Summary
The interactions of a number of possible energy sources for in-vitro development of 2-cell mouse ova were examined using statistical experimental designs. These experiments indicated that glucose has no beneficial effect on development when employed with the optimum concentration of pyruvate. Optimum concentrations of pyruvate and oxaloacetate when employed together resulted in a significantly lower response than when either compound was employed alone. It was found that the best medium for the development of 2-cell mouse ova into blastocysts contained 2·5 to 5·0 × 10−4 M-pyruvate + 2·5 to 5·0 × 10−2 M-lactate.
INTRODUCTION
A large number of possible energy sources were examined by Brinster (1965b) for their ability to support the development of 2-cell mouse ova. It was found that when such compounds as glucose, fructose, ribose, glucose-6-phosphate, fructose 1–6 diphosphate, acetate, citrate, isocitrate, α-ketoglutarate, succinate, fumarate and malate were employed alone as the only possible energy source in media for the cultivation of 2-cell ova, the ova degenerated within 24 hr and little or no cleavage occurred. However, pyruvate, phosphoenolpyruvate, oxaloacetate and lactate when employed alone as the only energy source in the medium did allow development of 2-cell mouse ova into blastocysts.
From the above findings two questions arise: (1) What pathways are employed by the 2-cell ova for energy metabolism? (2) What is the best combination of energy sources in which to cultivate 2-cell mouse ova ? The first question is of great theoretical interest, whereas the second question is of great practical interest. Information pertinent to both questions can be obtained by the use of statistical experimental factorial designs in conjunction with the basic cultivation method. Of particular interest is whether glucose and malate, although they do not support development alone, have any effect on development when employed with a compound which does support growth of 2-cell ova to blastocyst. In addition, it is important to know if the compounds which can supply energy to the ova, in particular pyruvate, oxaloacetate and lactate, interact when employed together. Experiments designed to provide information pertinent to the above problems are described in this article.
MATERIALS AND METHODS
The basic procedures employed to obtain and cultivate ova have been described in detail elsewhere (Brinster, 1963, 1965a). The biometrical problems associated with the analysis of the results of experiments with mouse embryos have been discussed by Biggers & Brinster (1965). Therefore, only those techniques which have not been described or which have special applicability to the present experiments are described below.
The 2-cell ova used for cultivation were obtained from 6- to 10-week-old random-bred Swiss mice. These mice were superovulated by an intraperitoneal injection of 10 to 15 i.u. of pregnant mare serum gonadotrophin (PMS) (Gestyl, Organon), followed 40 to 48 hr later by 10 to 15 i.u. of human chorionic gonadotrophin (HCG) (Pregnyl, Organon). The injected females were placed with males, and females which had mated were used the day following the observation of the vaginal plug. Since ovulation occurs 11 to 14 hr after injection of HCG (Edwards & Gates, 1959) the age of the ova could be determined by the interval between the injection of Pregnyl and harvest of the ova. In the experiments reported here the ova were obtained 43 or 45 hr after HCG injection.
The ova were flushed from the fallopian tubes, placed in a common pool and then randomly distributed to the experimental drops of medium. The basic medium used in these experiments is shown in Table 1. For handling the ova and storage of the ova, the medium contained 3·16 × 10−4 M-pyruvate. Before the ova were placed in experimental drops, they were washed three times in medium completely free of any energy source. Preparation of media of various compositions for ovum cultivation has been described by Brinster (1965a, b). The pH of all media was 7·38, and the osmolarity was 0·308 osmolar. The pH of prepared medium was kept constant by neutralizing acid energy sources, such as lactic, malic and oxaloacetic acid, with NaOH before preparation of the medium. The osmolarity was kept constant by substitution of added energy sources for sodium chloride in proper osmolar quantities. The lactic acid used was 85 to 90% D-L lactic acid (Mallinkrodt), and the molar quantities are based on total D-L lactic acid content. The insulin used was Protamine zinc insulin from beef pancreas, obtained from California Biochemical Company.
Table 1.
Basic solution for ovum culture
| Component | mg/l | M × 10−3 |
|---|---|---|
|
| ||
| NaCl | 6·975 | 119·32 |
| KCl | 0·356 | 4·78 |
| CaCl2 | 0·189 | 1·71 |
| KH2PO4 | 0·162 | 1·19 |
| MgSO4·7H2O | 0·294 | 1·19 |
| NaHCO3 | 2·106 | 25·07 |
| Penicillin G (potassium) | 100 u/ml | — |
| Streptomycin sulphate | 50 μg/ml | — |
| Crystalline bovine serum albumin | 1·000 | — |
The ova were observed at 24 hr intervals to determine the effect of the treatments. The development of a normal blastocyst by the 3rd day of cultivation was the criterion of a positive response. Blastocysts which were abnormal were not counted as a positive response. These included collapsed blastocysts, blastocysts with isolated blastomeres and blastocysts in which the cavitation was not clearly defined. The ratio of the number of normal blastocysts after 3 days cultivation to the number of 2-cell ova at the beginning of cultivation was transformed by means of the arc sine transformation. The transformed data was then treated by ordinary statistical methods (see Biggers & Brinster, 1965). In certain cases when the effect of a treatment was severe, all the ova degenerated in 24 hr and little or no cleavage occurred. In such cases statistical treatment of the data was unnecessary.
