T-helper cell subsets and diseases
Keywords: epigenetics, memory Th2 cells, pathogenic Th2 (Tpath2) cells, T-cell plasticity, Th1/Th2 paradigm
Abstract
CD4+ T cells are crucial for directing appropriate immune responses during host defense and for the pathogenesis of inflammatory diseases. In addition to the classical biphasic model of differentiation of T-helper 1 (Th1) and Th2 cells, unexpected increases in the numbers of CD4+ T-cell subsets, including Th17, Th9, T follicular-helper (Tfh) and T-regulatory (Treg) cells, have been recognized. In the present review, we focus on how these various T-helper cell subsets contribute to the pathogenesis of immune-mediated inflammatory diseases. In particular, we focus on multiple sclerosis, psoriasis and asthma as typical model diseases in which multiple T-helper cell subsets have recently been suggested to play a role. We will also discuss various unique sub-populations of T-helper cells that have been identified. First, we will introduce the heterogeneous T-helper cell subsets, which are classified by their simultaneous expression of multiple key transcription factors. We will also introduce different kinds of memory-type Th2 cells, which are involved in the pathogenesis of chronic type-2 immune-related diseases. Finally, we will discuss the molecular mechanisms underlying the generation of the plasticity and heterogeneity of T-helper cell subsets. The latest progress in the study of T-helper cell subsets has forced us to reconsider the etiology of immune-mediated inflammatory diseases beyond the model based on the Th1/Th2 balance. To this end, we propose another model—the pathogenic T-helper population disease-induction model—as a possible mechanism for the induction and/or persistence of immune-mediated inflammatory diseases.
Introduction
CD4+ T cells are critical for immune responses during host defense against harmful microorganisms. In addition to their key roles as helper cells, they may play a pathogenic role as drivers of autoimmune diseases and allergies, including asthma. We herein discuss the diverse effects of T-helper cells on the pathology of immune-related disorders. Our discussion is based on two concepts: (i) the classical T-helper 1 (Th1)/Th2 paradigm and (ii) a new concept, about disease induction, based on recent evidence of the existence of heterogeneous memory T-helper cell subsets. We summarize the association between inflammatory diseases and the complexity of the CD4+ T-cell subsets, including Th1, Th2, Th17, Th9, Th22, T follicular-helper (Tfh) cells and T-regulatory (Treg) cells. We need to consider the impact of this complex fate decision on the multifaceted pathology of immune-related diseases.
Moreover, we review the epigenetic modifications including super-enhancers that influence cell identity, plasticity of the T-helper cell subsets and genetic risk of diseases. The new insights are especially relevant to the development of new therapeutic strategies for immune-mediated inflammatory diseases, as we still face difficulties in treating most immune-mediated inflammatory diseases.
Beyond the Th1/Th2 paradigm
The Th1/Th2 paradigm and immune responses during host defense against microorganisms
For more than two decades, T-helper cells were thought to be limited to two major subsets, Th1 and Th2 cells. This paradigm is based on their production of specific cytokines (IFN-γ and IL-4, respectively) (1). The cytokines IL-12 and IL-4 are known to signal the induction of Th1 and Th2 cell differentiation, respectively. Th1 cells are crucial for host defense against intracellular pathogens including Leishmania major and Mycobacterium tuberculosis, whereas Th2 cells are known to be important for the elimination of helminthic parasites such as Nippostrongylus brasiliensis (2, 3). The Th1/Th2 paradigm is useful for classifying the immune responses that occur in the elimination of microbial pathogens.
CD4+ T cell subsets and immune-mediated inflammatory diseases
The Th1 cell subset and inflammatory diseases.
IFN-γ-producing Th1 cells have long been recognized to contribute to the pathogenicity of organ-specific autoimmune diseases such as autoimmune type 1 diabetes and multiple sclerosis (4–6). For instance, the pathogenic roles of Th1 cells have been well described in several mouse disease models, including experimental autoimmune encephalomyelitis (EAE), which is a mouse model of multiple sclerosis (6). The adoptive transfer of Th1 cells was found to exacerbate EAE (7). Moreover, the genetic abrogation of T-bet, which is a key transcription factor for Th1 cell differentiation, resulted in resistance to EAE (8).
The Th17 cell subset as a new player in the pathology of inflammatory diseases.
The finding of the pathogenic role of IL-23 in EAE suggested that another T-helper cell subset, distinct from Th1 cells, is involved in this inflammatory neural disease (9). Both IL-12 and IL-23 are heterodimeric cytokines and consist of p40–p35 and p40–p19, respectively. Mice that lack the IL-12p35 subunit have been shown to develop much more severe disease. In sharp contrast, the genetic abrogation of the p19 subunit of IL-23 resulted in resistance to EAE (9).
Around the same period, it was appreciated that the selective production of IL-17 by a distinct T-helper cell subset was induced by IL-23 stimulation (10), although the pathophysiological roles of the cytokine IL-17 in human arthritis have been in focus since the late 1990s (11). These findings led to the identification of an IL-17-producing population of CD4+ T cells, termed Th17 cells (9, 12–14), and the recognition of Th17 cells has helped to understand the contrasting findings in EAE. IL-27 is an important negative regulator of Th17 differentiation, which also induces Th1 differentiation (15–18). The hyper-susceptibility of IL-27R-deficient mice with elevated numbers of Th17 cells to EAE also suggests the importance of Th17 cells in the pathology of EAE (19).
Psoriasis, a chronic inflammatory immune-mediated disease of the skin and joints, is another example of a disease in which both Th1 and Th17 cells are involved (20). Patients with psoriasis develop erythematous scaly papules and plaques. Around one-third of patients develop a so-called psoriatic joint, which is sometimes accompanied by severe joint destruction (21). For more than 20 years, type 1 responses were thought to play a central role in the pathology of psoriasis, because of the presence of IL-12-expressing dendritic cells and Th1 cells, which secrete IFN-γ and TNF (22, 23). Recently, however, accumulating evidence suggests that IL-17-producing Th17 cells may play a crucial role in the pathology of psoriasis (20), and monoclonal antibodies that interfere with IL-17 action such as ixekizumab and secukinumab (both of which bind IL-17A) appear to be effective for reducing the symptoms of psoriasis (24, 25).
