Tfh and Tfr in autoimmunity
Keywords: autoimmune diseases, T follicular helper cell, T follicular regulatory cell
Abstract
CD4+ T follicular helper (Tfh) cells are recognized as a distinct T-cell subset, which provides help for germinal center (GC) formation, B-cell development and affinity maturation, and immunoglobulin class switching, as an indispensable part of adaptive immunity. Tfh cell differentiation depends on various factors including cell-surface molecule interactions, extracellular cytokines and multiple transcription factors, with B-cell lymphoma 6 (Bcl-6) being the master regulator. T follicular regulatory (Tfr) cells are also located in the GC and share phenotypic characteristics with Tfh cells and regulatory T cells, but function as negative regulators of GC responses. Dysregulation of either Tfh or Tfr cells is linked to the pathogenesis of autoimmune diseases such as systemic lupus erythematosus. This review covers the basic Tfh and Tfr biology including their differentiation and function, and their close relationship with autoimmune diseases.
Introduction
Naive CD4+ T cells can differentiate into various subsets of helper T (Th) cells such as Th type 1 (Th1), Th2, and Th17 cells, which are characterized by the expression of unique transcription factors, the production of different cytokines and the involvement of diverse immunological responses (1), whereas CD4+ regulatory T (Treg) cells play a dominant role in controlling immune homeostasis to maintain tolerance (2).
A new subset named T follicular helper (Tfh) cells was discovered over a decade ago; this subset is essential for germinal center (GC) formation and B-cell function (3–5). These cells are characterized by a high expression level of CXC chemokine receptor 5 (CXCR5), a receptor that can guide Tfh cells to migrate to B-cell follicles where CXCL13 (the ligand for CXCR5) is expressed; there, Tfh cells function as helpers for humoral immune responses (3, 6). In addition, a specialized subset of Treg cells, T follicular regulatory (Tfr) cells, were also recently found in GCs, where they play a suppressive role in GC reactions (7–9).
The GC reaction is responsible for the generation of high-affinity antibodies and long-lived plasma cells, which are the bases of humoral immune responses against pathogen invasion (10). However, uncontrolled Tfh or Tfr activity can result in the loss of immune tolerance and abnormal production of high levels of auto-antibodies, which can contribute to the development of autoimmune responses. Thus, the two T-cell subsets in GCs—Tfh and Tfr cells—are indispensable for the balance between immune activation and tolerance, and the breakdown of such a balance can result in autoimmunity (11). In this review, we will cover studies of the differentiation and function of Tfh and Tfr cells, and their potential roles in autoimmune diseases.
Tfh cell development
The chemokine receptor CXCR5 helped the identification of the special B-cell helpers, Tfh cells. In 2000 and 2001, a new population of CD4+ T cells was reported to possess high levels of CXCR5, but low levels of CC chemokine receptor 7 (CCR7) compared with naive CD4+ T cells, in human tonsils (3–5). CXCR5, which was found to be indispensable for B-cell homing to B-cell follicles, was also identified to be important for T-cell migration to B-cell follicles (3). By up-regulating CXCR5 and down-regulating CCR7, these distinct CD4+ T cells can move towards the T–B border, interact with B cells for further maturation and then provide help for B-cell proliferation and GC reactions. Thus, the name of Tfh cells is based on their localization and function (3, 5).
A breakthrough discovery about Tfh cells was the identification of a master transcription factor, B-cell lymphoma 6 (Bcl-6), by three independent groups (12–14). Bcl-6 was previously reported to be essential for B-cell fate, since it can inhibit GC B-cell differentiation into plasma cells or memory cells by repression of Prdm1, the gene encoding Blimp-1 (B lymphocyte-induced maturation protein 1) (15–17). Therefore, it is interesting that Bcl-6 can also act as a master transcription factor in Tfh cells. It was reported that Bcl-6 deficiency in CD4+ T cells resulted in impaired Tfh cell development and impaired GC reactions, whereas enforced expression of Bcl-6 in CD4+ T cells could restore the defective phenotype (12–14).
The expression of hallmarks like CXCR5 and programmed cell death 1 (PD-1) in Tfh cells can be also promoted by enforced expression of Bcl-6, whereas the production of IFN-γ and IL-17 was repressed (14). Bcl-6, as a sequence-specific repressor of transcription, can bind to the promoter of Tbx21 and Rorc, which encode T-bet and Rorγt, respectively, and are the master transcription factors of Th1 and Th17 cells, respectively, and thus represses their expression (14). Similar to the finding that Bcl-6 and Blimp-1 play opposing roles in B cells (17, 18), Bcl-6 and Blimp-1 are antagonistic regulators during the process of Tfh cell differentiation (12).