RESULTS
Glucose and malate
Several experiments were performed to determine the effect of glucose on development of 2-cell mouse ova into blastocysts. In the first experiment, insulin was added in different concentrations to media containing only glucose and to media containing glucose and lactate as energy sources. The composition of the media and the results of the experiment are shown in Table 2. Most of the ova in those treatments which did not contain lactate failed to cleave regardless of the concentration of insulin and began to degenerate during the first 24 hr of culture. There was no apparent difference between Treatments 8 to 14 in their ability to support growth of 2-cell mouse ova. Treatments 1 to 7 demonstrate that the concentrations of insulin and glucose used did not interfere with development if lactate was present in the medium.
Table 2.
Effect of insulin on the ability of 2-cell mouse ova to utilize glucose for development to blastocysts in vitro
| Treatment | Concentration
|
Response | ||
|---|---|---|---|---|
| Lactate × 10−3 M | Glucose × 10−3 M | Insulin (u/l) | ||
|
| ||||
| 1 | 10·0 | 8·33 | 4760·00 | 3 |
| 2 | 10·0 | 8·33 | 476·00 | 2 |
| 3 | 10·0 | 8·33 | 47·60 | 3 |
| 4 | 10·0 | 8·33 | 4·76 | 4 |
| 5 | 10·0 | 8·33 | 0·476 | 4 |
| 6 | 10·0 | 8·33 | 0·0476 | 5 |
| 7 | 10·0 | 8·33 | 0·0000 | 4 |
| 8 | 0 | 8·33 | 4760·00 | 0 |
| 9 | 0 | 8·33 | 476·00 | 0 |
| 10 | 0 | 8·33 | 47·60 | 0 |
| 11 | 0 | 8·33 | 4·76 | 0 |
| 12 | 0 | 8·33 | 0·476 | 0 |
| 13 | 0 | 8·33 | 0·0476 | 0 |
| 14 | 0 | 8·33 | 0·0000 | 0 |
Response is the number of normal blastocysts from twelve 2-cell ova after 3 days cultivation. Two-cell ova obtained 45 hr after HCG injection.
Because of the fundamental importance of glucose in energy metabolism of most mammalian cells, its effect on the development of mouse ova was examined more closely in several experiments. The first experiment employed glucose with pyruvate in a 2 × 2 factorial design. The concentration of pyruvate employed was based on previous work (Brinster, 1965b), and the glucose was absent or present in the normal concentration of blood (1 mg/ml). The treatments, results and the analysis of variance of the data are shown in Table 3. There is a significant interaction between pyruvate and glucose in the concentrations used in this experiment (0·05 > P > 0·01). This occurs despite the fact that glucose alone will not support the development of 2-cell ova. The major contribution to this significant interaction appears to arise from the positive action of glucose at the low concentration of pyruvate, but there appears to be a slight decrease in response when glucose is added to media containing pyruvate at the high concentration.
Table 3.
| (a) Pyruvate–glucose interaction
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Concentration
|
Response
|
Mean angular response | Response (%) | ||||
| Pyruvate | Glucose | Drop number | ||||||
| 1 | 2 | 3 | 4 | |||||
| 1 | 3·16 × 10−4 M | 1 mg/ml | 4 | 5 | 6 | 4 | 38·95 | 39·5 |
| 2 | 3·16 × 10−4 M | 0 | 5 | 5 | 4 | 8 | 42·60 | 45·8 |
| 3 | 6·00 × 10−5 M | 1 mg/ml | 3 | 3 | 2 | 3 | 28·53 | 22·8 |
| 4 | 6·00 × 10−5 M | 0 | 0 | 0 | 1 | 0 | 10·43 | 3·3 |
| (b) Analysis of variance
| ||
|---|---|---|
| Source of variation | d.f | m.s. |
|
| ||
| Between drops | 3 | 9·122 |
| Between treatments | (3) | (832·208) |
| Pyruvate | 1 | 1814·760* |
| Glucose | 1 | 208·803 |
| P × G | 1 | 473·062† |
| Error | 9 | 36·551 |
| Total | 15 | |
| Theoretical variance | ∞ | 75·3 |
Response is the number of normal blastocysts from twelve 2-cell ova after 3 days cultivation in vitro.
Two-cell ova obtained 43 hr after HCG injection.
P< 0·001.
