Inflammatory cytokines in autoimmunity
Keywords: autoimmune diseases, IL-6, IL-17, IL-23, Th17, TNFα
Abstract
Inflammatory cytokines are key regulators of immune responses. Persistent and excessive production of inflammatory cytokines underscores the development of autoimmune diseases. Therefore, neutralizing inflammatory cytokines or antagonizing their receptor function is considered as a useful therapeutic strategy to treat autoimmune diseases. To achieve the success of such a strategy, understanding of the complex actions of these cytokines and cytokine networks is required. In this review we focus on four inflammatory cytokines—tumor necrosis factor α (TNFα), interleukin-6 (IL-6), IL-23 and IL-17—and dissect how the dysregulation of these cytokines regulates autoimmune diseases. On the basis of pre-clinical and clinical data, we specifically discuss the therapeutic rationale for targeting these cytokines and describe the potential adverse effects.
Introduction
Since interferon (IFN) was discovered in 1957 (1, 2), more than 90 inflammatory cytokines and their corresponding receptors have been identified (3, 4). These cytokines are produced by various cell types, and regulate immune responses, wound healing, angiogenesis, hematopoiesis and tissue remodeling. An appropriate inflammatory response is vital to host defense, whereas excessive or persistent production of inflammatory cytokines results in immunopathology such as inflammatory or autoimmune diseases.
Although inflammatory cytokines exert variable effects at different stages of different autoimmune diseases, not all of them are effective or promising targets for the treatment of these diseases. Here we outline some of the most effective treatments for autoimmunity including those targeting tumor necrosis factor α (TNFα), interleukin-6 (IL-6), IL-23 and IL-17, and discuss the underlying biological mechanisms. We also discuss the future challenges in the development of cytokine-targeted drugs on the basis of pre-clinical and clinical data.
Cytokines in autoimmune diseases
Upon infection or injury, keratinocytes, macrophages, dendritic cells (DCs) and other cells are activated to produce inflammatory cytokines (5–9). These inflammatory cytokines in turn act back on macrophages and DCs to induce more inflammatory cytokines, chemokines and other antimicrobial mediators (10–12). Subsequently, chemokines recruit myeloid DCs, neutrophils and T cells to the site of infection or injury to further amplify inflammatory responses to protect hosts from microbial infections or to repair tissue damage (13, 14). However, under certain circumstances, possibly due to the presence of autoreactive T cells in the case of autoimmunity, there is uncontrolled production of inflammatory cytokines. Work for the past 10 years has revealed a vital role of T helper 17 (Th17) cells in autoimmune diseases.
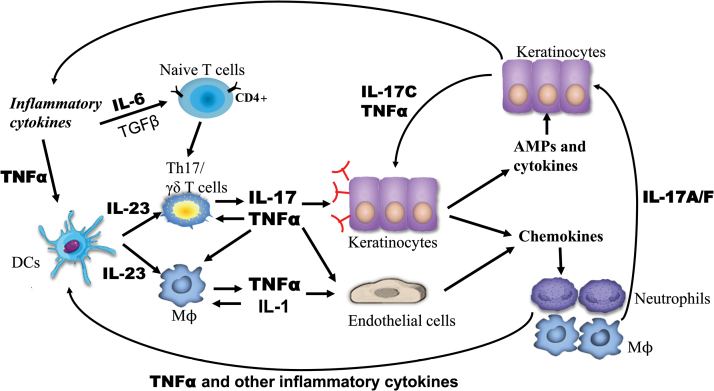
For example, in psoriasis, IL-6, together with TGFβ, induces naive CD4+ T cells to differentiate into IL-17-producing T cells (15–17), and the persistent production of TNFα and other inflammatory cytokines activates macrophages and keratinocytes to constantly produce chemokines such as CCL20 and CCL27 to recruit immune cells including myeloid DCs, neutrophils and Th17 cells to lesional skin of patients with psoriasis (18–22). Among these immune cells, DCs are further activated by TNFα to produce IL-23 (23, 24). IL-23, on one hand, directly activates a subset of IL-23-receptor-expressing macrophages and DCs, resulting in the production of inflammatory cytokines such as TNFα and IL-1 (25–27). On the other hand, IL-23 not only promotes Th17 cells to become highly pathogenic but also activates γδ T cells; both cell types produce IL-17A, IL-17F, IL-6 and TNFα (26, 28–30). IL-17 activates epithelial cells (e.g. keratinocytes), endothelial cells and fibroblasts to produce a variety of inflammatory cytokines (31–33), chemokines (34) and antimicrobial peptides/proteins (AMPs) (35–37).
In addition, IL-17 synergizes with other inflammatory cytokines including TNFα and IL-1β to further induce the expression of inflammatory cytokines and chemokines (38). The inflammatory cytokines induced by IL-17 promote the expansion of IL-17-producing γδ T or Th17 cells whereas the chemokines recruit more neutrophils or IL-17-producing T cells to sites of inflammation in the skin (30, 39, 40); these processes result in a feed-forward mechanism to further amplify local inflammatory responses to incite cytokine storms, thus leading to the inflammatory manifestation of psoriatic skin (Fig. 1).
Fig. 1.