Various studies have documented the regulation of Bcl-6 expression. It was reported that IL-6 and IL-21 regulate Bcl-6 expression (13). Moreover, the transcription factor Batf was reported to bind to the Bcl6 locus and activate its transcription (19). In addition, the transcription factor achaete-scute homologue 2 (Ascl2) can up-regulate CXCR5 expression and initiate Tfh development (20). Two recent studies reported the function of the transcription factors T-cell factor 1 (TCF-1) and lymphocyte enhancer factor 1 (LEF-1) in Tfh cells: TCF-1 binds to the Bcl6 locus and activates its expression while repressing Prdm1 expression by binding to its 5′ regulatory regions (21); and TCF-1 and LEF-1 play redundant roles in Bcl-6 expression (22).
Cell-surface co-stimulators are also important in Tfh cell development, for example, interactions via inducible co-stimulator (ICOS) and ICOS ligand (ICOSL). An early study revealed that ICOS signaling plays a vital role in T-cell activation and differentiation (23). Subsequent studies reported that ICOS–/– or ICOSL–/– mice exhibit impaired development of Tfh cells and GC reactions (24, 25). Sanroque mice exhibit enhanced Tfh cell development and GC reactions, and exhibit spontaneous lupus-like disease, due to the mutation in the Roquin gene, a negative regulator of ICOS mRNA stability or posttranscriptional repression (26–29). It is well established that ICOS signaling via PI3K is important for Tfh differentiation (30, 31).
It is well established that ICOS signaling via PI3K is important for Tfh cell differentiation (26–31). One recent study showed that the Akt-mediated inactivation of forkhead box o1 (Foxo1), a downstream target of the ICOS–PI3K signaling pathway, contributes to the up-regulation of Bcl-6 expression and enhanced Tfh cell differentiation (32). Consistent with this finding, another study reported that the E3 ubiquitin ligase Itch was required for Tfh differentiation at both the early and late stages by targeting the degradation of Foxo1 (33). Activation of ICOS can promote the interaction between p85α and intracellular osteopontin, and then lead to osteopontin nuclear translocation and binding to Bcl-6, which protects the latter from proteasome degradation (34).
It was also shown that ICOS expressed on activated T cells and ICOSL on bystander B cells are required for the recruitment of Tfh cells to follicles (35). Some other co-stimulators also play roles during the process, such as OX40 that can up-regulate Tfh-related gene expression (36).
Cytokines such as IL-6 and IL-21 can promote Bcl-6 production and thus Tfh differentiation via signal transducer and activator of transcription 1 (STAT1) and STAT3 signaling (25, 37, 38). Other transcription factors are also involved in Tfh differentiation; an example is c-Maf, which can bind to the IL-21 promoter and induce IL-21 production (39, 40). Two recent studies reported the role of microRNAs (miRs) in Tfh cell development (41, 42), in that the miR-17~92 family promotes Tfh differentiation during viral infection or protein immunization. The miR-17~92 family functions through repressing the phosphatase PHLPP2 (PH domain and leucine-rich repeat protein phosphatase 2), which is a negative regulator of PI3K–Akt signaling, or suppressing Rora (which encodes Rorα) to prevent subset-inappropriate gene expression (41, 42).
Tfh cell function as a B-cell helper
As mentioned above, the most important role for Tfh cells is to provide help for GC formation, and generation of long-lived memory B cells, as well as high-affinity plasma cells. The GC is the place where B cells go through rounds of proliferation, affinity maturation and selection (Fig. 1). GC B cells present antigen to Tfh cells in the follicle light zone, which then send signals to the cognate B cells for their survival and proliferation; GC B cells that receive the signals from Tfh cells can migrate to the follicle dark zone where they will undergo rounds of proliferation and somatic hypermutation. Then B cells with higher affinity for antigen will be selected by Tfh cells and receive signals again for another round of selection (43).