0·05 > P > 0·01.
It was established earlier that 2-cell mouse ova will not develop in media containing only glucose as an energy source, but a number of workers have reported that glucose alone will allow growth of 8-cell mouse ova (Hammond, 1949; Whitten, 1956). The pyruvate–glucose interaction experiment indicates a beneficial effect of glucose at low concentrations of pyruvate. Two experiments were performed to determine if glucose would support the growth of 4-cell ova. The concentration of glucose employed and the results of these experiments are shown in Table 4. Glucose in a concentration between 0·5 mg/ml and 5·0 mg/ml will support development of 4-cell mouse ova into blastocysts. The beneficial effect of glucose at low concentrations of pyruvate probably results from the ability of glucose alone to support development of mouse ova from the 4-cell stage to the blastocyst, there being sufficient pyruvate for the second cleavage.
Table 4.
Effect of glucose on the development of 4-cell mouse ova
| Treatment | Concentration | Response
|
|
|---|---|---|---|
| Experiment 1 | Experiment 2 | ||
|
| |||
| 1 | 5·0 | 8 | 6 |
| 2 | 1·0 | 3 | 4 |
| 3 | 0·5 | 6 | 1 |
| 4 | 0 | 0 | 0 |
Response is the number of normal blastocysts from twelve 4-cell ova after 3 days cultivation in vitro. Four-cell ova obtained 51 hr after HCG injection.
Because of the interaction of the effect of glucose and pyruvate concentration, an experiment was designed to examine more closely the effect of adding glucose at several concentrations to media already containing sodium pyruvate at the optimum concentration of 3·16 x 10−4 M (Brinster, 1965b). The concentrations of glucose employed and the results of the experiment, which was repeated twice, are shown in Table 5. The analysis of variance indicated that there was no significant difference between the treatments. The addition of glucose, in the concentrations tested, to the medium containing pyruvate had no significant effect on the development of the ova.
Table 5.
The effect of adding glucose to medium containing pyruvate at optimum concentration
| Treatment | Glucose concentration (mg/ml) | Experiment 1 Response
|
Experiment 2 Response
|
Mean angular response | Response (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drop number | Drop number | ||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||||
| 1 | 0 | 1 | 1 | 1 | 1 | 3 | 5 | 3 | 3 | 24·68 | 18·75 |
| 2 | 1 | 1 | 2 | 5 | 3 | 2 | 4 | 1 | 3 | 27·16 | 21·88 |
| 3 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 22·28 | 14·58 |
| 4 | 3 | 1 | 3 | 1 | 2 | 1 | 3 | 2 | 1 | 21·93 | 14·58 |
| 5 | 4 | 4 | 4 | 2 | 6 | 3 | 1 | 3 | 4 | 31·48 | 28·13 |
| 6 | 8 | 0 | 2 | 4 | 0 | 1 | 2 | 2 | 3 | 21·38 | 14·58 |
Response is the number of normal blastocysts from twelve 2-cell ova after 3 days cultivation in vitro. Two-cell ova obtained 43 hr after HCG injection.
The analysis of variance indicated no significant treatment effect.
Two closely related compounds, oxaloacetate and malate, differ markedly in their ability to supply energy for the developing 2-cell mouse ova. Oxaloacetate alone supports good development of the ova to blastocysts, whereas malate alone will not support development of the ova for even 24 hr (Brinster, 1965b). To determine if there could be even a slight effect of malate on development, a 2 × 2 factorial experiment was performed using pyruvate and malate at two doses each. The concentrations employed were based on previous work (Brinster, 1965b). The experimental treatments and the results are shown in Table 6. An analysis of variance of the data indicated (1) no significant interaction between pyruvate and malate, (2) no significant effect of malate on development, and (3) a highly significant (P < 0·001) effect of pyruvate on development. From this data it appears that malate has little or no influence on the development of 2-cell mouse ova into blastocysts.
Table 6.
Pyruvate–malate interaction at pH 7·38 and osmolarity 0·308
| Treatment | Molar concentration
|
Response
|
Mean angular response | Response (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Pyruvate | Malate | Drop number | ||||||
| 1 | 2 | 3 | 4 | |||||
| 1 | 3·16 x 10−4 | 3·16 x 10−4 | 6 | 4 | 5 | 5 | 40·18 | 41·67 |
| 2 | 3·16 x 10−4 | 6·00 x 10−5 | 9 | 5 | 4 | 6 | 45·13 | 50·00 |
| 3 | 6·00 x 10−5 | 3·16 x 10−4 | 0 | 0 | 0 | 0 | 8·30 | 0 |
| 4 | 6·00 x 10−5 | 6·00 x 10−5 | 0 | 0 | 1 | 0 | 10·43 | 2·08 |
Response is the number of normal blastocysts from twelve 2-cell ova after 3 days cultivation in vitro. Two-cell ova obtained 43 hr after HCG injection.