Thus, Th17 cells have been demonstrated to have critical pathogenic roles in the pathogenesis of human autoimmune diseases and a variety of mouse models of autoimmune diseases. Th17 cells also contribute to the host defense against extracellular bacteria such as Staphylococcus aureus and Klebsiella pneumonia and against fungi (26, 27).
The Th2, Th9 and Th17 cell subsets and allergic diseases.
The pathophysiological importance of cytokines produced by Th2 cells, namely IL-4, IL-5 and IL-13, has been demonstrated in allergic inflammation, such as human asthma, as well as in animal models of allergic airway inflammation (28–31); the involvement of Th2 cytokines in the pathophysiology of airway inflammation, eosinophilia, the hyper-production of mucus, fibrosis and other responses is well recognized (32–34). In addition to Th2 cells, IL-9-producing T-helper cells, namely Th9 cells, make up another key T-helper cell subset, which is involved in the pathology of airway inflammation (35, 36).
In addition to the pathogenic role of Th2 and Th9 cytokines in airway inflammation, emerging evidence suggests that Th17 cells are also key players in chronic lung inflammation, including asthma (37–41). The massive infiltration of neutrophils in the lung is a well-known clinical feature of steroid-resistant asthma and is an important pathogenic action of IL-17; the recruitment of neutrophils to the lung during airway inflammation likely plays a key role in steroid-resistant asthma (37–40, 42–45). IL-17 also directly affects the airway smooth muscle by inducing allergen-induced airway hyper-responsiveness (40).
Recent research has highlighted that the expression patterns of the Th2 and Th17 signature genes in the bronchial epithelial cells of asthmatics are mutually exclusive, which suggests that the etiology of asthma is heterogeneous and indicates the reciprocal regulation of the Th2 and Th17 inflammatory pathways in asthma (46). Interestingly, IL-21, a cytokine that is known to be a potent inducer of Th17 cell differentiation (47), can also promote Th2 cell responses together with IL-25 in a mouse model of house-dust asthma (48).
Th17 cells can also produce IL-22, which has been shown to have protective roles in the epithelial barrier functions of the lung and the gut (49). There are positive correlations between the expression level of IL-22 and the severity of allergic diseases such as asthma and atopic dermatitis (50, 51). In humans, the identification of a population of CD4+ T cells that produce IL-22 without the expression of IL-17, IL-4 or IFN-γ has led to the notion of ‘Th22’ cells (52). However, recent research using a fate-mapping mouse model has suggested that, in mice, IL-22 induction might not be strongly associated with a particular T-helper cell subset (53).
Taken together, these findings indicate that the clinical features of asthma are heterogeneous (46, 54, 55), and that the involvement of several T-helper cell subsets in asthma might be one of the reasons for the heterogeneity of this disease.
The Tfh cell subset and immune-mediated inflammatory diseases.
Tfh cells contribute to both host protection and immune-mediated inflammatory diseases by providing B-cell help and antibody production. These cells are identified on the basis of their location in the germinal centers in vivo and by the surface expression of the CXC chemokine receptor 5 (CXCR5) and programmed cell death 1 molecules (56). Tfh cells are critical, for example, for the production of neutralizing antibodies against HIV (57, 58).
Tfh cells are also involved in various immune-related diseases. As lineage-specific cytokines including IFN-γ, IL-4 and IL-17 can be produced by Tfh cells (56), it may be possible that Tfh cells contribute to immune-mediated inflammatory diseases both redundantly and distinctly. For example, IgE is well known as a key player in the pathophysiology of allergies and asthma (59) and Tfh cells are important for providing B-cell help to generate IgE-producing plasma cells. Moreover, patients with systemic lupus erythematous (SLE) have increased numbers of IL-17-producing T cells, which stimulate B-cell antibody production and infiltrate the kidney (60, 61). The expression of OX40 ligand on antigen-presenting cells has been recently revealed to control the activity of Tfh cells and the pathogenicity of SLE (62).
The Treg cell subset as a regulator of immune-mediated diseases.
In contrast to effector T-helper cell subsets, Treg cells are essential for the maintenance of immunological self-tolerance and the prevention of various autoimmune diseases (63–67). The importance of immunological tolerance is particularly evident in mice and humans that are missing transcription factor forkhead box P3 (Foxp3), which drives the specification of this subset (64–67). Treg cells can be divided into thymus-derived Treg (tTreg) cells and peripherally induced Treg (pTreg) cells (68); these two sub-populations of Treg cells are distinguished by several markers, including Neuropilin 1 and Helios (69–71). pTreg cells are known to control allergic inflammation in the lung and gut (65). Various mechanisms, such as the consumption of IL-2 by Treg cells and CTLA-4 expression on Treg cells, are suggested to be crucial for their suppressive activity (64, 72).
Taken together, it has become clear that CD4+ T cells execute host defense immune responses and that they are involved in immune-mediated inflammatory diseases through their multiple distinct fates. These new insights will provide a more sophisticated understanding of immune-mediated inflammatory diseases and new curative approaches.
Heterogeneity of CD4+ T-cell subsets and immune-mediated inflammatory diseases
Heterogeneity of CD4+ T-cell subsets and key transcriptional factors.
As mentioned above, the diversity of CD4+ T cells—Th1, Th2, Th17, Th9, Th22, Tfh cells and Treg cells—is now widely acknowledged (36, 56, 64, 73, 74). Extrinsic factors, in particular cytokines, are crucial for the appropriate differentiation of these CD4+ T-cell subsets because they activate transcription factors, including signal transducers and activators of transcription (STATs) (75). The combination of TCR and STAT signals is important for the induction of subset-specific transcription factors (75). Th1, Th2, Th17, Th9, Tfh and Treg cells express T-bet (encoded by Tbx21), GATA-binding protein 3 (GATA3), retinoic acid receptor-related orphan receptor γt (Rorγt), SFFV proviral integration 1 (PU.1), B-cell lymphoma 6 (Bcl6), and FoxP3, respectively (14, 76–80). Beyond these transcription factors, there are several key transcription factors for the differentiation of CD4+ T cells. For instance, Runt-related transcription factor (Runx3) is crucial for Th1 differentiation and Blimp-1 (encoded by Prdm1) is a crucial negative regulator for Tfh differentiation (79, 81).