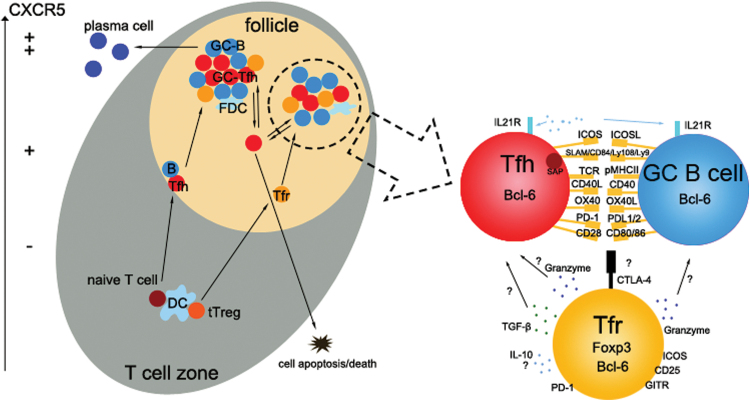
Fig. 1.
Interactions among Tfh, Tfr and B cells in GCs. Left, after priming by dendritic cells (DCs) in the T-cell zone, Tfh cells will up-regulate CXCR5 expression and then migrate to the T–B border to interact with cognate B cells. Interaction with B cells helps further commitment of Tfh cells and enhances CXCR5 expression. Similarly, thymus-derived Treg cells migrate to the GC and differentiate to Tfr cells to interact with Tfh and B cells. Right, an enlarged view of cell–cell interactions in the follicle. GC Tfh cells provide help, via various mechanisms such as interaction between cell-surface molecules or production of cytokines like IL-21 for B-cell proliferation and somatic hypermutation, allowing them to differentiate into high-affinity plasma cells or memory B cells. In contrast, Tfr cells express the transcription factors Foxp3 and Bcl-6 and other molecules. Tfr cells constrain GC reactions via co-inhibitory receptors such as CTLA-4, secretion of inhibitory cytokines such as IL-10 and TGF-β, and cytotoxic effects by granzyme release. FDC, follicular dendritic cell.
The helper signals from Tfh cells include cytokines and co-stimulation. IL-21R signaling can induce Bcl-6 expression and promote B-cell proliferation (13, 44–46). In addition, in vivo generation of CD40 signaling showed that CD40L–CD40 ligation can augment plasma cell differentiation but inhibit GC B-cell late expansion (47, 48). Moreover, four signaling lymphocytic activation molecule (SLAM)-family receptors expressed on Tfh cells can bind to SLAM-associated protein to trigger different but overlapping signals for GC responses. The CD84–CD84 (a SLAM family member) interaction is able to enhance T–B interaction and then help high-affinity GC B-cell selection, whereas SLAM–SLAM pairs exert their function on IL-4 production (49–51).
In addition to the co-stimulation signals, inhibitory signals are also essential to ensure that Tfh cells provide appropriate help to B cells and accomplish affinity selection correctly. Interactions between PD-1 and its ligands (PD-L1/PD-L2) can be enhanced by repeated T–B interaction or enhanced TCR signals, and their interactions, in turn, negatively regulate TCR signals and Tfh activities. However, mice deficient in PD-1 or PD-1 ligands have reduced B-cell responses after protein immunization, because loss of PD-1 signals impaired the interaction between Tfh cells and GC B cells (52, 53). Thus, balanced Tfh help and B-cell responses can be achieved via collaboration among various cytokines and co-stimulatory molecules, as well as via antagonism between stimulatory and inhibitory signals.
Tfr cells
A specialized subset of Treg cells, named Tfr cells, has been identified, which shares common characteristics with Tfh and conventional Treg cells and which can inhibit GC responses through controlling the number of Tfh and GC B cells (7–9).
CXCR5-expressing Treg cells were first described in 2004 in human tonsil GCs (54). In 2011, three independent groups demonstrated that a specialized subset of Treg cells could stably exist in GCs and suppress the GC reaction after immunization (7–9). This subset highly expresses Bcl-6, CXCR5, PD-1 and ICOS, which is similar to Tfh cells. However, this population of cells also exerts suppressive capacity comparable with conventional Treg cells in vitro and in vivo, and the transcriptional signature was closer to Treg cells characterized by the expression of the master transcription Foxp3 (2). In addition, this subset originated from thymic-derived Treg cells (tTreg cells), but not from naive T cells, indicating that this population was a subset of Treg cells. Tfr cells also have unique characteristics distinguishable from Tfh cells, such as the high expression of CTLA-4 (CTL-associated antigen 4), GITR (glucocorticoid-induced TNFR-related protein), CD25 and IL-10, whereas they barely express CD40L, IL-21 and IL-4 (7, 9). Therefore, it is generally believed that Tfr cells resemble Tfh cells due to their follicular localization but function like Treg cells.