Pyruvate, oxaloacetate and lactate
Of the four possible energy sources which 2-cell mouse ova can utilize for development (Brinster, 1965b), three were selected for further examination. The dose response for lactate and its optimum concentration (5·00 × 10−2 M) had been accurately determined (Brinster, 1964, 1965b). However, the dose response for pyruvate and oxaloacetate and their optimum concentrations were determined with less accuracy than in the case of lactate (see Brinster, 1965b). Consequently, the pyruvate and oxaloacetate dose response experiments were repeated using smaller concentration intervals in order to determine more accurately the optimum concentration for each compound. The experimental treatments and the results are shown in Table 7. These experiments indicate the optimum concentration of pyruvate and oxaloacetate to be 5·00 × 10−4 M rather than 3·16 × 10−4 M as determined in previous experiments (Brinster, 1965b).
Table 7.
Effect of pyruvate and oxaloacetate on the development of 2-cell mouse ova
| Concentration × 10−4M | Experiment 1 Response
|
Experiment 2 Response
|
Mean angular response | Response (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drop number | Drop number | ||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||||
| Pyruvate | 1·0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8·30 | 0·00 |
| 3·0 | 4 | 3 | 2 | 5 | 6 | 1 | 2 | 4 | 31·35 | 28·13 | |
| 5·0 | 5 | 6 | 7 | 3 | 9 | 5 | 5 | 7 | 44·40 | 48·96 | |
| 7·0 | 5 | 2 | 1 | 3 | 3 | 3 | 0 | 4 | 26·84 | 21·88 | |
| 9·0 | 0 | 1 | 3 | 1 | 2 | 2 | 1 | 1 | 19·21 | 11·46 | |
|
| |||||||||||
| Oxaloacetate | 1·0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8·30 | 0·00 |
| 3·0 | 2 | 5 | 4 | 2 | 1 | 1 | 0 | 6 | 26·33 | 21·88 | |
| 5·0 | 5 | 5 | 6 | 4 | 5 | 5 | 0 | 6 | 36·80 | 37·50 | |
| 7·0 | 2 | 4 | 2 | 3 | 2 | 2 | 2 | 6 | 28·85 | 23·96 | |
| 9·0 | 3 | 4 | 3 | 4 | 1 | 1 | 0 | 0 | 22·60 | 16·67 | |
Response is the number of normal blastocysts from twelve 2-cell ova after 3 days cultivation in vitro. Two-cell ova obtained 43 hr after HCG injection. The analysis of variance of the treatment effect for each energy source was significant (P < 0·001).
The relationships between pyruvate, oxaloacetate, and lactate were examined in a 23 factorial design to determine (1) differences in maximum response, and (2) if interactions existed between the effects of the compounds. The treatments employed, the results and the analysis of variance of the data are shown in Table 8. There is a significant difference between the experiments in overall response (P < 0·001). This does not affect the treatment effect since the experiment × treatment interaction is not significant. No second order interaction among the three energy sources was demonstrated, but there was a highly significant first order interaction between pyruvate and oxaloacetate. The main effects of pyruvate and oxaloacetate cannot be interpreted because of the interaction which occurs between these two compounds, but previous experiments have, in fact, demonstrated a highly significant effect of each of these three energy sources on response. When the mean angular responses of the treatments are compared, it appears that the response with pyruvate + lactate or oxaloacetate + lactate is significantly better than when any of the three energy sources are employed alone. In addition, it appears that pyruvate and oxaloacetate, when employed together at their optimum concentration, decrease development when compared with either compound employed alone.
Table 8.
