Abstract
Chronic hepatitis B virus (HBV) infection is estimated to affect >350 million people worldwide and represents a significant cause of morbidity and mortality related to cirrhosis and hepatocellular carcinoma. Mother-to-child transmission (MTCT) of HBV remains an important source of incident cases of HBV. Current barriers to eradication of incident HBV infections via MTCT include underutilization of immunoprophylaxis with hepatitis B vaccination and hepatitis B immune globulin in certain endemic regions as well as failure of immunoprophylaxis.
Keywords: hepatitis B, pregnancy, perinatal transmission, vaccination HBIG
The risk for development of chronic hepatitis B virus (HBV) infection is strongly linked to the age of exposure. Risk for chronic infection after exposure varies from approximately 90% in infants, to 50% in toddlers and young children, and 5% in adults [1]. Mother-to-child transmission (MTCT) rates also vary significantly according to the mother's hepatitis B e antigen (HBeAg) status (70%–90% transmission rate for HBeAg-positive mothers vs 10%–40% for HBeAg-negative mothers). Standard active-passive immunoprophylaxis with hepatitis B immune globulin (HBIG) and hepatitis B vaccination administered immediately after birth (within 12 hours) to infants of hepatitis B surface antigen (HBsAg)–positive mothers, followed by 2 additional doses of vaccine within 6–12 months, prevents transmission in approximately 95%. However, a recent review of the published literature from 1975 to 2011 demonstrated that active-passive immunoprophylaxis fails to prevent HBV transmission in 8%–30% of children born to highly viremic mothers [2]. Postulated causes of immunoprophylaxis failure include high levels of maternal viremia, intrauterine infection, or mutations of the HBV surface protein [3, 4]. Thus, a clinical need remains to identify all causes of immunoprophylaxis failure and to determine safe and effective means of reducing MTCT rates.
HBV TRANSMISSION AND DNA
High maternal viremia is correlated with the highest risk for the transmission of HBV in pregnancy. In a large, nested case-control study of 773 HBsAg-positive women in Taiwan, high levels of HBV DNA (≥1.4 ng/mL or approximately 3.8 × 108 copies/mL) in HBeAg-positive women was associated with an odds ratio of 147 for chronic infection in infants compared to those women with HBV DNA <0.005 ng/mL (1.6 × 106 copies/mL) [5]. Even in the era of immunoprophylaxis, viremia remains a strong predictor of MTCT. In a study of 138 babies born to HBsAg-positive women, Wiseman et al found the immunoprophylaxis failure rate to be 9%, all occurring with mothers who were HBeAg-positive with HBV DNA ≥8 log10 copies/mL [6]. Recent literature also provides useful data to risk-stratify the magnitude of MTCT risk and immunoprophylaxis failure according to varying thresholds of maternal HBV DNA. These data suggest that HBV DNA levels of 6–6.99 log copies/mL portend a 3% risk of transmission, 7–7.99 log copies/mL a 7% risk of transmission, and ≥8 log copies/mL in the mother an 8% risk of MTCT of HBV [7].
HBV TRANSMSSION AND MODE OF DELIVERY
Older data assessing the MTCT rate in infants born via cesarean delivery vs vaginal delivery failed to conclusively show a significant difference in neonatal HBV infection. Expert opinion has suggested that there were insufficient data to recommend changes in the mode of delivery for HBV-infected women [8]. Some more recent data support reconsideration of elective cesarean delivery to reduce MTCT, including a meta-analysis that suggested a 17.5% absolute risk reduction compared to immunoprophylaxis alone. However, other studies report no benefit to elective caesarean delivery [9]. Data from Beijing from 1409 infants born to HBsAg-positive mothers from 2007 to 2011, all of whom received appropriate immunoprophylaxis at birth, reported MTCT rates of 1.4% with elective cesarean delivery compared to 3.4% with vaginal delivery and 4.2% with urgent cesarean delivery (P < .05) [10]. When mothers in this study were stratified according to HBV DNA, in those with low HBV DNA (<1 000 000 copies/mL), delivery modality did not impact MTCT. This suggests a potential role for elective cesarean delivery among women with HBV DNA >1 000 000 copies/mL. However, before definitive recommendations can be made, validation studies are needed to determine the relative safety and efficacy of elective cesarean delivery and immunoprophylaxis vs immunoprophylaxis alone in reducing MTCT without compromising fetal outcomes [9, 10].