The development and function of Tfr cells
Similar to Tfh cells, the differentiation of Tfr cells is also a multistage and multifactorial process. Tfr cell differentiation absolutely requires priming by dendritic cells at an early stage (55) and help from B cells for further development and expansion (9, 56, 57), co-stimulatory signals such as CD28 and ICOS (9, 58), and the expression of transcription factors such as Bcl-6 (7, 9). However, there are still some differences between Tfr and Tfh cell development. For example, the initial up-regulation of CXCR5 in Tfr cell is dependent on the transcription factor NFAT2 (nuclear factor of activated T cells 2) (59) but is not dependent on Ascl2, which is critical for the initial expression of CXCR5 in Tfh cells (20). Furthermore, NFAT2 deletion in T cells specifically resulted in reduced numbers of Tfr cells, because of diminished homing into B-cell follicles accompanied by enhanced GC responses, whereas it had little effect on Tfh cell migration.
Co-inhibitory signals are also essential to control the pool of Tfr cells, since PD-1 or PD-L1 (but not PD-L2)-deficient mice exhibited a largely increased percentage of Tfr cells and GC output, and similarly CTLA-4 deletion in all cells or only in Treg cells resulted in a profound increase of Tfr cells (57, 58, 60). Other molecules are also important in Tfr cell differentiation, such as tumor necrosis factor receptor-associated factor 3 (TRAF3), which may modulate Tfr cell differentiation via extracellular signal-regulated kinase signaling; ablation of TRAF3 attenuated the number of Tfr cells and was accompanied by increased antibody production (61). Helix–loop–helix proteins inhibitor of DNA binding 2 (Id2) and Id3 are also involved in Tfr cell development, since Id2 and Id3 deletion in Treg cells could enhance Tfr cell formation by inducing a Tfr-specific transcription signature including CXCR5 and IL-10 expression at an early stage, whereas it is also proposed that Id2 and Id3 are indispensable for the maintenance of the mature Tfr cell population through modulating Bcl-6 and Blimp-1 protein abundance (62). Together, it seems that there are some correlations in Tfr and Tfh cell differentiation, while some special cues are required for Tfr cell development/maintenance.
As mentioned above, Tfr cells show suppressive capacity in vivo and in vitro; however, studies on the function of Tfr cells in vivo have been controversial. A significant decrease of high-affinity antibodies and antigen-specific GC B-cell numbers was found in mixed bone-marrow chimeric mice when Tfr cell numbers were reduced, which suggested that Tfr cell may regulate the selection of antigen-specific B cells (9). However, two other groups, when transferring Cxcr5 –/– or Bcl6 –/– Treg cells into Tcrb –/– or Tcra –/– (T-cell deficient) recipient mice, found increased levels of antibodies, of both high and low affinity (7, 8). These conflicting results suggest that Tfr cells exert different responses to different foreign antigens or different microenvironments.
Another point to be considered is the timing and duration of Treg cell depletion. A recent study showed that short-term depletion of Treg cells could enhance the number of antigen-specific Tfh cells; however, the percentage of antigen-specific B cells was decreased when Treg cells were depleted for a prolonged time, which is probably related to immune homeostasis (60).
Even though the influence of Tfr cells on antigen-specific B cells remains contentious, it is clear that Tfr cells can control the size of the GC reaction (7–9). There are several potential mechanisms by which Tfr cells modulate the GC reaction. One of the hypotheses is that Tfr cells exert their suppressive capacity through CTLA-4. Two recent studies demonstrated that CTLA-4 depletion after Tfr cell formation impaired the suppressive capacity compared with the control Tfr cells in vitro and in vivo (57, 60). Based on the fact that CTLA-4 has a higher affinity binding to CD80/CD86 expressed on GC B cells than CD28, it is possible that CTLA-4 may exert its role by competitive binding with the co-stimulatory molecules.
Secretion of inhibitory cytokines such as IL-10 or TGF-β is also involved in the regulation of the GC reaction by Tfr cells. Although IL-10 is important for B-cell survival and proliferation, a marked increase of IL-10 had been found in patients with systemic lupus erythematosus (SLE) (63–68). Mice deprived of IL-10R signaling had aberrant accumulation of Tfh cells, and these Tfh cells highly expressed IL-17 and IL-21 to enhance antibody production (69).However, others also showed that IL-10 in supernatants was reduced when Tfr cells were added to Tfh–B-cell cultures, so the effect of IL-10 on the GC reaction may result from a non-Tfr Treg subset (57). Obviously, further investigation is needed to unravel the role of IL-10 in the regulation of the GC reaction and autoimmune diseases. In addition, Tfr cells may also secrete TGF-β to control the GC response, since aberrant accumulation of Tfh cells and self-reactive B cells could be restrained by TGF-β signaling (70). Since TGF- β has different isoforms (β1, β2 and β3) (71), it remains to be investigated whether they play the same or different functions in Tfr cells. Other hypotheses such as mechanical disruption of Tfh and B-cell contacts or direct B-cell killing via the release of granzymes by Tfr cells have also been proposed (72, 73). Thus, Tfr cells utilize a number of mechanisms to modulate the GC reaction, as summarized in Fig. 1. However, the detailed mechanisms remain to be investigated.