| (a) Interaction between pyruvate, oxaloacetate and lactate at pH 7·38 and osmolarity 0·308
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Pyruvate ×10−4M | Oxaloacetate ×10−4M | Lactate ×10−2M | Day 1 Response
|
Day 2 Response
|
Mean angular response | Response (%) | ||||||
| Drop number | Drop number | ||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||||||
| 1 | 5·0 | 5·0 | 5·0 | 3 | 1 | 2 | 5 | 8 | 6 | 7 | 10 | 40·81 | 43·75 |
| 2 | 5·0 | 5·0 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 3 | 4 | 19·23 | 12·50 |
| 3 | 5·0 | 0 | 5·0 | 6 | 4 | 3 | 6 | 12 | 9 | 6 | 8 | 49·59 | 56·25 |
| 4 | 5·0 | 0 | 0 | 4 | 1 | 5 | 4 | 4 | 3 | 5 | 7 | 35·36 | 34·38 |
| 5 | 0 | 5·0 | 5·0 | 5 | 6 | 5 | 5 | 11 | 9 | 8 | 9 | 51·69 | 60·42 |
| 6 | 0 | 5·0 | 0 | 2 | 2 | 2 | 2 | 6 | 3 | 5 | 4 | 30·86 | 27·08 |
| 7 | 0 | 0 | 5·0 | 2 | 0 | 2 | 1 | 4 | 5 | 4 | 6 | 28·64 | 25·00 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8·30 | 0·00 |
| (b) Pyruvate × Oxaloacetate two-way table demonstrating interaction of effects (mean angular response)
| ||
|---|---|---|
| Oxaloacetate (M) | Pyruvate (M)
|
|
| 0 | 5 × 10−4 | |
|
| ||
| 0 | 18·47 | 42·48 |
| 5 × 10−4 | 41·28 | 30·02 |
| (c) Analysis of variance
| ||
|---|---|---|
| Source of variation | d.f. | m.s. |
|
| ||
| Between experiments | 1 | 4022·730* |
| Drops within experiments | 6 | 98·102 |
| Between drops | (7) | |
| Between treatments | (7) | |
| Pyruvate (P) | 1 | 650·250† |
| Oxaloacetate (O) | 1 | 428·490‡ |
| Lactate (L) | 1 | 5925·150* |
| P × O | 1 | 4973·775* |
| O × L | 1 | 61·623 |
| L × P | 1 | 28·623 |
| P × O × L | 1 | 47·266 |
| Experiments × treatments | 7 | 148·922 |
| Error | 42 | 51·711 |
| Total | 63 | |
| Theoretical variance | ∞ | 75·3 |
Response is the number of normal blastocysts from twelve 2-cell ova after 3 days cultivation in vitro. Two-cell ova obtained 43 hr after HCG injection. The standard error for the difference of two means in this example is 4·339.
P< 0·001.
0·05 > P> 0·01.
0·01 > P> 0·001.
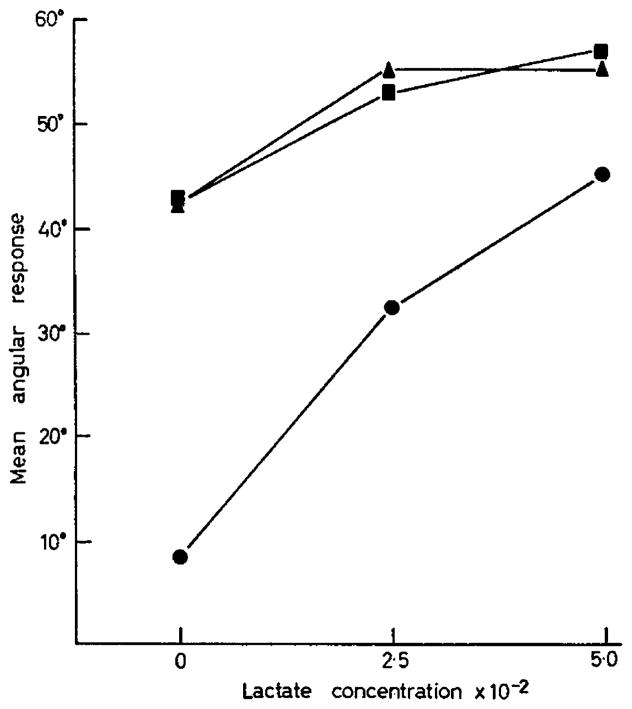
To investigate the relationship between pyruvate and lactate more closely, a 32 factorial experiment was designed in which three concentrations of pyruvate and three concentrations of lactate were employed in all possible combinations. The concentrations used were based on previous experiments. The treatments employed, the results and the analysis of variance appear in Table 9. In this experiment, when three concentrations of each pyruvate and lactate are used, a significant interaction in the effect of the two compounds appears. The interactions arise not from an inhibition of response, as in the case of oxaloacetate and pyruvate, but from the fact that the effect of pyruvate and lactate are not completely additive. The complex interaction between pyruvate quadratic and lactate linear probably results from a combination of (1) the non-additivity of the two response effects, and (2) the quadratic characteristics of the dose response lines (especially for pyruvate) in the concentrations studied. Again in this experiment, as in the previous experiment with pyruvate, oxaloacetate and lactate, it is demonstrated that a combination of pyruvate and lactate at their optimum concentrations is superior to either compound alone at its optimum concentration. In addition, it appears that any combination of these two compounds between half-optimum and optimum results in approximately equal response (Text-fig. 1).