The inflammatory circuit of TNFα, IL-6, IL-23 and IL-17 in the pathogenesis of psoriasis. The persistent production of TNFα and other inflammatory cytokines activates DCs to produce IL-23, and IL-6, together with TGFβ, induces naive CD4+ T cells to differentiate into IL-17-producing T cells. IL-23 directly activates macrophages (Mϕ) to produce inflammatory cytokines such as TNFα and IL-1; IL-23 also promotes Th17 cell differentiation into highly pathogenic Th17 cells and activates γδ T cells, both cell types constantly produce IL-17A, IL-17F, IL-6 and TNFα. IL-17 activates epithelial cells (e.g. keratinocytes) and endothelial cells to produce a variety of inflammatory cytokines, chemokines and AMPs. The inflammatory cytokines induced by IL-17 promote the expansion of IL-17-producing γδ T or Th17 cells whereas the chemokines recruit more neutrophils or IL-17-producing T cells to sites of inflammation in the skin, which produces a positive feed-forward mechanism and further amplifies local inflammatory responses to incite cytokine storms in psoriasis.
Taken together, the inflammatory circuit of TNFα, IL-6, IL-23 and IL-17 plays a critical role in host defense and tissue repair, whereas the dysregulation of this inflammatory circuit leads to the development of autoimmune diseases such as psoriasis.
Targeting inflammatory cytokines in autoimmune diseases
Given the importance of TNFα, IL-6, IL-23 and IL-17 in the development and pathogenesis of inflammatory and/or autoimmune diseases, lots of efforts have been spent on targeting these cytokines to treat these diseases. Accumulating pre-clinical and clinical studies show that blocking TNFα, IL-6, IL-23, IL-17 or their corresponding receptors by use of neutralizing antibodies is highly effective in the treatment of multiple autoimmune diseases such as psoriasis, rheumatoid arthritis (RA) and inflammatory bowel disease (IBD; Table 1). In particular, targeting the IL-23–IL-17 axis has been the most successful strategy for the treatment of psoriasis in the past decade.
Table 1.
Antibody-based biologics targeting TNFα, IL-23, IL-17 or their corresponding receptors
| Biologic | Developers | Type of agent | Indications | Stage |
|---|---|---|---|---|
| TNFα | ||||
| Infliximab (Remicade®) | Janssen Biologics (Philadelphia, PA, USA) | Chimeric TNFα-specific antibody | Rheumatoid arthritis, psoriatic arthritis, psoriasis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis | Approved |
| Etanercept (Enbrel®) | AMGen (Thousand Oaks, CA, USA) / Pfizer (New York, NY, USA) | Human TNFR2–Fc fusion protein | Rheumatoid arthritis, ankylosing spondylitis, psoriasis, ankylosing spondylitis | Approved |
| Adalimumab (Humira®) | Abbott Laboratories (Chicago, IL, USA) | Human TNFα-specific antibody | Rheumatoid arthritis, psoriatic arthritis, psoriasis, ankylosing spondylitis, Crohn’s disease | Approved |
| Certolizumab pegol (Cimzia®) | UCB (Brussels, Belgium) | Human pegylated FAb TNFα- specific antibody | Rheumatoid arthritis, Crohn’s disease | Approved |
| Golimumab (Simponi®) | Janssen Biologics | Human TNFα-specific antibody | Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis | Approved |
| IL-6 | ||||
| Tocilizumab (Actemra®/ RoActemra®) | Hoffmann—La Roche (Basel, Switzerland) | Humanized IL-6R-specific mAb | Rheumatoid arthritis, juvenile rheumatoid arthritis, Crohn’s disease, Castleman’s disease | Approved |
| Siltuximab (Sylvant®) | Janssen Biologics | Chimeric (mouse–human) IL-6- specific mAb | Multicentric Castleman’s disease | Approved |
| Sarilumab | Sanofi (Paris, France) | Fully human IL-6Rα-specific mAb | Rheumatoid arthritis | Phase III |
| Ankylosing spondylitis | Phase II | |||
| Olokizumab | UCB | Humanized IL-6-specific mAb | Rheumatoid arthritis | Phase II |
| Sirukumab | Janssen Biologics | Fully human IL-6-specific mAb | Rheumatoid arthritis | Phase II |
| IL-23 | ||||
| Ustekinumab (Stelera®) | Janssen Biologics | Human IL-12/IL-23- specific antibody targeting the p40 subunit | Psoriasis, psoriatic arthritis | Approved |
| Crohn’s disease | Phase III | |||
| Rheumatoid arthritis | Phase II | |||
| Tildrakizumab | Merck (Kenilworth, NJ, USA) / Sun Pharmaceuticals (Mumbai, India) | Fully human mAb targeting IL-23 p19 | Psoriasis | Phase III |
| Crohn’s disease | Phase I | |||
| Guselkumab | Janssen Biologics | Fully human mAb targeting IL-23 p19 | Psoriasis | Phase III |
| Psoriatic arthritis | Phase II | |||
| BI-655066 | Boehringer Ingelheim GmbH (Ingelheim am Rhein, Germany) | Humanized mAb targeting IL-23 p19 | Psoriasis, Crohn’s disease, ankylosing spondylitis | Phase II |
| LY3074828 | Eli Lilly & Co. (Indianapolis, IN, USA) | Humanized mAb targeting IL-23 p19 | Psoriasis | Phase I |
| IL-17 | ||||
| Secukinumab (Cosentyx®) | Novartis International AG (Basel, Switzerland) | Fully human mAb targeting IL-17A | Psoriasis | Approved |
| Psoriatic arthritis, ankylosing spondylitis | Phase III | |||
| Rheumatoid arthritis, multiple sclerosis | Phase II | |||
| Ixekizumab | Eli Lilly | Humanized mAb targeting IL-17A | Psoriasis, psoriatic arthritis | Phase III |
| Rheumatoid arthritis | Phase II | |||
| Brodalumab | AMGen / AstraZenenca (London, UK) | Fully human mAb targeting IL-17RA | Psoriasis, psoriatic arthritis | Phase III |
| CNTO6785 | Janssen Biologics | Fully human mAb targeting IL-17A | Rheumatoid arthritis | Phase II |
| Bimekizumab | UCB | Humanized mAb targeting IL-17A and IL-17F | Psoriasis, psoriatic arthritis | Phase I |
| SCH-900117 | Merck | Fully human mAb targeting IL-17A | Rheumatoid arthritis | Phase I |
Tumor necrosis factor α
TNFα is one of most avidly studied and clinically targeted cytokines in the treatment of autoimmune diseases. To date, TNFα blockers are still the best-selling cytokine-targeting drug type on the market. TNFα is expressed by several cell types including keratinocytes, macrophages, monocytes, neutrophils, and T lymphocytes while its receptors, TNF receptor 1 (TNFR1; also known as p60) and TNFR2 (also known as p75), are ubiquitously expressed. Deregulated production of TNFα and its TNFRs is detrimental and has been associated with sepsis and several other inflammatory and autoimmune diseases including RA, psoriasis and colitis.