At the same time, the flexibility and heterogeneity of CD4+ T-cell subsets have recently been described (82–84). In fact, CD4+ T cells can express multiple ‘subset-specific’ transcription factors. For example, it is now known that T-bet is expressed in GATA3+ Th2 cells, Rorγt+ Th17 cells, Foxp3+ Treg cells and Bcl6+ Tfh cells (85–88).
The expression of multiple transcription factors can help to provide specialized functions to sub-populations of CD4+ T-cell subsets. In the case of Treg cells, diverse and unique sub-populations, in which Foxp3 is co-expressed with T-bet, GATA3, Bcl6 or STAT3, work to regulate the Th1, Th2, Tfh or Th17 responses in vivo (87, 89–92). For example, IL-10R-expressing Treg cells can respond to IL-10 signaling to activate STAT3 and are crucial for the regulation of Th17 immune responses in vivo (91).
A member of the IL-1 family, IL-33 is expressed constitutively in epithelial cells and works as an alarmin in response to tissue damage (93). Colonic Treg cells expressing the IL-33 receptor (ST2) can also express GATA3 and control intestinal inflammation via IL-33 signaling (92). After stimulation by IL-33, ST2+ Treg cells show a protective role against viral infection in the lung through the induction of amphiregulin, which is a member of the epidermal growth factor family and is known to be a key protein for tissue repair (94, 95). Another type of Treg cell with a unique in vivo activity are Rorγt+Foxp3+ pTreg cells, which are induced by the microbiota and vitamin A in the gut and which regulate type-2 immune responses (96, 97).
Heterogeneity of the memory-type T-helper cell subsets and organ-specific chronic inflammatory diseases.
Recent technological progress has allowed us to investigate genetic expression at the single-cell level among T-cell subsets (98, 99). These investigations have shown that the populations of T-cell subsets, including memory CD8+ T cells, are more heterogeneous than we anticipated (99). In the case of CD4+ T cells, various types of effector and memory Th2 cells are involved in the type-2 immune responses (98, 100–103).
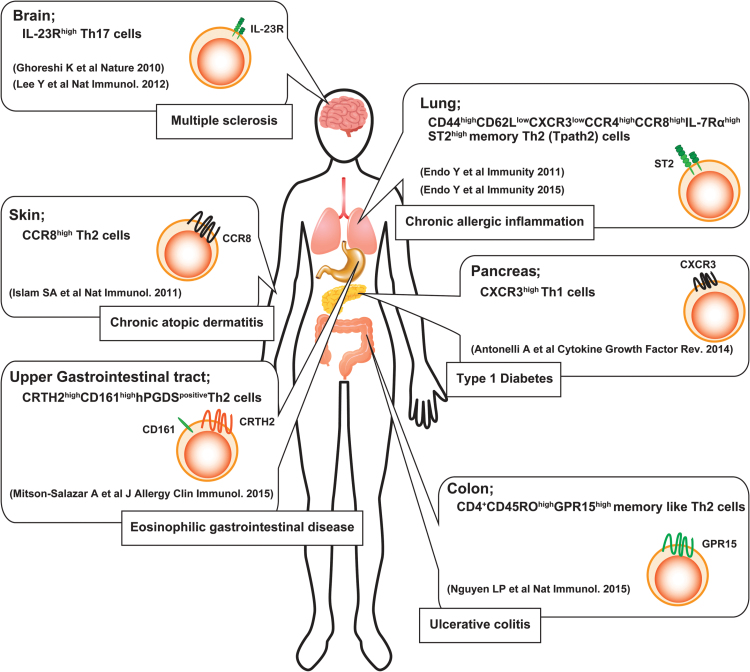
Among these sub-populations, IL-5-producing Th2 cells are known to contribute to the pathogenicity of immune-mediated inflammatory diseases in both humans and mice (100–107). For example, a certain population of memory Th2 cells, which shows low expression of both CXCR3 and CD62L, plays a crucial role in the pathogenicity of chronic allergic airway inflammation, since this memory Th2 subset, namely pathogenic Th2 (Tpath2) cells, has the ability to produce a large amount of IL-5 (100, 101). IL-33 confers the induction of memory-type Tpath2 cells from effector Th2 cells through the enhancement of ST2 expression on T cells both in vitro and in vivo (101), whereas CCR8+ memory-type Th2 cells are key pathogenic players, which induce the production of high levels of IL-5 during chronic skin inflammation (102, 108). G-protein-coupled receptor 15 (GPR15) is known as a lymphocyte trafficking receptor (109). It has recently been demonstrated that CD4+CD45RO+GPR15+ memory-type Th2 cells are found in increased numbers in the colon of patients with ulcerative colitis (106).
These results indicate that the heterogeneity of memory-type Th2 cells among different organs could be crucial for the pathology of chronic immune-mediated diseases (Fig. 1). Interestingly, a recent single-cell analysis revealed that a certain population of effector Th2 cells had a suppressive function through the de novo synthesis of steroids (98).
Fig. 1.
The heterogeneity of pathogenic T-helper (Tpath) cells. Recent advances in research point out several lines of evidence of the heterogeneity of Tpath cells, which play a critical role in the pathogenesis of various immune-mediated diseases in an organ-specific manner.
Taken together, the evidence from recent studies implies that T-helper cells show far more plasticity and heterogeneity than we previously imagined. Precise assessments regarding the nature of T-helper cells will be indispensible in the development of new therapeutic approaches for these immune-related diseases.