Table 9.
| (a) Interaction between Pyruvate and Lactate
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Molar concentration
|
Day 1 Response
|
Day 2 Response
|
Mean angular response | Response (%) | |||||||
| Pyruvate × 10−4M | Lactate × 10−2M | Drop number | Drop number | |||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||||
| 1 | 5·0 | 5·0 | 8 | 4 | 10 | 10 | 9 | 9 | 9 | 7 | 56·45 | 68·75 |
| 2 | 5·0 | 2·5 | 8 | 5 | 8 | 5 | 8 | 9 | 7 | 11 | 53·44 | 63·54 |
| 3 | 5·0 | 0 | 5 | 5 | 7 | 6 | 5 | 7 | 4 | 6 | 43·19 | 46·88 |
| 4 | 2·5 | 5·0 | 10 | 8 | 9 | 8 | 6 | 7 | 7 | 9 | 54·99 | 66·67 |
| 5 | 2·5 | 2·5 | 8 | 8 | 8 | 8 | 8 | 8 | 9 | 7 | 54·75 | 66·67 |
| 6 | 2·5 | 0 | 5 | 8 | 6 | 6 | 6 | 5 | 6 | 3 | 43·14 | 46·88 |
| 7 | 0 | 5·0 | 7 | 5 | 5 | 9 | 7 | 5 | 2 | 8 | 44·88 | 50·00 |
| 8 | 0 | 2·5 | 4 | 2 | 4 | 3 | 3 | 5 | 3 | 4 | 32·53 | 29·17 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8·30 | 0·00 |
| (b) Analysis of variance
| ||
|---|---|---|
| Source of variation | d.f. | m.s. |
|
| ||
| Between experiments | 1 | 2·961 |
| Drops within experiments | 6 | 65·249 |
| Between drops | (7) | |
| Between treatments | (8) | |
| Pyruvate (P) | ||
| linear (l) | 1 | 6052·521* |
| quadratic (q) | 1 | 1993·623* |
| Lactate (L) | ||
| linear (l) | 1 | 5073·797* |
| quadratic (q) | 1 | 413·106† |
| Pl × Ll | 1 | 1086·945* |
| Pl × Lq | 1 | 14·338 |
| Pq × Ll | 1 | 455·466† |
| Pq × Lq | 1 | 2·940 |
| Experiments × treatments | 8 | 70·095 |
| Error | 50 | 49·931 |
| Theoretical variance | ∞ | 75·3 |
Response is the number of normal blastocysts from twelve 2-cell ova after 3 days cultivation in vitro. Two-cell ova obtained 45 hr after HCG injection. The standard error for the difference of two means in this example is 4·339.
P< 0·001.
0·05 > P > 0·01.
Fig 1.
Interaction of pyruvate and lactate. Pyruvate concentration: ■, 5·0 × 10−4 M; ▲, 2·5 × 10−4 M; ●, 0.
DISCUSSION
Glucose and malate
The fact that glucose does not support growth of 2-cell mouse ova through to blastocysts, and does not supply sufficient energy for most ova to cleave once, suggests that glucose is not metabolized by the ova or that the ova are not permeable to glucose. Several cell culture media contain insulin in addition to glucose and it has been reported that insulin exerts a beneficial effect in some cell culture systems. The range of insulin which has been employed varies considerably between different media. Trowell’s medium contains 400 u/1, whereas Baker’s medium for monocytes contains only 0·12 u/1 (Parker, 1961). The range of concentrations of insulin employed with the ova includes both these concentrations, but no beneficial effect of insulin was seen. Insulin did not allow glucose to support the development of any 2-cell ova to blastocysts and, in fact, did not prevent the characteristic degeneration of the ova which takes place in the absence of an adequate energy source. Since insulin is one of the most potent facilitators of glucose transport across the cell membrane, this experiment furnishes some evidence that the inability of glucose to support development is not a result of impermeability, but rather the lack of necessary enzyme systems.
The experiments in which both glucose and pyruvate were in the media provide information about the necessity of glucose for development of the mouse ova. An examination of the data in Table 3 indicates that the significant interaction of glucose and pyruvate in these experiments is predominately due to the positive effect of glucose at the low pyruvate concentration. This positive effect of glucose at low pyruvate concentration is no doubt a result of the fact that glucose will support the development of 4-cell ova to blastocysts. The low concentration of pyruvate enables the ova to obtain sufficient energy to develop to the 4-cell stage at which time they can metabolize glucose. But the low pyruvate concentration will not allow complete development to blastocysts of the 2-cell ova. Such an interpretation is further substantiated by the data in Table 5. If the pyruvate concentration is optimum for growth of the ova, the addition of glucose does not affect the response either positively or negatively. Apparently the ova are not adversely affected by the necessity to synthesize carbon chains longer than the three carbons of pyruvate, or some increase in response would have been noted when six carbon glucose was supplied. We know from the experiments in Table 4 that the ova are definitely permeable to glucose after the 4-cell stage, therefore there is no question of the lack of glucose effect being a result of impermeability.