Tfh and Tfr in autoimmune diseases
Auto-antibodies are involved in many autoimmune diseases, such as rheumatoid arthritis (RA), SLE, Sjögren’s syndrome and myasthenia gravis, and patients with those autoimmune diseases often have enhanced Tfh cell numbers in the blood and enhanced GC responses (74–77). SLE, an autoimmune disease characterized by red speckles on the skin, is due to the production of autoreactive antibodies that account for different clinical symptoms and organ or tissue damage (78). Recent studies showed that there is a dramatic increase in the frequency of the circulating CXCR5+ICOS+ Tfh-like cells in patients with SLE (78–80). In addition, a positive correlation can be derived between the SLE disease-activity index or serum auto-antibody levels and the frequency of Tfh cells circulating in the blood (78). CD40L–CD40 signaling may contribute to the pathogenesis since the level of IgG secretion is reduced by adding anti-CD40L antibody in vitro (78). Excessive OX40 signaling can also lead to SLE pathogenesis via promoting Tfh responses (36).
RA is another autoimmune disorder, with the immune system attacking joints, causing inflammation and thickening of the joint capsule. Several groups have reported that there is a higher frequency of circulating CXCR5+ICOS+ or CXCR5+ICOShi CD4+ T cells in patients suffering from RA (81, 82). Furthermore, serum IL-21 is also higher in RA patients compared with that in healthy controls (81, 83). Another study showed evidence about IL-21 in the pathogenesis of autoimmune arthritis (84) in that less-severe joint inflammation was observed, together with a reduced Tfh cell population in draining lymph nodes, in IL-21 receptor (IL-21R)-deficient KBx/N mice. Moreover, IL-21 blockade in mouse RA models reduces disease severity and RA progression (84). In addition, a positive correlation between Tfh or Tfh-like cell numbers in the blood and auto-antibodies has been detected in autoimmune thyroid diseases (85), juvenile dermatomyositis and Sjögren’s syndrome (11).
Since Tfr cells are regulators of the GC response, it can be expected that they are also related to autoimmune diseases. A recent study showed the importance of the Tfr/Tfh balance in autoimmune responses in BXD2 mice, which display spontaneous autoreactive GC formation (86). IL-21 selectively promotes Tfh cell differentiation but inhibits Tfr cell differentiation in BXD2 mice. IL-21 depletion then leads to an increased frequency of Tfr cells and transfer of those Tfr cells into young BXD2 mice largely restricts the size of the GC response and the production of autoreactive antibodies (86). In addition, intravenous immunoglobulin administration to mice with collagen-induced arthritis augments the number of Tfr cells and represses the subsequent maturation of GC B cells (87), which also supported the idea of a critical role for Tfr cells in autoimmune diseases.
Conclusions
In summary, CD4+ Tfh cells are a distinct cell subset from Th1, Th2, Th17 and Treg cells. In addition to the master regulator Bcl-6, other transcription factors have recently been identified to be essential for Tfh differentiation and function. Diverse regulatory mechanisms such as the control of the expression of Bcl-6 and other factors such as CXCR5, co-stimulatory or inhibitory receptors and cytokines, also play an indispensable role in Tfh cell development, maintenance and function.
Providing help for B-cell development and GC reactions is the most crucial function of Tfh cells via direct interaction and/or cytokine secretion, leading to B-cell maturation and high-affinity antibody production to defend against invading pathogens. On the other hand, Tfr cells function to constrain the “help” provided by Tfh cells and thus maintain the balance for GC reactions. Obviously, there are still many outstanding questions remaining unsolved, including their fate and differentiation, their interactions with other cell types and their detailed transcriptional or epigenetic programs.