In the early 1970s, TNFα was discovered as a potent muscle-wasting (or cachexic) factor with potent anticancer activity (41, 42). This discovery stimulated great enthusiasm in the treatment of cancer. Unexpectedly, the administration of TNFα to patients caused hypotension and hepatic damage (43). The failure of TNFα in cancer treatment made scientists shift their attention to the field of sepsis as macrophage-derived TNFα was found to be one of two primary inflammatory cytokines in the pathogenesis of septic or endotoxin shock (44). However, the inhibition of TNFα with neutralizing antibodies or soluble TNFα receptor in patients with sepsis was either ineffective or made survival outcomes worse (45, 46).
Although anti-TNFα therapies failed in the treatment of cancer and sepsis, the first clinical trial of a TNFα inhibitor succeeded in the treatment of RA in 1992 (47). This success subsequently led to five approved TNFα-blocking biologicals (Table 1)—infliximab, etanercept, adalimumab, certolizumab and golimumab (48). Infliximab is an IgG1 mouse–human chimeric antibody and was the first TNFα-targeted drug to be approved, in 1998 (49). This was followed by the approval of the TNFR2–Fc (crystallizable fragment of immunoglobulins) IgG1 chimera protein etanercept (50). Adalimumab was the first fully human antibody targeting TNFα and was approved in 2002 (51). Certolizumab pegol is a new, polyethylene glycol-conjugated, humanized FAb (antigen-binding fragment of immunoglobulins) of a TNFα-specific monoclonal antibody (mAb) and was approved in 2008 (52). Golimumab, another fully human antibody, was approved in 2009 (53).
It is reasonable to speculate that all these five TNFα-blocking biologicals would be effective in the treatment of autoimmune diseases associated with overproduction of TNFα, such as psoriasis, RA, multiple sclerosis (MS) and IBD (54, 55). However, not all patients have responded equally to each medication. Despite the impressive positive effects of blockade of TNFα in psoriasis, psoriatic arthritis and RA (51), anti-TNFα agents worsened disease in patients with MS (56) and etanercept failed to treat patients with IBD (57).
The basis for these differential effects of TNFα blockade might be due to the heterogeneity of RA, MS and IBD. In addition, all five TNFα-blocking biologicals have been reported to be effective in the treatment of RA, but about one-third of patients with RA treated with anti-TNFα agents did not respond (58). This difference might be dependent on the genetic makeup of patients and different mechanisms underlying the pathogenesis of RA. Moreover, TNFα is at the upstream of the inflammatory cytokine cascade. This early release inflammatory cytokine is at key steps of disease development but may not be fundamental to the disease pathogenesis in some cases as recent detailed studies indicate that both the timing and the duration of TNFα expression are important in determining the pathogenic roles of TNFα (59, 60).
Thus, determining the appropriate stage of disease at which to intervene is crucial. Moreover, the systemic blockade of soluble TNFα and membrane-bound TNFα by using neutralizing antibodies has been indicated to enhance cancer risk and susceptibility to infections (61, 62).
Interleukin-6
IL-6, like TNFα, is a key mediator in the immune system and produced by various haematopoietic and non-haematopoietic cells. Its receptor consists of a membrane-bound IL-6Rα and the accessory molecule glycoprotein 130 (gp130; also known as IL-6Rβ). The expression of membrane-bound IL-6Rα is restricted mainly to cells of the immune system and to hepatocytes, whereas gp130 is ubiquitously expressed. IL-6 also has a key role in the development of autoimmune models such as experimental autoimmune encephalomyelitis (EAE), collagen-induced arthritis (CIA), RA, IBD and psoriasis. In IL6-deficient mice, arthritis development was completely inhibited (63) and the severity of IBD was strikingly reduced (64), suggesting that targeting IL-6 holds therapeutic potential in the treatment of these autoimmune diseases. Therefore, multiple neutralizing antibodies targeting IL-6 and its receptor have been developed (Table 1). To date, tocilizumab (humanized IL-6R-specific mAb) has been approved, in combination with methotrexate, to treat RA (65); and siltuximab (chimeric IL-6-specific mAb) has been approved for multi-center trials of Castleman’s disease treatment (66). Sirukumab (fully human IL-6-specific mAb), olokizumab (humanized IL-6-specific mAb) and sarilumab (fully human IL-6Rα mAb) are under Phase-II or -III study in patients with RA (67–69).