It is interesting that malate, although very similar structurally to oxaloacetate, does not support development of the 2-cell ova, nor was it possible to demonstrate an interaction between pyruvate and malate. It appears that glucose and malate differ in respect to their interaction with pyruvate. Although both glucose and malate will support development of 8-cell mouse ova to blastocysts (Whitten, 1957), perhaps glucose is more directly involved in the energy metabolism of the 2-cell ovum. Before the morula stage in the rabbit embryo, the pentose phosphate pathway appears to be the main pathway of energy metabolism (Fridhandler, 1961). Glucose is more closely related to this pathway than malate. In fact, glucose-6-phosphate is a necessary intermediate in the pentose phosphate pathway. If this pathway is important in metabolism of the developing mouse ovum, then it might be expected that effects of glucose would be more easily demonstrated than effects of malate. In addition, the enzymes necessary for the metabolism of glucose develop at the 4-cell stage.
Pyruvate, oxaloacetate and lactate
The dose response lines of pyruvate and oxaloacetate were re-examined to assure that the most accurate optimum concentrations of these compounds could be used in assessing the relationship between pyruvate, oxaloacetate and lactate. This was necessary to ensure that, if additive effects of the compounds were found, they could not be a result of adding less than true optimum concentrations of two compounds together. For instance, if the individual concentrations of pyruvate and lactate are considerably less than optimum, then the two compounds employed together would yield a better response due simply to their interconversion. However, if they are both at optimum concentrations, then their effects must be related to factors other than their interconversion. In addition, no evidence has been found to indicate that the optimum dose of the energy sources changes with different groups of ova.
From the pyruvate–oxaloacetate–lactate interaction experiment, two main facts emerge. First, optimum concentrations of pyruvate and oxaloacetate, when employed together, are inhibitory to the development of 2-cell mouse ova. Either of these two compounds employed alone yields better development than when they are employed together. This accounts for the significant interaction between these two compounds in the analysis of variance. The reason for this interaction was not readily apparent. However, preliminary experiments, conducted on enzyme activity in the mouse ova, indicate that oxaloacetate may be converted to pyruvate in sufficient quantities to account for the fact that oxaloacetate will support development of the 2-cell mouse ova (Brinster, unpublished). This decarboxylation of oxaloacetate to pyruvate is catalysed by malic enzyme in some mammalian cells. If the decarboxylation of oxaloacetate to pyruvate is the reason for the ability of oxaloacetate to support development, then this adequately accounts for the inhibition noted when optimum concentrations of oxaloacetate and pyruvate are employed together. This pyruvate from oxaloacetate added to the pyruvate already present raises the level of pyruvate to an inhibitory level. The ΔF of the reaction
is approximately −6·4 kcal (Burton & Krebs, 1953). The equilibrium constant (K) for the reaction is given by
and is approximately 3·3 × 104 M. This means that at equilibrium almost all of the oxaloacetate is converted to pyruvate. Therefore, the effective pyruvate concentration would actually be between 9·0 and 10·0 × 10−4 M. This is easily an inhibitory level (see Table 7 and Brinster, 1965b).
The second main fact to emerge from the pyruvate–oxaloacetate–lactate experiment is the additive effect of pyruvate + lactate and oxaloacetate + lactate. It is probably safe to assume that the oxaloacetate is, indeed, decarboxylated to pyruvate, and, consequently, we can consider that the additivity of the effect of oxaloacetate and lactate just reflects a basic additivity of pyruvate and lactate. Since the concentrations of pyruvate and lactate were optimal concentrations, the effect is difficult to explain on the basis of interconversion of sub-optimal doses (see above). If we consider the method of interconversion of pyruvate and lactate by the lactate dehydrogenase reaction:
we might postulate that the addition of either pyruvate or lactate alone might exert an equilibrium pressure on the above reaction. Such a pressure would alter the ratio of DPN to DPNH and thereby the oxidation–reduction potential of the entire ovum. This alteration in the oxidation–reduction potential could adversely affect the development of the ovum. It has been calculated (Brinster, 1965b) that the optimum concentrations of pyruvate and lactate could provide a ratio of DPN to DPNH within the range of values (1 : 1 to 10 : 1) reported for other tissues (Long, 1961). Therefore, the use of both pyruvate and lactate at optimum concentrations may cause less stress on the oxidation–reduction potential of the ovum and thereby improve the probability of development of the ovum into a blastocyst.
The experiments on the interaction of pyruvate and lactate are in agreement with the findings of the previous experiments and in addition indicate an interaction of pyruvate and lactate. The probable reasons for the statistical interactions have already been covered in the results section. The biochemical explanation for the enhanced effects of pyruvate and lactate together, as discussed above, applies equally well to these experiments. One of the primary purposes of these last experiments on pyruvate–lactate interaction was to establish empirical limits for pyruvate and lactate concentration within which media for the optimum growth of 2-cell mouse ova could be prepared. From the results of the experiments shown in Table 9 it can be seen that the best medium would contain 2·5 to 5·0 × 10−4 M pyruvate + 2·5 to 5·0 × 10−2 M lactate. Concentrations within this range provide the maximum response. From the data in Table 5 it is safe to conclude that the addition of glucose to the medium does not increase the response of the 2-cell mouse ova.