Given that Tfh and Tfr cells are reciprocal and antagonistic regulators of GC responses, a balance of their actions is critical for immune homeostasis. An aberrant or disordered Tfh/Tfr ratio results in the disruption of this balance, excessive antibody production, and the development of autoimmune diseases. However, future mechanistic studies are needed to elucidate how the maintenance and function of Tfh or Tfr cells in GCs contribute to the initiation and expansion of humoral autoimmunity. Further understanding of Tfh and Tfr biology and their roles in disease development will benefit the discovery of new therapeutic targets for human diseases.
Conflict of interest statement: The authors declared no conflict of interests.
References
- 1. Zhu J. Yamane H. and Paul W. E. 2010. Differentiation of effector CD4 T cell populations (*). Annu. Rev. Immunol. 28:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakaguchi S. Yamaguchi T. Nomura T. and Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133:775. [DOI] [PubMed] [Google Scholar]
- 3. Breitfeld D., Ohl L., Kremmer E., et al. 2000. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 192:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim C. H. Rott L. S. Clark-Lewis I. Campbell D. J. Wu L. and Butcher E. C. 2001. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J. Exp. Med. 193:1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schaerli P. Willimann K. Lang A. B. Lipp M. Loetscher P. and Moser B. 2000. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 192:1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gunn M. D. Ngo V. N. Ansel K. M. Ekland E. H. Cyster J. G. and Williams L. T. 1998. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature 391:799. [DOI] [PubMed] [Google Scholar]
- 7. Chung Y., Tanaka S., Chu F., et al. 2011. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 17:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wollenberg I., Agua-Doce A., Hernandez A., et al. 2011. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J. Immunol. 187:4553. [DOI] [PubMed] [Google Scholar]
- 9. Linterman M. A., Pierson W., Lee S. K., et al. 2011. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621. [DOI] [PubMed] [Google Scholar]
- 11. Ma C. S. and Deenick E. K. 2014. Human T follicular helper (Tfh) cells and disease. Immunol. Cell Biol. 92:64. [DOI] [PubMed] [Google Scholar]
- 12. Johnston R. J., Poholek A. C., DiToro D., et al. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nurieva R. I., Chung Y., Martinez G. J., et al. 2009. Bcl6 mediates the development of T follicular helper cells. Science 325:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu D., Rao S., Tsai L. M., et al. 2009. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 31:457. [DOI] [PubMed] [Google Scholar]
- 15. Allman D., Jain A., Dent A., et al. 1996. BCL-6 expression during B-cell activation. Blood 87:5257. [PubMed] [Google Scholar]
- 16. Reljic R. Wagner S. D. Peakman L. J. and Fearon D. T. 2000. Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J. Exp. Med. 192:1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shaffer A. L. Yu X. He Y. Boldrick J. Chan E. P. and Staudt L. M. 2000. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 13:199. [DOI] [PubMed] [Google Scholar]
- 18. Shaffer A. L., Lin K. I., Kuo T. C., et al. 2002. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17:51. [DOI] [PubMed] [Google Scholar]
- 19. Ise W., Kohyama M., Schraml B. U., et al. 2011. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat. Immunol. 12:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X., Chen X., Zhong B., et al. 2014. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature 507:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu L., Cao Y., Xie Z., et al. 2015. The transcription factor TCF-1 initiates the differentiation of T cells during acute viral infection. Nat. Immunol. 16:991. [DOI] [PubMed] [Google Scholar]
- 22. Choi Y. S., Gullicksrud J. A., Xing S., et al. 2015. LEF-1 and TCF-1 orchestrate T differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat. Immunol. 16:980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong C., Juedes A. E., Temann U. A., et al. 2001. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409:97. [DOI] [PubMed] [Google Scholar]
- 24. Akiba H., Takeda K., Kojima Y., et al. 2005. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 175:2340. [DOI] [PubMed] [Google Scholar]
- 25. Nurieva R. I., Chung Y., Hwang D., et al. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vinuesa C. G., Cook M. C., Angelucci C., et al. 2005. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435:452. [DOI] [PubMed] [Google Scholar]
- 27. Athanasopoulos V., Barker A., Yu D., et al. 2010. The ROQUIN family of proteins localizes to stress granules via the ROQ domain and binds target mRNAs. FEBS J. 277:2109. [DOI] [PubMed] [Google Scholar]
- 28. Glasmacher E., Hoefig K. P., Vogel K. U., et al. 2010. Roquin binds inducible costimulator mRNA and effectors of mRNA decay to induce microRNA-independent post-transcriptional repression. Nat. Immunol. 11:725. [DOI] [PubMed] [Google Scholar]