Despite the great potential of targeting IL-6 and IL-6R, caution is warranted given that IL-6 bound to soluble IL-6Rα, a form generated by proteolytic cleavage of membrane-bound IL-6Rα by the metalloproteinases TNFα-converting enzyme or alternatively spliced mRNA, can induce trans-signaling by associating with the accessory molecule gp130 in a multitude of cell types that do not express membrane-bound IL-6Rα (4). Therefore, the antibodies directed against IL-6Rα will target both membrane-bound and soluble forms of the receptor, which may be outweighed in some cases by adverse effects. In such cases, targeting signaling molecules such as JAKs downstream of IL-6R might be more effective. Thus, tofacitinib, a small-molecule therapeutic targeting JAK1 and JAK3, has proved to be significantly effective (70). Moreover, IL-6 plays a critical role in host defense against microbial infections (71, 72) and plays a protective role during neural and liver injury (73, 74). Long-term IL-6 neutralization may increase susceptibility to bacterial and viral infections or may cause mortality in patients with ischemic neural damage or alcoholic cirrhosis.
The IL-23–IL-17 axis
The discovery of the IL-23–IL-17 immune axis has led to defining a new lineage of T helper cells—Th17 cells. This new T-cell subset plays a key role in the pathogenesis of multiple autoimmune diseases as Th17 cells are one of major producers of IL-17 (75).
Interleukin-23.
IL-23 is a heterodimeric cytokine composed of the p19 and the p40 subunits, and shares the p40 subunit with IL-12, which contains another subunit—p35. Given its key role in driving pathogenic Th17 and γδ T cells to produce high levels of IL-17 (Fig. 1), neutralizing antibodies have been developed that target IL-23. The anti-p40 subunit therapy targeting IL-12 was first evaluated in patients with Crohn’s disease (CD) in 2000 and provided benefit in this disease (76). Subsequent studies demonstrated that, in patients with psoriasis, levels of mRNA for the p40 of IL-12 or IL-23 and for the p19 of IL-23 were higher in lesions of psoriasis patients than in non-lesional and normal skin, whereas mRNA for p35 of IL-12 was present but decreased in lesional skin (77). Moreover, pre-clinical models of psoriasis illustrated that IL-23 exposure in murine skin drove the excessive growth and abnormal differentiation of keratinocytes, whereas IL-12 did not promote the same pathology (77, 78).
The evidence suggests that targeting the p40 of IL-12 indeed inhibits IL-23 and that inhibiting p40 might be effective to treat psoriasis, too. Therefore, two therapeutic mAbs targeting the p40 subunit, Ustekinumab and briakinumab, were used in treatment of psoriasis (Table 1). Both Ustekinumab and briakinumab were effective in Phase III clinical trials for the treatment of psoriasis and psoriatic arthritis (4). Ustekinumab also showed benefits in the treatment of moderate to severe CD when it was administered as maintenance therapy (79), but failed to prevent inflammation in patients with MS (80).
To date, Ustekinumab has been approved to treat psoriasis and psoriatic arthritis (81). However, briakinumab was withdrawn pending further analysis as major adverse cardiac events occurred in a higher number of patients receiving briakinumab compared with those receiving placebo in some Phase III trials (81, 82). Besides Ustekinumab and briakinumab, several IL-23-specific antibodies antagonizing the p19 subunit including tildrakizumab, guselkumab, LY2525623, AMG139, BI-655066 and LY3074828 (Table 1) have been developed (81). Tildrakizumab and guselkumab have completed Phase II trials for psoriasis. LY2525623 was terminated in Phase II for complexities in development but not safety concerns. AMG139, BI-655066 and LY3074828 are in early stages of development (81).
Although IL-23 is redundant in host defense to many pathogens, it is important for host responses against Mycobacterium and Candida infections as IL-23-dependent IL-17A and IL-17F play key roles in protecting hosts from these infections (83, 84). Therefore, treatment of autoimmune diseases with IL-23 antagonists carries the risk of impaired host defense responses to pathogens. Moreover, IL-12 can act on both innate and adaptive lymphoid cells such as natural killer cells and CD8+ cytotoxic T lymphocytes, and these cells then produce IFNγ to prevent tumor initiation, growth and metastasis (85, 86). In contrast, IL-23 increases tumor cell proliferation, survival and invasion by activating signal transducer and activator of transcription 3 (STAT3) and the overexpression of IL-23 in mice is sufficient to induce rapid (3–4 weeks) de novo development of intestinal adenomas (85, 87, 88). Therefore, the risk of breakdown in tumor surveillance by antagonizing the IL-12/IL-23 p40 subunit is of particular concern. To achieve both efficacy and safety in the treatment of autoimmune diseases by targeting IL-23, the intricate cellular and molecular mechanisms of those diseases warrant further investigation. Meanwhile, clinical testing is required to determine whether a specific disease mechanism also operates in humans.
Interleukin-17.