The importance of pyruvate and lactate and the relative unimportance of glucose for the development of the early cleavage stages of the mouse ovum seems quite surprising. The metabolism of the oviduct and the ova within the oviduct may be intimately related. Bishop (1957) found high concentrations of lactate in oviduct fluid from the rabbit. Mastroianni & Wallach (1961) also found high concentrations of lactate in rabbit oviduct fluid and found that the concentration of lactate was increased during the first 3 days after ovulation. If such high concentrations of lactate occur in the fallopian tube of the mouse during this time, it would be extremely beneficial in view of the unusual energy source requirements of the mouse ovum. The presence of high concentrations of lactate in tubal fluid may result from the unusual metabolic characteristics of the tissues lining the tube. Mastroianni, Winternitz & Lowi (1958) found that the mucosa of the human oviduct, when studied under aerobic conditions in the Warburg apparatus, converted over 60% of the glucose in the medium into lactic acid. If the fallopian tube of the mouse is similar to that of the human and rabbit, then the energy requirements which have been found in the cultivation experiments with mouse ova fit in well with the concept that the metabolism of the cleavage stages and the oviduct are closely related.
Acknowledgments
The author would like to thank Professor John D. Biggers for his helpful suggestions. The work was supported by a grant from the Population Council, Inc. and Grant CA-06638 from the National Cancer Institute, U.S. Public Health Service. The author was a Pennsylvania Plan Scholar at the Department of Physiology, School of Medicine, University of Pennsylvania, Philadelphia at the time this work was begun. The expert technical assistance of Mrs Merilee Heffron, Mr Dorsey Williams and Mrs Pamela Yates is gratefully acknowledged.
References
- Biggers JD, Brinster RL. Biometrical problems in the study of early mammalian embryos in vitro. J exp Zool. 1965;158:39. doi: 10.1002/jez.1401580104. [DOI] [PubMed] [Google Scholar]
- Bishop D. Metabolic conditions within the oviduct of the rabbit. Int J Fertil. 1957;2:11. [Google Scholar]
- Brinster RL. A method for in vitro cultivation of mouse ova from two-cell to blastocyst. Exp Cell Res. 1963;32:205. doi: 10.1016/0014-4827(63)90093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL. PhD thesis. University of Pennsylvania; 1964. Studies on the development of mouse embryos in vitro. [Google Scholar]
- Brinster RL. Studies on the development of mouse embryos in vitroI The effect of osmolarity and hydrogen ion concentration. J exp Zool. 1965a;158:49. doi: 10.1002/jez.1401580105. [DOI] [PubMed] [Google Scholar]
- Brinster RL. Studies on the development of mouse embryos in vitroII The effect of energy source. J exp Zool. 1965b;158:59. doi: 10.1002/jez.1401580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K, Krebs HA. The free-energy changes associated with the individual steps of the tricarboxylic acid cycle, glycolysis and alcoholic fermentation and with the hydrolysis of the pyrophosphate groups of adenosinetriphosphate. Biochem J. 1953;54:94. doi: 10.1042/bj0540094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RG, Gates AH. Timing of the stages of the maturation divisions, ovulation, fertilization and the first cleavage of eggs of adult mice treated with gonadotrophins. J Endocrin. 1959;18:292. doi: 10.1677/joe.0.0180292. [DOI] [PubMed] [Google Scholar]
- Fridhandler L. Pathways of glucose metabolism in fertilized rabbit ova at various pre-implantation stages. Exp Cell Res. 1961;22:303. doi: 10.1016/0014-4827(61)90109-4. [DOI] [PubMed] [Google Scholar]
- Hammond J. Recovery and culture of tubal mouse ova. Nature, Lond. 1949;163:28. doi: 10.1038/163028b0. [DOI] [PubMed] [Google Scholar]
- Long C. Biochemist’s handbook. 1. Van Nostrand; Princeton, New Jersey: 1961. [Google Scholar]
- Mastroianni L, Wallach RC. Effect of ovulation and early gestation on oviduct secretions in the rabbit. Amer J Physiol. 1961;200:815. [Google Scholar]
- Mastroianni L, Winternitz WW, Lowi NP. The in vitro metabolism of the human endosalpinx. Fertil Steril. 1958;9:500. [PubMed] [Google Scholar]
- Parker RC. Methods of tissue culture. 3. Harper; New York: 1961. [Google Scholar]
- Whitten WK. Culture of tubal mouse ova. Nature, Lond. 1956;176:96. doi: 10.1038/177096a0. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Culture of tubal ova. Nature, Lond. 1957;179:1081. doi: 10.1038/1791081a0. [DOI] [PubMed] [Google Scholar]