TREATMENT OF HBV TO PREVENT TRANSMISSION
There is a growing body of literature to support both the safety and efficacy of antiviral therapy initiated in late pregnancy for reduction of MTCT among women in the highest risk for immunoprophylaxis failure (those with HBV DNA levels in the range of 107 log copies/mL and higher) (Table 1). Han conducted a prospective, open-label trial of women aged 20–40 years who were HBeAg positive with HBV DNA >7 log10 copies/mL between gestational weeks 20 and 32. All women were offered antiviral therapy, and 135 who accepted received telbivudine 600 mg daily. The comparison arm consisted of 94 women who consented to participate in the trial but declined antiviral therapy. All infants were administered appropriate immunoprophylaxis. Mean viral load at enrollment was approximately 8 log10 copies/mL in both arms and was reduced to 2.44 log10 copies/mL in the telbivudine-treated arm prior to delivery. The reported MTCT rate was 0% with telbivudine therapy compared to 8% without antiviral therapy. One infant in each group had low birth weight, and 6 infants (4%) in the telbivudine group compared with 5 infants (5%) in the control group had pneumonia by age 7 months. No congenital abnormalities were identified [11]. In a similar study, Han and colleagues compared 53 women with HBeAg-positive HBV with viral loads >6 log10 copies/mL and elevated alanine aminotransferase (ALT) level treated with telbivudine initiated in the second or third trimester to 35 similar women who declined therapy. Immunoprophylaxis failure rate in this study was 0% with telbivudine therapy compared to 8.6% in controls, with no significant difference in adverse event rates out to 28 weeks postpartum [7].
Table 1.
Treatment Options for Hepatitis B Virus in Pregnancy
| Antiviral Agent | FDA Pregnancy Category | Defects/Live Birth When Exposed During First Trimester, % (no./No.) | Defects/Live Birth When Exposed During Second/Third Trimester, % (no./No.) | Advantages/Disadvantages of Using During Pregnancy |
|---|---|---|---|---|
| Adefovir | C | 0 (0/48) | 0 (0/0) |
|
| Entecavir | C | 0 (2/58) | 0 (0/2) |
|
| Lamivudine | C | 3.1 (143/4566) | 2.8 (204/7193) |
|
| Telbivudine | B | 0 (0/10) | 0 (0/10) |
|
| TDF | B | 2.3 (60/2608) | 2.2 (24/1112) |
|
Abbreviations: FDA, US Food and Drug Administration; TDF, tenofovir disoproxil fumarate.
Source: Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International Interim Report for 1 January 1989 through 31 July 2015. Wilmington, NC: Registry Coordinating Center, 2015. Available at: http://www.apregistry.com/forms/interim_report.pdf. Accessed 6 February 2016.
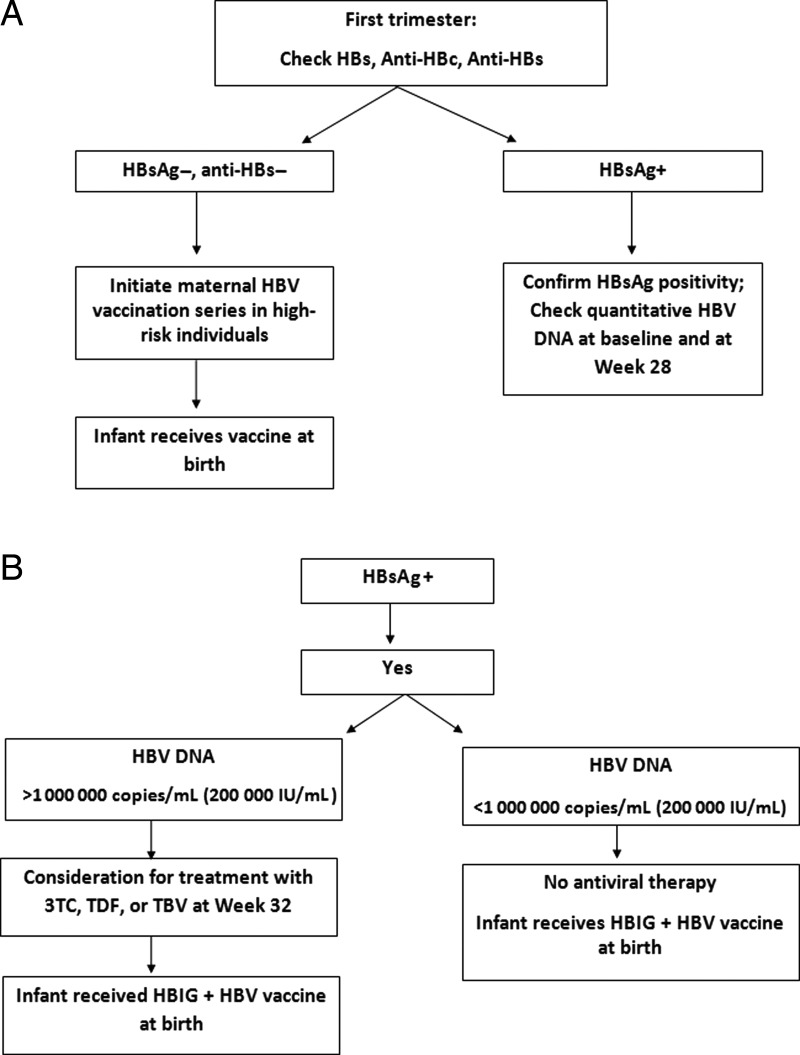
In one multicenter, prospective study from Australia, 58 women with HBV DNA >7 log10 IU/mL commencing therapy with tenofovir dipivoxil at 32 weeks’ gestation were compared to women (n = 52) treated with lamivudine and untreated historical controls (n = 20). Perinatal transmission was reduced to 0% and 2% in the lamivudine and tenofovir cohorts compared to 20% in the untreated groups. No differences were noted in obstetric or infant safety outcomes [12]. Although some studies have suggested a favorable safety profile for antiviral therapy even in the first and second trimesters of pregnancy, when utilized purely for purposes of reducing MTCT, antiviral therapy should be initiated in the third trimester (thus minimizing the risk associated with fetal exposure to these medications). In one recent study, however, 74 infants exposed to tenofovir in late pregnancy were compared to 69 tenofovir-unexposed infants with an assessment of their