- 29. Yu D., Tan A. H., Hu X., et al. 2007. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature 450:299. [DOI] [PubMed] [Google Scholar]
- 30. Gigoux M., Shang J., Pak Y., et al. 2009. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc. Natl Acad. Sci. USA 106:20371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rolf J. Fairfax K. and Turner M. 2010. Signaling pathways in T follicular helper cells. J. Immunol. 184:6563. [DOI] [PubMed] [Google Scholar]
- 32. Stone E. L., Pepper M., Katayama C. D., et al. 2015. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity 42:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao N. Eto D. Elly C. Peng G. Crotty S. and Liu Y. C. 2014. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat. Immunol. 15:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leavenworth J. W. Verbinnen B. Yin J. Huang H. and Cantor H. 2015. A p85α-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat. Immunol. 16:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu H., Li X., Liu D., et al. 2013. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 496:523. [DOI] [PubMed] [Google Scholar]
- 36. Jacquemin C., Schmitt N., Contin-Bordes C., et al. 2015. OX40 ligand contributes to human lupus pathogenesis by promoting T follicular helper response. Immunity 42:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi Y. S. Eto D. Yang J. A. Lao C. and Crotty S. 2013. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 190:3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vogelzang A. McGuire H. M. Yu D. Sprent J. Mackay C. R. and King C. 2008. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29:127. [DOI] [PubMed] [Google Scholar]
- 39. Bauquet A. T., Jin H., Paterson A. M., et al. 2009. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hiramatsu Y., Suto A., Kashiwakuma D., et al. 2010. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J. Leukoc. Biol. 87:703. [DOI] [PubMed] [Google Scholar]
- 41. Baumjohann D., Kageyama R., Clingan J. M., et al. 2013. The microRNA cluster miR-17 approximately 92 promotes TFH cell differentiation and represses subset-inappropriate gene expression. Nat. Immunol. 14:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang S. G., Liu W. H., Lu P., et al. 2013. MicroRNAs of the miR-17 approximately 92 family are critical regulators of T(FH) differentiation. Nat. Immunol. 14:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Victora G. D. and Nussenzweig M. C. 2012. Germinal centers. Annu. Rev. Immunol. 30:429. [DOI] [PubMed] [Google Scholar]
- 44. Zotos D., Coquet J. M., Zhang Y., et al. 2010. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ozaki K., Spolski R., Ettinger R., et al. 2004. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J. Immunol. 173:5361. [DOI] [PubMed] [Google Scholar]
- 46. Linterman M. A., Beaton L., Yu D., et al. 2010. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kishi Y., Aiba Y., Higuchi T., et al. 2010. Augmented antibody response with premature germinal center regression in CD40L transgenic mice. J. Immunol. 185:211. [DOI] [PubMed] [Google Scholar]
- 48. Bolduc A., Long E., Stapler D., et al. 2010. Constitutive CD40L expression on B cells prematurely terminates germinal center response and leads to augmented plasma cell production in T cell areas. J. Immunol. 185:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yusuf I., Kageyama R., Monticelli L., et al. 2010. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J. Immunol. 185:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cannons J. L., Qi H., Lu K. T., et al. 2010. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity 32:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yan Q., Malashkevich V. N., Fedorov A., et al. 2007. Structure of CD84 provides insight into SLAM family function. Proc. Natl Acad. Sci. USA 104:10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Good-Jacobson K. L. Szumilas C. G. Chen L. Sharpe A. H. Tomayko M. M. and Shlomchik M. J. 2010. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat. Immunol. 11:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fife B. T., Pauken K. E., Eagar T. N., et al. 2009. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 10:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lim H. W. Hillsamer P. and Kim C. H. 2004. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J. Clin. Invest. 114:1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gerner M. Y. Torabi-Parizi P. and Germain R. N. 2015. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 42:172. [DOI] [PubMed] [Google Scholar]
- 56. Kerfoot S. M., Yaari G., Patel J. R., et al. 2011. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 34:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sage P. T. Alvarez D. Godec J. von Andrian U. H. and Sharpe A. H. 2014. Circulating T follicular regulatory and helper cells have memory-like properties. J. Clin. Invest. 124:5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sage P. T. Francisco L. M. Carman C. V. and Sharpe A. H. 2013. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 14:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vaeth M., Muller G., Stauss D., et al. 2014. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. J. Exp. Med. 211:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wing J. B. Ise W. Kurosaki T. and Sakaguchi S. 2014. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity 41:1013. [DOI] [PubMed] [Google Scholar]