Molecular mechanisms underlying CD4+ T-cell stability—the intersection of epigenetics and immune-mediated inflammatory diseases
The term ‘epigenetic’ was originally used to refer to inheritable changes in genetic expression that are not due to changes in the DNA sequence (110). At present, however, the term ‘epigenetic change’ refers to different chromatin alterations, such as histone tail modifications, DNA methylation and chromatin conformation and interaction. Regarding histone tail modifications, histone H3 lysine 36 trimethylation (H3K36me3) is a marker of an actively transcribed coding region and histone H3 lysine 4 trimethylation (H3K4me3) is associated with active promoters. Histone H3 lysine 4 monomethylation (H3K4me1) and histone H3 lysine 4 dimethylation (H3K4me2) are enhancer-related marks, whereas histone H3 lysine 27 trimethylation (H3K27me3) and histone H3 lysine 9 trimethylation (H3K9me3) encompass silenced genes.
Consistent with the plasticity of T-helper cell differentiation, genes encoding key transcription factors, including Tbx21, Gata3, Bcl6, Runx3 and Prdm1 exhibit both accessible and repressive marks (111). These bivalent modifications might be one of the mechanisms underlying the plasiticity of the T-helper cell subsets. These histone marks are mediated by a variety of enzyme complexes including the Polycomb and Trithorax complexes (112–116).
It is now well appreciated that epigenetic regulation through the Trithorax and Polycomb complexes is important for the functions of T-helper cells (113, 114). For example, CD4+ T cells that lack the Polycomb proteins Mel-18, Bmi-1 and Ring1B exhibit impaired Th2 cell differentiation (117–119). The deletion of the Polycomb protein Ezh2 results in spontaneous cytokine production of both IFN-γ and Th2 cytokines (120). Moreover, the loss of Ezh2 has been associated with enhanced pathology and the accumulation of memory Th2 cells in an allergic airway inflammation model (120). The disruption of Trithorax impairs the maintenance of the Th2 cell signature in memory Th2 cells (121).
Another important factor in determining whether or not genes can be ‘read’ is DNA methylation. For example, the DNA-methylation adaptor protein, Uhrf1, controls the homeostasis of intestinal Treg cells through the silencing of Cdkn1a (122).
Cis-regulatory elements are well appreciated as the DNA elements that control genetic expression and which constitute an integral part of gene structure. Among the many regulatory elements in non-coding DNA, enhancers play key roles in appropriate gene expression. Enhancers are non-coding DNA sequences that enhance the transcription of cognate target genes. Originally evidence of enhancer function relied on certain conserved non-coding sequences (CNSs) and DNase hypersensitivity sites (123). In T-helper cells, the Th2 cytokine loci have been well investigated as part of the complex architecture of reasonably regulated lineage-specific genes (124, 125). In particular, the IL4/Il4 loci are composed of a number of enhancers and a silencer element (125).
In addition to CNS and DNase hypersensitivity sites, it is now appreciated that H3K4me1high and H3K4me3low are chromatin signatures of ‘active’ and ‘poised’ enhancers in human cells (126). H3K4me2 modification also reveals both ‘active’ and ‘poised’ enhancers and this histone tail marker allows for the more precise localization of enhancers in comparison to that which is provided by an analysis of H3K4me1 (127).
Additional indicators such as the binding of acetyltransferase p300 or H3K27Ac have been related to ‘active’ enhancers. Emerging data show that these epigenetic modifications of the cis-regulatory region are closely related to human diseases including asthma, ulcerative colitis and allergy (128–131). For example, a genome-wide study of histone modifications identified disease-associated enhancers in CD4+ T cells in samples from asthmatic patients (129–131). Enhancers that gain the H3K4me2 mark during Th2 cell differentiation in asthmatic patients reveal the enhanced enrichment of asthma-associated single nucleotide polymorphisms (130). Moreover, super-enhancers, which consist of clusters of enhancers, were recently identified as key DNA regulatory elements for the control of cell-type-specific gene expression and genetic risk of diseases (131–133).
In summary, all of these epigenetic modulators work together to influence the accessibility of genes to the actions of transcription factors and promote appropriate gene expression. The breakdown of the orchestrated systems causes immune-mediated inflammatory diseases. It is therefore crucial for us to elucidate the detailed molecular mechanisms underlying the T-helper cell fate decisions.
The pathogenic T-helper population disease-induction model—toward the development of specific immune therapy
In accordance with the increased complexity and heterogeneity of T-helper cell subsets, we realized that we have to reconsider the pathogenicity of inflammatory diseases beyond the ‘classical Th1/Th2 balance disease-induction model’. Specifically, the heterogeneity of T-helper cells implies that a specific population of T-helper cells dictates the pathology of immune-mediated inflammatory diseases. We proposed a ‘pathogenic T-helper population disease-induction model’ in 2014 (134). In this model, regardless of the balance of Th1/Th2, the pathogenesis of so-called Th1-mediated or Th2-mediated diseases is largely dependent on the ‘pathogenic sub-populations’ of each T-helper cell subset that are generated in vivo and possess a distinct feature of effector function. In particular, in Th2-type pathology such as asthma or chronic dermatitis, IL-5-producing or IL-17-producing Th2 cell sub-populations are considered to be pathogenic populations.
Consistent with this new disease-induction model, several groups including us have reported that various distinct sub-populations of T-helper cells are identified in mice and humans, and play a big role in the pathogenesis of certain types of immune-mediated inflammatory diseases (Fig. 1). For example, Tpath2 cells are involved in chronic allergic inflammation of the airway, eosinophilic chronic rhinosinusitis, chronic atopic dermatitis, ulcerative colitis and eosinophilic gastrointestinal diseases in both mice and humans (100–103, 106). In the case of Th1 cells, CXCR3high Th1 cells are known to be crucial for the pathogenicity of type 1 diabetes (135). Moreover, pathogenic Th17 cells are induced under conditions without TGF-β1 or with TGF-β3 (86, 136). A recent report shows that AIM (CD5L) restrains Th17 cell pathogenicity through regulation of lipid biosynthesis (137).