bone mineral content (BMC) at 1 month after delivery. Tenofovir-exposed newborns did not differ from unexposed newborns on mean gestational age (38.2 vs 38.1 weeks) or mean length (−0.41 vs −0.18) or weight (−0.71 vs −0.48) z scores. The mean BMC of tenofovir-exposed infants was 12% lower than for unexposed infants (56.0 [standard deviation {SD}, 11.8] g vs 63.8 [SD, 16.6] g; P = .002). The adjusted mean BMC was 5.3 g lower (95% confidence interval, −9.5 to −1.2; P = .013) in the tenofovir-exposed infants. The long-term follow-up conclusions of this cohort and clinical significance are not clear, but emphasize the need for minimization of exposure and assessment and discussion of clear risk and benefit [13, 14]. Treatment at levels <106 log copies/mL do not appear to be indicated unless the pregnant woman has liver disease for which viral suppression is indicated (Figure 1). The end point of antiviral therapy administered to reduce risk of MTCT typically is immediately in the postpartum period for mothers who plan to breastfeed their infants, unless treatment continuation is indicated for the clinical benefit of the mother. Discontinuation of therapy at any point during or after pregnancy requires careful monitoring due to the potential for HBV flares upon antiviral therapy withdrawal. It is most prudent to monitor for flares at least once monthly in the postpartum period with HBV DNA and ALT and bilirubin, and it would be expected to have viral levels rebound to baseline in the weeks following medication discontinuation.
Figure 1.
Suggested management strategy for hepatitis B surface antigen (HBsAg)–positive women in pregnancy. Abbreviations: 3TC, lamivudine; anti-HBc, hepatitis B core antibody; anti-HBs, hepatitis B surface antibody; HBIG, hepatitis B immune globulin; HBs, hepatitis B surface; HBV, hepatitis B virus; TBV, telbivudine; TDF, tenofovir disoproxil fumarate.
Transmission of HBV with breastfeeding is low risk in infants who receive appropriate immunoprophylaxis. Current World Health Organization recommendations are to allow breastfeeding as there is no evidence for additional risk, even without immunization [15]. Breastfeeding should be avoided in the presence of breast pathology such as cracked or bleeding nipples. Oral nucleos(t)ide analogues have been shown to be excreted in breast milk, albeit at low levels, and there are limited data on the effect of these medications on infants [16].
In summary, the data have evolved to clearly show that reduction of viral replication in pregnant women with the highest levels of viremia (>108 copies/mL or 2 × 107 IU/mL) is of likely benefit to reduce MTCT of HBV. Levels between 2 × 105 IU/mL and 2 × 107 IU/mL have slightly lower risk (3%–5%), but new recommendations by the American Association for the Study of Liver Diseases suggest using a viral cutoff of 2 × 105 IU/mL [17]. Current first-line therapies are tenofovir and telbivudine given their FDA pregnancy category B status, but lamivudine may be considered as well given its robust human exposure data in human immunodeficiency virus–infected women. Resistance, although a theoretical concern, is lower risk given the short duration of therapy (3 months) when given in this circumstance. While tenofovir at this time is the first-line choice given its relatively safer profile, low resistance, and efficacy, further long-term data will need to be gathered on clinical effect of bone mineral density. A new molecule in development at this time, tenofovir alafenamide, which is reported to have similar efficacy with lower drug exposure, may be an even more viable treatment option.
