endocr Rev. 2014;35(2):195–233. doi: 10.1210/er.2013-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leal LF, Szarek E, Faucz F, Stratakis CA. Phosphodiesterase 8B and cyclic AMP signaling in the adrenal cortex. Endocrine. 2015 Sep;50(1):27–31. doi: 10.1007/s12020-015-0621-y. [DOI] [PubMed] [Google Scholar]
- 36.Szarek E, Stratakis CA. Phosphodiesterases and adrenal Cushing in mice and humans. Horm Metab Res. 2014 Nov;46(12):863–868. doi: 10.1055/s-0034-1389916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beuschlein F, Fassnacht M, Assié G, Calebiro D, Stratakis CA, Osswald A, Ronchi CL, Wieland T, Sbiera S, Faucz F, Schaak K, Schmittfull A, Schwarzmayr T, Barreau O, Vezzosi D, Rizk-Rabbin M, Zabel U, Szarek E, Salpea P, Forlino A, Vetro A, Zuffardi O, Kisker C, Diener S, Meitinger T, Lohse MJ, Reincke M, Bertherat J, Strom TM, Allolio B. Constitutive activation of PRKACA in adrenal Cushing's syndrome. N Engl J Med. 2014;370(11):1019–1028. doi: 10.1056/NEJMoa1310359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stratakis CA. E pluribus unum? The main protein kinase A catalytic subunit (PRKACA), a likely oncogene, and cortisol-producing tumors. J Clin Endocrinol Metab. 2014 Oct;99(10):3629–3633. doi: 10.1210/jc.2014-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lodish MB, Yuan B, Levy I, Braunstein GD, Lyssikatos C, Salpea P, Szarek E, Karageorgiadis AS, Belyavskaya E, Raygada M, Faucz FR, Izatt L, Brain C, Gardner J, Quezado M, Carney JA, Lupski JR, Stratakis CA. Germline PRKACA amplification causes variable phenotypes that may depend on the extent of the genomic defect: molecular mechanisms and clinical presentations. Eur J Endocrinol. 2015 Jun;172(6):803–811. doi: 10.1530/EJE-14-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carney JA, Lyssikatos C, Lodish MB, Stratakis CA. Germline PRKACA amplification leads to Cushing syndrome caused by 3 adrenocortical pathologic phenotypes. Hum Pathol. 2015 Jan;46(1):40–49. doi: 10.1016/j.humpath.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Espiard S, Drougat L, Libé R, Assié G, Perlemoine K, Guignat L, Barrande G, Brucker-Davis F, Doullay F, Lopez S, Sonnet E, Torremocha F, Pinsard D, Chabbert-Buffet N, Raffin-Sanson ML, Groussin L, Borson-Chazot F, Coste J, Bertagna X, Stratakis CA, Beuschlein F, Ragazzon B, Bertherat J. ARMC5 mutations in a large cohort of primary macronodular adrenal hyperplasia: clinical and functional consequences. J Clin Endocrinol Metab. 2015 Jun;100(6):E926–E935. doi: 10.1210/jc.2014-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berthon A, Stratakis CA. From β-catenin to ARM-repeat proteins in adrenocortical disorders. Horm Metab Res. 2014 Nov;46(12):889–896. doi: 10.1055/s-0034-1389993. [DOI] [PubMed] [Google Scholar]
- 43.Faucz FR, Zilbermint M, Lodish MB, Szarek E, Trivellin G, Sinaii N, Berthon A, Libé R, Assié G, Espiard S, Drougat L, Ragazzon B, Bertherat J, Stratakis CA. Macronodular adrenal hyperplasia due to mutations in an armadillo repeat containing 5 (ARMC5) gene: a clinical and genetic investigation. J Clin Endocrinol Metab. 2014 Jun;99(6):E1113–E1119. doi: 10.1210/jc.2013-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Correa R, Zilbermint M, Berthon A, Espiard S, Batsis M, Papadakis GZ, Xekouki P, Lodish MB, Bertherat J, Faucz FR, Stratakis CA. The ARMC5 gene shows extensive genetic variance in primary macronodular adrenocortical hyperplasia. Eur J Endocrinol. 2015 Oct;173(4):435–440. doi: 10.1530/EJE-15-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]