Taken together, we consider a key to understand the pathogenicity of immune-mediated inflammatory diseases is a ‘pathogenic T-helper population disease-induction model’. In the model, a pathogenic sub-population of T-helper cells, which is induced under certain conditions, is rather critical for the pathogenesis of immune-mediated diseases than the balance among the T-helper cell subsets (134) (Fig. 2). Therefore, pathogenic T cells (Tpath cells; Tpath1, Tpath2 and Tpath17) could be good specific-therapeutic targets for immune-mediated intractable inflammatory diseases.
Fig. 2.
A schematic representation of the ‘classical Th1/Th2 balance disease-induction model’ and the ‘pathogenic T-helper population disease-induction model’. Since the 1980s, the balance among the T-helper cell subsets has been considered a key component for the pathogenesis of immune-mediated inflammatory diseases such as allergic diseases and autoimmune diseases. In the ‘pathogenic T-helper population disease-induction model’, regardless of the balance among T-helper cell subsets, a pathogenic sub-population of T-helper cells plays a critical role in the pathogenesis of these immune-mediated inflammatory diseases.
Conclusions
We have briefly reviewed the recent progress of research on the association between various CD4+ T-cell subsets and immune-mediated inflammatory diseases. There has been increasing evidence regarding the heterogeneity of T-helper cell subsets, particularly Tpath cells. The heterogeneity of Tpath cells might be involved in treatment resistance (to various types of treatment) that is observed in various immune-mediated inflammatory diseases. In order to develop new therapeutic strategies for the intractable immune-mediated inflammatory diseases, it will therefore become increasingly important for immunologists and clinicians to understand the precise features of Tpath cells.
Funding
This work was supported by AMED-CREST, The Japan Agency for Medical Research and Development (AMED), and a grant from the Ministry of Education, Culture, Sports, Science and Technology (MEXT Japan; Program for Leading Graduated School, Grants-in-Aid for Scientific Research (S) #26221305), the Astellas Foundation for Research on Metabolic Disorders, The Uehara Memorial Foundation, Osaka Foundation for Promotion of Fundamental Medical Research, Kanae Foundation for the Promotion of Medical Science, Takeda Science Foundation and The Naito Foundation.
References
- 1. Mosmann T. R. and Coffman R. L. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145. [DOI] [PubMed] [Google Scholar]
- 2. Reiner S. L. and Locksley R. M. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151. [DOI] [PubMed] [Google Scholar]
- 3. Pulendran B. and Artis D. 2012. New paradigms in type 2 immunity. Science 337:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szabo S. J. Sullivan B. M. Peng S. L. and Glimcher L. H. 2003. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 21:713. [DOI] [PubMed] [Google Scholar]
- 5. Christen U. and von Herrath M. G. 2004. Manipulating the type 1 vs type 2 balance in type 1 diabetes. Immunol. Res. 30:309. [DOI] [PubMed] [Google Scholar]
- 6. Sospedra M. and Martin R. 2005. Immunology of multiple sclerosis. Annu. Rev. Immunol. 23:683. [DOI] [PubMed] [Google Scholar]
- 7. McDonald A. H. and Swanborg R. H. 1988. Antigen-specific inhibition of immune interferon production by suppressor cells of autoimmune encephalomyelitis. J. Immunol. 140:1132. [PubMed] [Google Scholar]
- 8. Szabo S. J. Kim S. T. Costa G. L. Zhang X. Fathman C. G. and Glimcher L. H. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655. [DOI] [PubMed] [Google Scholar]
- 9. Cua D. J., Sherlock J., Chen Y., et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744. [DOI] [PubMed] [Google Scholar]
- 10. Aggarwal S., Ghilardi N., Xie M. H., de Sauvage F. J., Gurney A. L. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910. [DOI] [PubMed] [Google Scholar]
- 11. Attur M. G. Patel R. N. Abramson S. B. and Amin A. R. 1997. Interleukin-17 up-regulation of nitric oxide production in human osteoarthritis cartilage. Arthritis Rheum. 40:1050. [DOI] [PubMed] [Google Scholar]
- 12. Veldhoen M. Hocking R. J. Atkins C. J. Locksley R. M. and Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179. [DOI] [PubMed] [Google Scholar]
- 13. Bettelli E., Carrier Y., Gao W., et al. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235. [DOI] [PubMed] [Google Scholar]
- 14. Ivanov I. I., McKenzie B. S., Zhou L., et al. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121. [DOI] [PubMed] [Google Scholar]
- 15. Yoshida H., Hamano S., Senaldi G., et al. 2001. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 15:569. [DOI] [PubMed] [Google Scholar]
- 16. Villarino A., Hibbert L., Lieberman L., et al. 2003. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19:645. [DOI] [PubMed] [Google Scholar]
- 17. Hirahara K., Ghoreschi K., Yang X. P., et al. 2012. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity 36:1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirahara K., Onodera A., Villarino A. V., et al. 2015. Asymmetric action of STAT transcription factors drives transcriptional outputs and cytokine specificity. Immunity 42:877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Batten M., Li J., Yi S., et al. 2006. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 7:929. [DOI] [PubMed] [Google Scholar]
- 20. Belge K. Bruck J. and Ghoreschi K. 2014. Advances in treating psoriasis. F1000prime Rep. 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perera G. K. Di Meglio P. and Nestle F. O. 2012. Psoriasis. Annu. Rev. Pathol. 7:385. [DOI] [PubMed] [Google Scholar]
- 22. Yawalkar N. Karlen S. Hunger R. Brand C. U. and Braathen L. R. 1998. Expression of interleukin-12 is increased in psoriatic skin. J. Invest. Dermatol. 111:1053. [DOI] [PubMed] [Google Scholar]
- 23. Ghoreschi K., Thomas P., Breit S., et al. 2003. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat. Med. 9:40. [DOI] [PubMed] [Google Scholar]
- 24. Langley R. G., Elewski B. E., Lebwohl M., et al. 2014. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 371:326. [DOI] [PubMed] [Google Scholar]
- 25. Griffiths C. E., Reich K., Lebwohl M., et al. 2015. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 386:541. [DOI] [PubMed] [Google Scholar]