As a key effector cytokine produced by pathogenic Th17 and γδ T cells, IL-17 plays a crucial role in the pathogenesis of multiple autoimmune diseases such as psoriasis, RA, MS, IBD and myocarditis (89–91) and has been thought as one of the best targets in the treatment of autoimmune diseases (92). Therefore, several monoclonal antibodies targeting IL-17A and IL-17RA have been developed (Table 1). The Phase II proof-of-concept studies for secukinumab (a fully human IL-17A-specific monoclonal antibody) (93), ixekizumab (a humanized IL-17A-specific antibody) (94) and brodalumab (a fully human IL-17RA-specific monoclonal antibody) (95) showed that each drug was highly effective and helped around 80% of treated patients to achieve a 75% reduction in the Psoriasis Area and Severity Index (PASI) (96).
To date, secukinumab has been approved for the treatment of plaque psoriasis, whereas isekizumab and brodalumab are in Phase III trials (92). Beyond psoriasis, secukinumab, isekizumab and brodalumab are currently in pipeline, with trials in psoriatic arthritis and RA still ongoing. Other drug candidates targeting IL-17A include CNTO6785, SCH-900117 and bimekizumab. All these candidates are in either Phase II or Phase I clinical trials (97).
Although both IL-17A and IL-17RA are good targets in the treatment of autoimmune diseases, a key question remains: targeting which one—the cytokine or the receptor—is more effective? From a pharmacokinetic point of view it may be easier to block the cytokine than the receptor. IL-17 is readily available in the circulation, whereas the receptor resides in the membrane of many cell types and the corresponding neutralizing antibodies need to gain access to the relevant tissue before the receptor can be neutralized (92). Therefore, it is reasonable to speculate that targeting the ligand would be more straightforward than the receptor. However, IL-17RA is the most common signaling subunit in the IL-17 pathway. It can form heterodimeric receptor complex with most other IL-17 receptors. Therefore, targeting IL-17RA might be an effective way to disrupt the pathway if multiple IL-17 cytokines are contributing to a disease rather than a particular cytokine. Recent positive results for brodalumab in Phase III trials in psoriasis have confirmed this hypothesis (97).
Similar to targeting IL-23, the rates of adverse events including infections need to be considered due to a crucial role of IL-17A in the control of extracellular but not intracellular bacterial infections and fungi (98). To avoid the adverse effects, blocking Th17 cell responses but not universal IL-17 cytokine signaling might be safer. Therefore, targeting RORγt, a key transcription factor that determines the lineage commitment of Th17 cells, might be an alternative option. Several small molecules including digoxin and ursolic acid have been developed to antagonize RORγt activity (99, 100). However, off-target effects of these small molecules must be under key consideration.
Conclusions
Over the past two decades, targeting inflammatory cytokines and their receptors has produced multiple highly successful drugs in the treatment of autoimmune diseases such as RA, psoriasis and colitis. Although the potential of inflammatory cytokines and their receptors as drug targets is clearly established, how to select the key players in specific autoimmune diseases or pivotal regulators of cytokine circuits is still under debate. Therefore understanding the intrinsic cellular and molecular mechanisms of each cytokine in different disease settings is required to select a specific inflammatory target with better efficacy and safety profiles in the treatment of each autoimmune disease. Additionally, although the general principles in defining good anti-inflammatory targets are well established (3), the redundancy in the actions of many inflammatory cytokines that will limit the efficacy of single neutralizing agents requires us to pay careful attention.
We should point out that not all agents work as expected; some work better than others in certain diseases. Thus, the heterogeneity and complexity of human autoimmune diseases needs to be considered. Since mouse models cannot exactly resemble all types of human diseases, innovative translational studies on human patients are urgently required.
In addition, we need to keep in mind that inflammatory cytokines are also critical to maintain effective host defense or prevent tumorigenesis. Therefore, blocking cytokines to prevent autoimmune diseases might make patients more susceptible to infection or risk causing decreased tumor surveillance.
Clearly, there is much to be done to dissect the complex and highly interactive effects of inflammatory cytokines. With most of cytokines unstudied in human diseases, we are optimistic that many new, even better and safer ways of treating autoimmune diseases will emerge.
Funding
National Natural Science Foundation of China (31222021, 31470878 and 31170867 to Y.L.); Science and Technology Commission of Shanghai Municipality (13JC1402301 and 11DZ2260300 to Y.L.); Shanghai Education Commission (13SG25 to Y.L.); Henry Fok Educational Foundation (141017 to Y.L.); Institute for Immunology and Center for Life Sciences at Tsinghua University (to C.D.).
Conflict of interest statement: The authors declared no conflict of interests.