- 61. Chang J. H., Hu H., Jin J., et al. 2014. TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J. Exp. Med. 211:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miyazaki M., Miyazaki K., Chen S., et al. 2014. Id2 and Id3 maintain the regulatory T cell pool to suppress inflammatory disease. Nat. Immunol. 15:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Conti P., Kempuraj D., Kandere K., et al. 2003. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol. Lett. 86:123. [DOI] [PubMed] [Google Scholar]
- 64. Hata H., Sakaguchi N., Yoshitomi H., et al. 2004. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J. Clin. Invest. 114:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Houssiau F. A. Lefebvre C. Vanden Berghe M. Lambert M. Devogelaer J. P. and Renauld J. C. 1995. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus 4:393. [DOI] [PubMed] [Google Scholar]
- 66. Llorente L., Richaud-Patin Y., Couderc J., et al. 1997. Dysregulation of interleukin-10 production in relatives of patients with systemic lupus erythematosus. Arthritis Rheum. 40:1429. [DOI] [PubMed] [Google Scholar]
- 67. Llorente L., Zou W., Levy Y., et al. 1995. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J. Exp. Med. 181:839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peng H. Wang W. Zhou M. Li R. Pan H. F. and Ye D. Q. 2013. Role of interleukin-10 and interleukin-10 receptor in systemic lupus erythematosus. Clin. Rheumatol. 32:1255. [DOI] [PubMed] [Google Scholar]
- 69. Cai G., Nie X., Zhang W., et al. 2012. A regulatory role for IL-10 receptor signaling in development and B cell help of T follicular helper cells in mice. J. Immunol. 189:1294. [DOI] [PubMed] [Google Scholar]
- 70. McCarron M. J. and Marie J. C. 2014. TGF-beta prevents T follicular helper cell accumulation and B cell autoreactivity. J. Clin. Invest. 124:4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Okamura T., Morita K., Iwasaki Y., et al. 2015. Role of TGF-beta3 in the regulation of immune responses. Clin. Exp. Rheumatol. 33:63. [PubMed] [Google Scholar]
- 72. Zhao D. M. Thornton A. M. DiPaolo R. J. and Shevach E. M. 2006. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood 107:3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sage P. T. and Sharpe A. H. 2015. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 36:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Crotty S. 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity 41:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. He J., Tsai L. M., Leong Y. A., et al. 2013. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 39:770. [DOI] [PubMed] [Google Scholar]
- 76. Simpson N., Gatenby P. A., Wilson A., et al. 2010. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 62:234. [DOI] [PubMed] [Google Scholar]
- 77. Szabo K. Papp G. Barath S. Gyimesi E. Szanto A. and Zeher M. 2013. Follicular helper T cells may play an important role in the severity of primary Sjögren’s syndrome. Clin. Immunol. 147:95. [DOI] [PubMed] [Google Scholar]
- 78. Xu H., Liu J., Cui X., et al. 2015. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol. 295:46. [DOI] [PubMed] [Google Scholar]
- 79. Choi J. Y., Ho J. H., Pasoto S. G., et al. 2015. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. 67:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang X., Lindwall E., Gauthier C., et al. 2015. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus 24:909. [DOI] [PubMed] [Google Scholar]
- 81. Ma J., Zhu C., Ma B., et al. 2012. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin. Dev. Immunol. 2012:827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang J., Shan Y., Jiang Z., et al. 2013. High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin. Exp. Immunol. 174:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liu R., Wu Q., Su D., et al. 2012. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res. Ther. 14:R255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jang E. Cho S. H. Park H. Paik D. J. Kim J. M. and Youn J. 2009. A positive feedback loop of IL-21 signaling provoked by homeostatic CD4+CD25- T cell expansion is essential for the development of arthritis in autoimmune K/BxN mice. J. Immunol. 182:4649. [DOI] [PubMed] [Google Scholar]
- 85. Zhu C., Ma J., Liu Y., et al. 2012. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 97:943. [DOI] [PubMed] [Google Scholar]
- 86. Ding Y., Li J., Yang P., et al. 2014. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol. 66:2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee S. Y., Jung Y. O., Ryu J. G., et al. 2014. Intravenous immunoglobulin attenuates experimental autoimmune arthritis by inducing reciprocal regulation of Th17 and Treg cells in an interleukin-10-dependent manner. Arthritis Rheumatol. 66:1768. [DOI] [PubMed] [Google Scholar]