- 26. Ye P., Rodriguez F. H., Kanaly S., et al. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stockinger B. Veldhoen M. and Martin B. 2007. Th17 T cells: linking innate and adaptive immunity. Semin. Immunol. 19:353. [DOI] [PubMed] [Google Scholar]
- 28. Wills-Karp M. 2004. Interleukin-13 in asthma pathogenesis. Immunol. Rev. 202:175. [DOI] [PubMed] [Google Scholar]
- 29. Cohn L. Elias J. A. and Chupp G. L. 2004. Asthma: mechanisms of disease persistence and progression. Annu. Rev. Immunol. 22:789. [DOI] [PubMed] [Google Scholar]
- 30. Umetsu D. T. and DeKruyff R. H. 2006. The regulation of allergy and asthma. Immunol. Rev. 212:238. [DOI] [PubMed] [Google Scholar]
- 31. Hirahara K., Yamashita M., Iwamura C., et al. 2008. Repressor of GATA regulates TH2-driven allergic airway inflammation and airway hyperresponsiveness. J. Allergy Clin. Immunol. 122:512. [DOI] [PubMed] [Google Scholar]
- 32. Finkelman F. D. Hogan S. P. Hershey G. K. Rothenberg M. E. and Wills-Karp M. 2010. Importance of cytokines in murine allergic airway disease and human asthma. J. Immunol. 184:1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rothenberg M. E. and Hogan S. P. 2006. The eosinophil. Annu. Rev. Immunol. 24:147. [DOI] [PubMed] [Google Scholar]
- 34. Wills-Karp M., Luyimbazi J., Xu X., et al. 1998. Interleukin-13: central mediator of allergic asthma. Science 282:2258. [DOI] [PubMed] [Google Scholar]
- 35. Kerzerho J., Maazi H., Speak A. O., et al. 2013. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J. Allergy Clin. Immunol. 131:1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaplan M. H. Hufford M. M. and Olson M. R. 2015. The development and in vivo function of T helper 9 cells. Nat. Rev. Immunol. 15:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McKinley L., Alcorn J. F., Peterson A., et al. 2008. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J. Immunol. 181:4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson R. H. Whitehead G. S. Nakano H. Free M. E. Kolls J. K. and Cook D. N. 2009. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 180:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alcorn J. F. Crowe C. R. and Kolls J. K. 2010. TH17 cells in asthma and COPD. Annu. Rev. Physiol. 72:495. [DOI] [PubMed] [Google Scholar]
- 40. Kudo M., Melton A. C., Chen C., et al. 2012. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 18:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watanabe Y., Onodera A., Kanai U., et al. 2014. Trithorax complex component Menin controls differentiation and maintenance of T helper 17 cells. Proc. Natl Acad. Sci. USA 111:12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Molet S., Hamid Q., Davoine F., et al. 2001. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 108:430. [DOI] [PubMed] [Google Scholar]
- 43. Gibson P. G. Simpson J. L. and Saltos N. 2001. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 119:1329. [DOI] [PubMed] [Google Scholar]
- 44. Laan M. Palmberg L. Larsson K. and Lindén A. 2002. Free, soluble interleukin-17 protein during severe inflammation in human airways. Eur. Respir. J. 19:534. [DOI] [PubMed] [Google Scholar]
- 45. Chakir J. Shannon J. Molet S. Fukakusa M. Elias J. Laviolette M. Boulet L. P. and Hamid Q. 2003. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J. Allergy Clin. Immunol. 111:1293. [DOI] [PubMed] [Google Scholar]
- 46. Choy D. F., Hart K. M., Borthwick L. A., et al. 2015. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci. Transl. Med. 7:301ra129. [DOI] [PubMed] [Google Scholar]
- 47. Korn T., Bettelli E., Gao W., et al. 2007. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coquet J. M., Schuijs M. J., Smyth M. J., et al. 2015. Interleukin-21-producing CD4(+) T cells promote type 2 immunity to house dust mites. Immunity 43:318. [DOI] [PubMed] [Google Scholar]
- 49. Sonnenberg G. F. Fouser L. A. and Artis D. 2011. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12:383. [DOI] [PubMed] [Google Scholar]
- 50. Zhao Y. Yang J. Gao Y. D. and Guo W. 2010. Th17 immunity in patients with allergic asthma. Int. Arch. Allergy Immunol. 151:297. [DOI] [PubMed] [Google Scholar]
- 51. Czarnowicki T., Gonzalez J., Shemer A., et al. 2015. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J. Allergy Clin. Immunol. 136:104. [DOI] [PubMed] [Google Scholar]
- 52. Duhen T. Geiger R. Jarrossay D. Lanzavecchia A. and Sallusto F. 2009. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10:857. [DOI] [PubMed] [Google Scholar]
- 53. Ahlfors H., Morrison P. J., Duarte J. H., et al. 2014. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J. Immunol. 193:4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Haldar P., Pavord I. D., Shaw D. E., et al. 2008. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 178:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moore W. C., Meyers D. A., Wenzel S. E., et al. 2010. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 181:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29:621. [DOI] [PubMed] [Google Scholar]
- 57. Locci M., Havenar-Daughton C., Landais E., et al. 2013. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39:758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamamoto T., Lynch R. M., Gautam R., et al. 2015. Quality and quantity of TFH cells are critical for broad antibody development in SHIVAD8 infection. Sci. Transl. Med. 7:298ra120. [DOI] [PubMed] [Google Scholar]
- 59. Busse W. W., Morgan W. J., Gergen P. J., et al. 2011. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N. Engl. J. Med. 364:1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Crispin J. C., Oukka M., Bayliss G., et al. 2008. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 181:8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Doreau A., Belot A., Bastid J., et al. 2009. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat. Immunol. 10:778. [DOI] [PubMed] [Google Scholar]
- 62. Jacquemin C., Schmitt N., Contin-Bordes C., et al. 2015. OX40 ligand contributes to human lupus pathogenesis by promoting T follicular helper response. Immunity 42:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sakaguchi S. Sakaguchi N. Asano M. Itoh M. and Toda M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151. [PubMed] [Google Scholar]
- 64. Sakaguchi S. Yamaguchi T. Nomura T. and Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133:775. [DOI] [PubMed] [Google Scholar]
