Abstract
Recent evidence has suggested that diabetic neuropathy (DN) is pathophysiologically related to both impaired angiogenesis and a deficiency of neurotrophic factors in the nerves. It is widely known that vascular and neural growths are intimately associated. Mesenchymal stem cells (MSCs) promote angiogenesis in ischemic diseases and have neuroprotective effects, particularly on Schwann cells. Accordingly, we investigated whether DN could be improved by local transplantation of MSCs by augmenting angiogenesis and neural regeneration such as remyelination. In sciatic nerves of streptozotocin (STZ)-induced diabetic rats, motor and sensory nerve conduction velocities (NCVs) and capillary density were reduced, and axonal atrophy and demyelination were observed. After injection of bone marrow-derived MSCs (BM-MSCs) into hindlimb muscles, NCVs were restored to near-normal levels. Histological examination demonstrated that injected MSCs were preferentially and durably engrafted in the sciatic nerves, and a portion of the engrafted MSCs were distinctively localized close to vasa nervora of sciatic nerves. Furthermore, vasa nervora increased in density, and the ultrastructure of myelinated fibers in nerves was observed to be restored. Real-time RT-PCR experiments showed that gene expression of multiple factors involved in angiogenesis, neural function, and myelination were increased in the MSC-injected nerves. These findings suggest that MSC transplantation improved DN through direct peripheral nerve angiogenesis, neurotrophic effects, and restoration of myelination.
Keywords: Bone marrow, Diabetic neuropathy (DN), Angiogenesis, Myelination, Mesenchymal stem cells (MSCs)
INTRODUCTION
Diabetic neuropathy (DN), the most prevalent complication of diabetes, affects up to 60% of diabetic patients and frequently leads to foot ulcers and may result in limb amputations (31). Despite a continuous increase in the incidence of diabetes mellitus, there is no effective treatment for DN, and the only available measures are glucose control and pain management (9).
Neuroaxonal dystrophy is considered the pathological hallmark of DN in humans and chronic experimental diabetes (45), and considerable attention has been paid to the altered growth factor signaling in the degeneration of sensory neurons in diabetes. Schwann cells (SCs) also undergo substantial degenerative changes in DN (10). Although there is some controversy about alterations of the peripheral nervous system in genetic or induced experimental models of DN (48), there are many reports in the literature of morphological abnormalities in the myelinated fibers of the sciatic and tibial nerves of streptozotocin (STZ)-diabetic rats, such as axonal atrophy, axon–myelin separation, and demyelination (6,11,33,49). Myelinating SCs require axon-derived signals for regulating myelin thickness, which is one determinant of nerve conduction velocity (39), but the morphologic abnormality of SCs can be independent of axonal degeneration in diabetic humans and felines (40).
DN is characterized by reduction of vascularity in peripheral nerves and deficiency in angiogenic and neurotrophic factors (19,26,47). Studies have reported that experimental DN is characterized by reduced microcirculation in peripheral nerves from the destruction of the vasa nervora, the blood vessels within the nerves, and thus administration of angiogenic factors such as vascular endothelial growth factors (VEGFs), sonic hedgehog (SHH), and statin could restore neural function by augmenting angiogenesis (16,29,47). In addition, deficiency of neurotrophic factors, including nerve growth factor (NGF), ciliary neurotrophic factor (CNTF), glial-derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF), were reported as an important mechanism that underlies DN (4,14,41,53). Growing evidence suggests that many representative growth factors have dual neurotrophic and angiogenic effects and were even named angioneurins (30,56). Some examples include VEGF (52), insulin-like growth factor (IGF) (34), NGF (46), BDNF (24), and fibroblast growth factor-2 (FGF2, also known as bFGF) (32). To reverse the manifestations of DN, clinical trials have attempted to deliver a single neurotrophic or angiogenic factor as treatment in the form of protein or gene therapy; however, the effects were reported to be very modest if not negligible, presumably due to the complex pathophysiologic nature of human DN (1,21).
Recent studies have demonstrated that bone marrow (BM)-derived cells can effectively treat ischemic cardiovascular diseases through their paracrine effects (8,22,26). Since BM-derived cells include multiple angiogenic and neurotrophic cytokines, and cells exert paracrine effects more durably than the protein forms of these same cytokines, attempts were made to treat experimental DN using these cells, and the DN improved. Our group reported that BM-derived endothelial progenitor cells (EPCs) and mononuclear cells exert favorable therapeutic effects via enhanced neural neovascularization and neuroprotection (19,26). These studies strongly support DN being a disorder with pathogenic involvement of both vascular and neural components, with myelination being an important determinant of peripheral nerve function. Prior studies did not address the effects of BM-EPCs on SCs and myelination, and based on their scope of action, it is likely that there is no effect.