References
- 1. Isaacs A. and Lindenmann J. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B. Biol. Sci. 147:258. [PubMed] [Google Scholar]
- 2. Isaacs A. Lindenmann J. and Valentine R. C. 1957. Virus interference. II. Some properties of interferon. Proc. R. Soc. Lond. B. Biol. Sci. 147:268. [PubMed] [Google Scholar]
- 3. Simmons D. L. 2006. What makes a good anti-inflammatory drug target? Drug Discov. Today 11:210. [DOI] [PubMed] [Google Scholar]
- 4. Kopf M. Bachmann M. F. and Marsland B. J. 2010. Averting inflammation by targeting the cytokine environment. Nat. Rev. Drug Discov. 9:703. [DOI] [PubMed] [Google Scholar]
- 5. Wong G. H. and Goeddel D. V. 1986. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. Nature 323:819. [DOI] [PubMed] [Google Scholar]
- 6. Bryant-Hudson K. M. Gurung H. R. Zheng M. and Carr D. J. 2014. Tumor necrosis factor alpha and interleukin-6 facilitate corneal lymphangiogenesis in response to herpes simplex virus 1 infection. J. Virol. 88:14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai Y., Di Nardo A., Nakatsuji T., et al. 2009. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 15:1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steenfos H. H. Hunt T. K. Scheuenstuhl H. and Goodson W. H. III. 1989. Selective effects of tumor necrosis factor-alpha on wound healing in rats. Surgery 106:171. [PubMed] [Google Scholar]
- 9. Mooney D. P. O’Reilly M. and Gamelli R. L. 1990. Tumor necrosis factor and wound healing. Ann. Surg. 211:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koch A. E., Kunkel S. L., Harlow L. A., et al. 1992. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J. Clin. Invest. 90:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jasny E., Eisenblatter M., Matz-Rensing K., et al. 2008. IL-12-impaired and IL-12-secreting dendritic cells produce IL-23 upon CD154 restimulation. J. Immunol. 180:6629. [DOI] [PubMed] [Google Scholar]
- 12. Ochoa O. Torres F. M. and Shireman P. K. 2007. Chemokines and diabetic wound healing. Vascular 15:350. [DOI] [PubMed] [Google Scholar]
- 13. Molofsky A. B. Savage A. K. and Locksley R. M. 2015. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity 42:1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McAleer J. P. and Kolls J. K. 2014. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol. Rev. 260:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park H., Li Z., Yang X. O., et al. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrington L. E. Hatton R. D. Mangan P. R. Turner H. Murphy T. L. Murphy K. M. and Weaver C. T. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123. [DOI] [PubMed] [Google Scholar]
- 17. Yang X. O. Panopoulos A. D. Nurieva R. Chang S. H. Wang D. Watowich S. S. and Dong C. 2007. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282:9358. [DOI] [PubMed] [Google Scholar]
- 18. Kennedy-Crispin M., Billick E., Mitsui H., et al. 2012. Human keratinocytes’ response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. J. Invest. Dermatol. 132:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morales J., Homey B., Vicari A. P., et al. 1999. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc. Natl Acad. Sci. USA 96:14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Homey B., Alenius H., Muller A., et al. 2002. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat. Med. 8:157. [DOI] [PubMed] [Google Scholar]
- 21. Russo C. and Polosa R. 2005. TNF-alpha as a promising therapeutic target in chronic asthma: a lesson from rheumatoid arthritis. Clin. Sci. (Lond.) 109:135. [DOI] [PubMed] [Google Scholar]
- 22. Aggarwal B. B. Shishodia S. Takada Y. Jackson-Bernitsas D. Ahn K. S. Sethi G. and Ichikawa H. 2006. TNF blockade: an inflammatory issue. Ernst Schering Res. Found. Workshop 56:161. [DOI] [PubMed] [Google Scholar]
- 23. Nakajima A., Matsuki T., Komine M., et al. 2010. TNF, but not IL-6 and IL-17, is crucial for the development of T cell-independent psoriasis-like dermatitis in Il1rn-/- mice. J. Immunol. 185:1887. [DOI] [PubMed] [Google Scholar]
- 24. Sheibanie A. F. Tadmori I. Jing H. Vassiliou E. and Ganea D. 2004. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 18:1318. [DOI] [PubMed] [Google Scholar]
- 25. Cua D. J., Sherlock J., Chen Y., et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744. [DOI] [PubMed] [Google Scholar]
- 26. Aggarwal S. Ghilardi N. Xie M. H. de Sauvage F. J. and Gurney A. L. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910. [DOI] [PubMed] [Google Scholar]
- 27. Parham C., Chirica M., Timans J., et al. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699. [DOI] [PubMed] [Google Scholar]
- 28. Sutton C. E. Lalor S. J. Sweeney C. M. Brereton C. F. Lavelle E. C. and Mills K. H. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31:331. [DOI] [PubMed] [Google Scholar]
- 29. Iwakura Y. and Ishigame H. 2006. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 116:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cai Y., Shen X., Ding C., et al. 2011. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 35:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teunissen M. B. Koomen C. W. de Waal Malefyt R. Wierenga E. A. and Bos J. D. 1998. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Invest. Dermatol. 111:645. [DOI] [PubMed] [Google Scholar]
- 32. Carrier Y., Ma H. L., Ramon H. E., et al. 2011. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J. Invest. Dermatol. 131:2428. [DOI] [PubMed] [Google Scholar]
- 33. Cai X. Y. Gommoll C. P. Jr Justice L. Narula S. K. and Fine J. S. 1998. Regulation of granulocyte colony-stimulating factor gene expression by interleukin-17. Immunol. Lett. 62:51. [DOI] [PubMed] [Google Scholar]