- 65. Josefowicz S. Z. Lu L. F. and Rudensky A. Y. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ohkura N. Kitagawa Y. and Sakaguchi S. 2013. Development and maintenance of regulatory T cells. Immunity 38:414. [DOI] [PubMed] [Google Scholar]
- 67. Ramsdell F. and Ziegler S. F. 2014. FOXP3 and scurfy: how it all began. Nat. Rev. Immunol. 14:343. [DOI] [PubMed] [Google Scholar]
- 68. Abbas A. K., Benoist C., Bluestone J. A., et al. 2013. Regulatory T cells: recommendations to simplify the nomenclature. Nat. Immunol. 14:307. [DOI] [PubMed] [Google Scholar]
- 69. Yadav M., Louvet C., Davini D., et al. 2012. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 209:1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Weiss J. M., Bilate A. M., Gobert M., et al. 2012. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 209:1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hansen W., Hutzler M., Abel S., et al. 2012. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J. Exp. Med. 209:2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wing K., Onishi Y., Prieto-Martin P., et al. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322:271. [DOI] [PubMed] [Google Scholar]
- 73. Korn T. Bettelli E. Oukka M. and Kuchroo V. K. 2009. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27:485. [DOI] [PubMed] [Google Scholar]
- 74. Akdis M. Palomares O. van de Veen W. van Splunter M. and Akdis C. A. 2012. TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J. Allergy Clin. Immunol. 129:1438. [DOI] [PubMed] [Google Scholar]
- 75. O’Shea J. J. Lahesmaa R. Vahedi G. Laurence A. and Kanno Y. 2011. Genomic views of STAT function in CD4+ T helper cell differentiation. Nat. Rev. Immunol. 11:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Szabo S. J., Sullivan B. M., Stemmann C., et al. 2002. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 295:338. [DOI] [PubMed] [Google Scholar]
- 77. Zheng W. and Flavell R. A. 1997. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89:587. [DOI] [PubMed] [Google Scholar]
- 78. Chang H. C., Sehra S., Goswami R., et al. 2010. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat. Immunol. 11:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Johnston R. J., Poholek A. C., DiToro D., et al. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hori S. Nomura T. and Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057. [DOI] [PubMed] [Google Scholar]
- 81. Djuretic I. M. Levanon D. Negreanu V. Groner Y. Rao A. and Ansel K. M. 2007. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat. Immunol. 8:145. [DOI] [PubMed] [Google Scholar]
- 82. O’Shea J. J. and Paul W. E. 2010. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327:1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kanno Y. Vahedi G. Hirahara K. Singleton K. and O’Shea J. J. 2012. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu. Rev. Immunol. 30:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bonelli M., Shih H. Y., Hirahara K., et al. 2014. Helper T cell plasticity: impact of extrinsic and intrinsic signals on transcriptomes and epigenomes. Curr. Topics Microbiol. Immunol. 381:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hegazy A. N., Peine M., Helmstetter C., et al. 2010. Interferons direct Th2 Cell reprogramming to generate a stable GATA-3+T-bet+ cell subset with combined Th2 and Th1 cell functions. Immunity 32:116. [DOI] [PubMed] [Google Scholar]
- 86. Ghoreschi K., Laurence A., Yang X. P., et al. 2010. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Koch M. A. Tucker-Heard G. Perdue N. R. Killebrew J. R. Urdahl K. B. and Campbell D. J. 2009. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Oestreich K. J. Huang A. C. and Weinmann A. S. 2011. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J. Exp. Med. 208:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wohlfert E. A., Grainger J. R., Bouladoux N., et al. 2011. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J. Clin. Invest. 121:4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chung Y., Tanaka S., Chu F., et al. 2011. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 17:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chaudhry A., Rudra D., Treuting P., et al. 2009. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326:986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schiering C., Krausgruber T., Chomka A., et al. 2014. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Palmer G. and Gabay C. 2011. Interleukin-33 biology with potential insights into human diseases. Nat. Rev. Rheumatol. 7:321. [DOI] [PubMed] [Google Scholar]
- 94. Arpaia N., Green J. A., Moltedo B., et al. 2015. A distinct function of regulatory T cells in tissue protection. Cell 162:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zaiss D. M. Gause W. C. Osborne L. C. and Artis D. 2015. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 42:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ohnmacht C., Park J. H., Cording S., et al. 2015. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 349:989. [DOI] [PubMed] [Google Scholar]
- 97. Sefik E., Geva-Zatorsky N., Oh S., et al. 2015. Mucosal immunology. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mahata B., Zhang X., Kolodziejczyk A. A., et al. 2014. Single-cell RNA sequencing reveals T helper cells synthesizing steroids de novo to contribute to immune homeostasis. Cell Rep. 7:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Arsenio J. Kakaradov B. Metz P. J. Kim S. H. Yeo G. W. and Chang J. T. 2014. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat. Immunol. 15:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Endo Y., Iwamura C., Kuwahara M., et al. 2011. Eomesodermin controls interleukin-5 production in memory T helper 2 cells through inhibition of activity of the transcription factor GATA3. Immunity 35:733. [DOI] [PubMed] [Google Scholar]
- 101. Endo Y., Hirahara K., Iinuma T., et al. 2015. The interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity 42:294. [DOI] [PubMed] [Google Scholar]
- 102. Islam S. A., Chang D. S., Colvin R. A., et al. 2011. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T(H)2 cells. Nat. Immunol. 12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mitson-Salazar A., Yin Y., Wansley D. L., et al. 2015. Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human T2 cell subpopulation with enhanced function. J. Allergy Clin. Immunol. [DOI] [PubMed] [Google Scholar]