CONCLUSIONS
Hepatitis B perinatal transmission remains a common mode of viral transmission, especially in highly endemic areas globally. The availability over the past decade of effective oral agents that suppress viral replication has allowed the consideration of third-trimester treatment to reduce the risk of this transmission. This is important, particularly in pregnant women with very high viral levels (>108 copies/mL or 2 × 107 IU/mL), in whom the risk is highest, but transmission can occur even at levels >200 000 IU/mL. Treatment decisions necessitate careful discussion of risks and benefits as emerging data suggest some possible effect on bone mineral concentration in tenofovir-exposed pregnant women, which must be balanced by a nearly 10% risk of chronic infection with an incurable virus. Pregnant women with HBV must be monitored for clinical flares, with or without medications, and breastfeeding should be allowed as well.
Notes
Supplement sponsorship. This article appears as part of the supplement “Hepatitis B,” sponsored by the CDC Foundation and Gilead.
Potential conflict of interest. Author certifies no potential conflicts of interest. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Edmunds WJ, Medly GF, Nokes DJ et al. The influence of age on the development of the hepatitis B carrier state. Proc R Soc Biol Sci 1993; 253:197–201. [DOI] [PubMed] [Google Scholar]
- 2.Pan CQ, Duan ZP, Bhamidimarri KR et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin J Gastroenterol Hepatol 2012; 10:452–9. [DOI] [PubMed] [Google Scholar]
- 3.Stevens CE, Beasley RP, Tsui J et al. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med 1975; 292:771–4. [DOI] [PubMed] [Google Scholar]
- 4.Ngui SL, O'Connell S, Eglin RP et al. Low detection rate and maternal provenance of hepatitis B virus S gene mutants in cases of failed postnatal immunoprophylaxis in England and Wales. J Infect Dis 1997; 176:1360–5. [DOI] [PubMed] [Google Scholar]
- 5.Burk RD, Hwang LY, Ho GY et al. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis 1994; 170:1418–23. [DOI] [PubMed] [Google Scholar]
- 6.Wiseman E, Fraser MA, Holden S et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust 2009; 109:489–92. [DOI] [PubMed] [Google Scholar]
- 7.Han G-R, Cao M-K, Zhao W et al. A prospective and open label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatology 2011; 55:1215–21. [DOI] [PubMed] [Google Scholar]
- 8.Zou H, Chen Y, Duan Z et al. A retrospective study for clinical outcome of caesarean section on perinatal transmission of hepatitis B virus in infants born to HBeAg positive mothers with chronic hepatitis. J Viral Hepat 2012; 18:e18–25.22239517 [Google Scholar]
- 9.Hu Y, Chen J, Wen J et al. Effect of elective cesarean section on the risk of mother-to-child transmission of hepatitis B virus. BMC Pregnancy Childbirth 2013; 13:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SD, Lo KJ, Tsai YT et al. Role of caesarean section in prevention of mother-infant transmission of hepatitis B. Lancet 1988; 2:833–4. [DOI] [PubMed] [Google Scholar]
- 11.Pan CQ, Zou H-b, Chen Y et al. Cesarean section reduces perinatal transmission of HBV infection from hepatitis B surface antigen-positive women to their infants. Clin Gastroenterol Hepatol 2013; 11:1349–55. [DOI] [PubMed] [Google Scholar]
- 12.Pan CQ, Han GR, Jiang HX et al. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clin Gastroenterol Hepatol 2012; 10:520–6. [DOI] [PubMed] [Google Scholar]
- 13.Siberry GK, Jacobson DL, Kalkwarf HJ et al. Pediatric HIV/AIDS Cohort Study. Lower newborn bone mineral content associated with maternal use of tenofovir disoproxil fumarate during pregnancy. Clin Infect Dis 2015; 61:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenup AJ, Tan PK, Nguyen V et al. Efficacy and safety of tenofovir disoproxil fumarate in pregnancy to prevent perinatal transmission of hepatitis B virus. J Hepatol 2014; 61:502–7. [DOI] [PubMed] [Google Scholar]
- 15.Protection against viral hepatitis. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep 1990; 39(RR-2):1–26. [PubMed] [Google Scholar]
- 16.Benboud S, Pruvost A, Coffie PA et al. Concentrations of tenofovir and emtricitabine in breastmilk of HIV-1 infected women in Abidjan, Cote d'Ivoire, in the ANRS 12109 TEmAA Study, step 2. Antimicrob Agents Chemother 2011; 55:1315–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terrault NA, Bzowej NH, Chang KM et al. AASLD practice guidelines for the treatment of hepatitis B. Hepatology 2016; 63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]