- 34. Chiricozzi A., Guttman-Yassky E., Suarez-Farinas M., et al. 2011. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Invest. Dermatol. 131:677. [DOI] [PubMed] [Google Scholar]
- 35. Hegyi Z., Zwicker S., Bureik D., et al. 2012. Vitamin D analog calcipotriol suppresses the Th17 cytokine-induced proinflammatory S100 “alarmins” psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J. Invest. Dermatol. 132:1416. [DOI] [PubMed] [Google Scholar]
- 36. Liang S. C. Tan X. Y. Luxenberg D. P. Karim R. Dunussi-Joannopoulos K. Collins M. and Fouser L. A. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lai Y., Li D., Li C., et al. 2012. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity 37:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jovanovic D. V., Di Battista J. A., Martel-Pelletier J., et al. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 160:3513. [PubMed] [Google Scholar]
- 39. Tortola L., Rosenwald E., Abel B., et al. 2012. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J. Clin. Invest. 122:3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ha H. L., Wang H., Pisitkun P., et al. 2014. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc. Natl Acad. Sci. USA 111:E3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carswell E. A. Old L. J. Kassel R. L. Green S. Fiore N. and Williamson B. 1975. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl Acad. Sci. USA 72:3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beutler B. and Cerami A. 1988. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu. Rev. Biochem. 57:505. [DOI] [PubMed] [Google Scholar]
- 43. Jones A. L. and Selby P. 1989. Tumour necrosis factor: clinical relevance. Cancer Surv. 8:817. [PubMed] [Google Scholar]
- 44. Cerami A. and Beutler B. 1988. The role of cachectin/TNF in endotoxic shock and cachexia. Immunol. Today 9:28. [DOI] [PubMed] [Google Scholar]
- 45. Abraham E., Anzueto A., Gutierrez G., et al. 1998. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet 351:929. [PubMed] [Google Scholar]
- 46. Fisher C. J., Jr, Agosti J. M., Opal S. M., et al. 1996. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N. Engl. J. Med. 334:1697. [DOI] [PubMed] [Google Scholar]
- 47. Feldmann M. and Maini R. N. 2003. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat. Med. 9:1245. [DOI] [PubMed] [Google Scholar]
- 48. Croft M. Benedict C. A. and Ware C. F. 2013. Clinical targeting of the TNF and TNFR superfamilies. Nat. Rev. Drug Discov. 12:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maini R. N., Breedveld F. C., Kalden J. R., et al. 1998. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 41:1552. [DOI] [PubMed] [Google Scholar]
- 50.Anonymous. 1998. Etanercept marketed for moderate, severe rheumatoid arthritis. Am. J. Health Syst. Pharm. 55:2593. [DOI] [PubMed] [Google Scholar]
- 51. den Broeder A. A., Joosten L. A., Saxne T., et al. 2002. Long term anti-tumour necrosis factor alpha monotherapy in rheumatoid arthritis: effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann. Rheum. Dis 61:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Danese S., Stefanelli T., Omodei P., et al. 2008. Successful treatment of fistulizing Crohn’s disease with certolizumab pegol. Inflamm. Bowel. Dis. 14:292. [DOI] [PubMed] [Google Scholar]
- 53. Keystone E. C., Genovese M. C., Klareskog L., et al. 2009. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann. Rheum. Dis. 68:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leonardi C. L., Powers J. L., Matheson R. T., et al. ; Etanercept Psoriasis Study Group. 2003. Etanercept as monotherapy in patients with psoriasis. N. Engl. J. Med. 349:2014. [DOI] [PubMed] [Google Scholar]
- 55. Lipsky P. E., van der Heijde D. M., St Clair E. W., et al. ; Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. 2000. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N. Engl. J. Med. 343:1594. [DOI] [PubMed] [Google Scholar]
- 56.Anonymous. 1999. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 53:457. [PubMed] [Google Scholar]
- 57. Sandborn W. J. and Hanauer S. B. 1999. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm. Bowel Dis. 5:119. [DOI] [PubMed] [Google Scholar]
- 58. O’Shea J. J. Ma A. and Lipsky P. 2002. Cytokines and autoimmunity. Nat. Rev. Immunol. 2:37. [DOI] [PubMed] [Google Scholar]
- 59. Christen U., Wolfe T., Mohrle U., et al. 2001. A dual role for TNF-alpha in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J. Immunol. 166:7023. [DOI] [PubMed] [Google Scholar]
- 60. Kollias G. Douni E. Kassiotis G. and Kontoyiannis D. 1999. The function of tumour necrosis factor and receptors in models of multi-organ inflammation, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Ann. Rheum. Dis. 58(Suppl. 1):I32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Horssen R. Ten Hagen T. L. and Eggermont A. M. 2006. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist 11:397. [DOI] [PubMed] [Google Scholar]
- 62. Wallis R. S. Broder M. S. Wong J. Y. Hanson M. E. and Beenhouwer D. O. 2004. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin. Infect. Dis. 38:1261. [DOI] [PubMed] [Google Scholar]
- 63. Hata H., Sakaguchi N., Yoshitomi H., et al. 2004. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J. Clin. Invest. 114:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yamamoto M. Yoshizaki K. Kishimoto T. and Ito H. 2000. IL-6 is required for the development of Th1 cell-mediated murine colitis. J. Immunol. 164:4878. [DOI] [PubMed] [Google Scholar]
- 65. Yamamoto K., Goto H., Hirao K., et al. 2015. Longterm safety of tocilizumab: results from 3 years of followup postmarketing surveillance of 5573 patients with rheumatoid arthritis in Japan. J. Immunol. 42:1368. [DOI] [PubMed] [Google Scholar]