- 104. Upadhyaya B. Yin Y. Hill B. J. Douek D. C. and Prussin C. 2011. Hierarchical IL-5 expression defines a subpopulation of highly differentiated human Th2 cells. J. Immunol. 187:3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wambre E. DeLong J. H. James E. A. LaFond R. E. Robinson D. and Kwok W. W. 2012. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J. Allergy Clin. Immunol. 129:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nguyen L. P., Pan J., Dinh T. T., et al. 2015. Role and species-specific expression of colon T cell homing receptor GPR15 in colitis. Nat. Immunol. 16:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Iinuma T., Okamoto Y., Yamamoto H., et al. 2015. Interleukin-25 and mucosal T cells in noneosinophilic and eosinophilic chronic rhinosinusitis. Ann. Allergy Asthma Immunol. 114:289. [DOI] [PubMed] [Google Scholar]
- 108. Islam S. A. and Luster A. D. 2012. T cell homing to epithelial barriers in allergic disease. Nat. Med. 18:705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Joost P. and Methner A. 2002. Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol. 3:RESEARCH0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Goldberg A. D. Allis C. D. and Bernstein E. 2007. Epigenetics: a landscape takes shape. Cell 128:635. [DOI] [PubMed] [Google Scholar]
- 111. Wei G., Wei L., Zhu J., et al. 2009. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schuettengruber B. Chourrout D. Vervoort M. Leblanc B. and Cavalli G. 2007. Genome regulation by polycomb and trithorax proteins. Cell 128:735. [DOI] [PubMed] [Google Scholar]
- 113. Nakayama T. and Yamashita M. 2008. Initiation and maintenance of Th2 cell identity. Curr. Opin. Immunol. 20:265. [DOI] [PubMed] [Google Scholar]
- 114. Nakayama T. and Yamashita M. 2009. Critical role of the Polycomb and Trithorax complexes in the maintenance of CD4 T cell memory. Semin. Immunol. 21:78. [DOI] [PubMed] [Google Scholar]
- 115. Onodera A. and Nakayama T. 2015. Epigenetics of T cells regulated by Polycomb/Trithorax molecules. Trends Mol. Med. 21:330. [DOI] [PubMed] [Google Scholar]
- 116. Onodera A., Tumes D. J., Watanabe Y., et al. 2015. Spatial interplay between Polycomb and Trithorax complexes controls transcriptional activity in T lymphocytes. Mol. Cell. Biol. 35:3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kimura M., Koseki Y., Yamashita M., et al. 2001. Regulation of Th2 cell differentiation by mel-18, a mammalian polycomb group gene. Immunity 15:275. [DOI] [PubMed] [Google Scholar]
- 118. Yamashita M., Kuwahara M., Suzuki A., et al. 2008. Bmi1 regulates memory CD4 T cell survival via repression of the Noxa gene. J. Exp. Med. 205:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Suzuki A., Iwamura C., Shinoda K., et al. 2010. Polycomb group gene product Ring1B regulates Th2-driven airway inflammation through the inhibition of Bim-mediated apoptosis of effector Th2 cells in the lung. J. Immunol. 184:4510. [DOI] [PubMed] [Google Scholar]
- 120. Tumes D. J., Onodera A., Suzuki A., et al. 2013. The polycomb protein Ezh2 regulates differentiation and plasticity of CD4(+) T helper type 1 and type 2 cells. Immunity 39:819. [DOI] [PubMed] [Google Scholar]
- 121. Yamashita M., Hirahara K., Shinnakasu R., et al. 2006. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity 24:611. [DOI] [PubMed] [Google Scholar]
- 122. Obata Y., Furusawa Y., Endo T. A., et al. 2014. The epigenetic regulator Uhrf1 facilitates the proliferation and maturation of colonic regulatory T cells. Nat. Immunol. 15:571. [DOI] [PubMed] [Google Scholar]
- 123. Bulger M. and Groudine M. 2011. Functional and mechanistic diversity of distal transcription enhancers. Cell 144:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yamashita M., Ukai-Tadenuma M., Kimura M., et al. 2002. Identification of a conserved GATA3 response element upstream proximal from the interleukin-13 gene locus. J. Biol. Chem. 277:42399. [DOI] [PubMed] [Google Scholar]
- 125. Ansel K. M. Djuretic I. Tanasa B. and Rao A. 2006. Regulation of Th2 differentiation and Il4 locus accessibility. Annu. Rev. Immunol. 24:607. [DOI] [PubMed] [Google Scholar]
- 126. Heintzman N. D., Hon G. C., Hawkins R. D., et al. 2009. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 459:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhang J. A. Mortazavi A. Williams B. A. Wold B. J. and Rothenberg E. V. 2012. Dynamic transformations of genome-wide epigenetic marking and transcriptional control establish T cell identity. Cell 149:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hawkins R. D., Larjo A., Tripathi S. K., et al. 2013. Global chromatin state analysis reveals lineage-specific enhancers during the initiation of human T helper 1 and T helper 2 cell polarization. Immunity 38:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gerasimova A., Chavez L., Li B., et al. 2013. Predicting cell types and genetic variations contributing to disease by combining GWAS and epigenetic data. PLoS One 8:e54359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Seumois G., Chavez L., Gerasimova A., et al. 2014. Epigenomic analysis of primary human T cells reveals enhancers associated with TH2 memory cell differentiation and asthma susceptibility. Nat. Immunol. 15:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Farh K. K., Marson A., Zhu J., et al. 2015. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hnisz D., Abraham B. J., Lee T. I., et al. 2013. Super-enhancers in the control of cell identity and disease. Cell 155:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vahedi G., Kanno Y., Furumoto Y., et al. 2015. Super-enhancers delineate disease-associated regulatory nodes in T cells. Nature 520:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Endo Y. Hirahara K. Yagi R. Tumes D. J. and Nakayama T. 2014. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol. 35:69. [DOI] [PubMed] [Google Scholar]
- 135. Antonelli A. Ferrari S. M. Corrado A. Ferrannini E. and Fallahi P. 2014. CXCR3, CXCL10 and type 1 diabetes. Cytokine Growth Factor Rev. 25:57. [DOI] [PubMed] [Google Scholar]
- 136. Lee Y., Awasthi A., Yosef N., et al. 2012. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13:991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wang C., Yosef N., Gaublomme J., et al. 2015. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]