- 66. van Rhee F., Wong R. S., Munshi N., et al. 2014. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 15:966. [DOI] [PubMed] [Google Scholar]
- 67. Smolen J. S. Weinblatt M. E. Sheng S. Zhuang Y. and Hsu B. 2014. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann. Rheum. Dis. 73:1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Genovese M. C., Fleischmann R., Kivitz A. J., et al. 2015. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol. 67:1424. [DOI] [PubMed] [Google Scholar]
- 69. Genovese M. C., Fleischmann R., Furst D., et al. 2014. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study. Ann. Rheum. Dis. 73:1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tanaka Y. 2015. Recent progress and perspective in JAK inhibitors for rheumatoid arthritis: from bench to bedside. J. Biochem. 158:173. [DOI] [PubMed] [Google Scholar]
- 71. Kopf M., Baumann H., Freer G., et al. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339. [DOI] [PubMed] [Google Scholar]
- 72. Romani L., Mencacci A., Cenci E., et al. 1996. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J. Exp. Med. 183:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ohtaki H., Nakamachi T., Dohi K., et al. 2006. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc. Natl Acad. Sci. USA 103:7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cressman D. E. Greenbaum L. E. DeAngelis R. A. Ciliberto G. Furth E. E. Poli V. and Taub R. 1996. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379. [DOI] [PubMed] [Google Scholar]
- 75. Miossec P. and Kolls J. K. 2012. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 11:763. [DOI] [PubMed] [Google Scholar]
- 76. Mannon P. J., Fuss I. J., Mayer L., et al. 2004. Anti-interleukin-12 antibody for active Crohn’s disease. N. Engl. J. Med. 351:2069. [DOI] [PubMed] [Google Scholar]
- 77. Lee E., Trepicchio W. L., Oestreicher J. L., et al. 2004. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J. Exp. Med. 199:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tonel G., Conrad C., Laggner U., et al. 2010. Cutting edge: a critical functional role for IL-23 in psoriasis. J. Immunol. 185:5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sandborn W. J., Gasink C., Gao L. L., et al. ; CERTIFI Study Group. 2012. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N. Engl. J. Med. 367:1519. [DOI] [PubMed] [Google Scholar]
- 80. Segal B. M. Constantinescu C. S. Raychaudhuri A. Kim L. Fidelus-Gort R. Kasper L. H. and Ustekinumab M. S. I. 2008. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Nat. Med. 7:796. [DOI] [PubMed] [Google Scholar]
- 81. Teng M. W. Bowman E. P. McElwee J. J. Smyth M. J. Casanova J. L. Cooper A. M. and Cua D. J. 2015. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 21:719. [DOI] [PubMed] [Google Scholar]
- 82. Gordon K. B., Langley R. G., Gottlieb A. B., et al. 2012. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J. Invest. Dermatol. 132:304. [DOI] [PubMed] [Google Scholar]
- 83. Puel A. Cypowyj S. Maródi L. Abel L. Picard C. and Casanova J. L. 2012. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr. Opin. Allergy Clin. Immunol. 12:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Khader S. A., Pearl J. E., Sakamoto K., et al. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 175:788. [DOI] [PubMed] [Google Scholar]
- 85. Dunn G. P. Koebel C. M. and Schreiber R. D. 2006. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6:836. [DOI] [PubMed] [Google Scholar]
- 86. Colombo M. P. and Trinchieri G. 2002. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 13:155. [DOI] [PubMed] [Google Scholar]
- 87. Ngiow S. F. Teng M. W. and Smyth M. J. 2013. A balance of interleukin-12 and -23 in cancer. Trends Immunol. 34:548. [DOI] [PubMed] [Google Scholar]
- 88. Chan I. H., Jain R., Tessmer M. S., et al. 2014. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 7:842. [DOI] [PubMed] [Google Scholar]
- 89. Bettelli E. Oukka M. and Kuchroo V. K. 2007. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8:345. [DOI] [PubMed] [Google Scholar]
- 90. Korn T. Bettelli E. Oukka M. and Kuchroo V. K. 2009. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27:485. [DOI] [PubMed] [Google Scholar]
- 91. Wilson N. J., Boniface K., Chan J. R., et al. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8:950. [DOI] [PubMed] [Google Scholar]
- 92. Tse M. T. 2013. IL-17 antibodies gain momentum. Nat. Rev. Drug Discov. 12:815. [DOI] [PubMed] [Google Scholar]
- 93. Rich P., Sigurgeirsson B., Thaci D., et al. 2013. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br. J. Dermatol. 168:402. [DOI] [PubMed] [Google Scholar]
- 94. Leonardi C., Matheson R., Zachariae C., et al. 2012. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N. Engl. J. Med. 366:1190. [DOI] [PubMed] [Google Scholar]
- 95. Papp K. A., Leonardi C., Menter A., et al. 2012. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N. Engl. J. Med. 366:1181. [DOI] [PubMed] [Google Scholar]
- 96. Chiricozzi A. and Krueger J. G. 2013. IL-17 targeted therapies for psoriasis. Expert Opin. Investig. Drugs 22:993. [DOI] [PubMed] [Google Scholar]
- 97. Bartlett H. S. and Million R. P. 2015. Targeting the IL-17-T(H)17 pathway. Nat. Rev. Drug Discov. 14:11. [DOI] [PubMed] [Google Scholar]
- 98. Dubin P. J. and Kolls J. K. 2008. Th17 cytokines and mucosal immunity. Immunol. Rev. 226:160. [DOI] [PubMed] [Google Scholar]
- 99. Huh J. R., Leung M. W., Huang P., et al. 2011. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature 472:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xu T. Wang X. Zhong B. Nurieva R. I. Ding S. and Dong C. 2011. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J. Biol. Chem. 286:22707. [DOI] [PMC free article] [PubMed] [Google Scholar]