BM-MSCs have emerged as an important source for cell therapy. Since MSCs are expandable in culture and easy to obtain in large numbers, they have advantages over mononuclear cells and EPCs for cell therapy. MSCs can differentiate into multiple cell types (18) and secrete various angiogenic and neurotrophic factors, including FGF2 and VEGF (28). Owing to robust paracrine effects on cell survival and angiogenesis, MSCs were reported to be effective for the treatment of ischemic diseases such as myocardial infarction (13,55) and limb ischemia (3,15,28). MSCs were also reported to transdifferentiate into SC-like cells when cultured under specific conditions (2,23,44). While there are many potential advantages of using MSCs for DN, no studies have investigated their direct effects on neural angiogenesis and myelination in DN.
Accordingly, in the present study, we sought to investigate whether local transplantation of MSCs could attenuate or reverse DN by modulating both angiogenesis and neural aspects. Here we provide the first evidence that intramuscularly injected BM-MSCs preferentially migrated to nerves and directly modulated the function of diabetic nerves. MSC transplantation augmented vascularity and restored myelination by increasing humoral factors, such as angiogenic and neurotrophic factors, as well as myelination-related genes.
MATERIALS AND METHODS
Induction of Diabetes
All animal protocols were approved by the Institutional Animal Care and Use Committees at Emory University School of Medicine. We induced diabetes in 8-week-old male Wistar rats (Harlan, Indianapolis, IN, USA) by IP injection of STZ (75 mg/kg in 0.9% sterile saline; Sigma-Aldrich, St. Louis, MO, USA). Serum glucose levels were measured using an Accu-Check gluco meter (Roche Diagnostics, Indianapolis, IN, USA) 2 weeks later, and all animals with glucose levels <300 mg/dl were excluded from the study. These diabetic (DM) rats were randomly assigned to BM-derived MSC or saline injection groups 12 weeks after the induction of diabetes. Age- and sex-matched rats were used as nondiabetic (NDM) controls and received the same treatment.
Isolation of MSCs and Cell Culture
Bone marrow mononuclear cells from Wistar rats were isolated using HISTOPAQUE®-1083 (Sigma-Aldrich) according to the manufacturer's instructions. Whole bone marrow mononuclear cells from normal rats were plated in low-glucose (100 mg/dl) Dulbecco's modified Eagle medium (DMEM; Life Technologies, Grand Island, NY, USA) supplemented with 15% FBS (Life Technologies) and antibiotics (antibiotic–antimycotic; Life Technologies). After 3 days of incubation in a humidified incubator at 37°C with 5% CO2, nonadherent hematopoietic cells were removed by changing the medium. The adherent MSCs were further cultured as previously described (25). Passages 2 to 4 cells were used for this study.
Measurements of Nerve Conduction Velocity
Nerve conduction velocities (NCVs) were measured in hindlimbs bilaterally using standard orthodromic surface recording techniques and a Teca TD-10 portable recording system (Oxford Instruments, Abingdon, Oxfordshire, UK) at baseline (before treatment) and 2, 4, and 8 weeks after treatment (19). Motor NCV was calculated by dividing the distance between stimulating electrodes by the average latency difference between the peaks of the compound muscle action potentials evoked from two sites (sciatic notch and ankle). Sensory NCV was calculated by dividing the distance between stimulating and recording electrodes by the latency of the signal from the stimulation artifact to the onset of the peak signal. For each nerve, maximal velocities were determined bilaterally.
Fluorescent Imaging of Blood Vessels in Sciatic Nerves
Diabetic nerves were harvested 4 weeks after injection of DiI-labeled MSCs, fixed in 4% paraformaldehyde (Sigma-Aldrich) at 4°C for 16 h, and incubated in 30% sucrose (Sigma-Aldrich) solution for 24 h. Frozen sections of the nerves were obtained at a 14-μm thickness. Vascularity of sciatic nerves from both nondiabetic and diabetic rats was assessed by in situ fluorescent staining using the EC-specific marker BS-1 lectin conjugated to fluorescein isothiocyanate (FITC; Vector Laboratories, Burlingame, CA, USA) (19). Hindlimbs were perfused with FITC-conjugated BS-1 lectin (1 mg per rat) by IV injection. Fifteen minutes later, the animals were sacrificed, and the sciatic nerves were harvested and fixed in 4% paraformaldehyde. After fixation, samples were either whole mounted for longitudinal analysis or embedded in OCT compound (Sakura Finetek USA, Inc., Torrance, CA, USA) for frozen sections. Samples were analyzed using a computer-assisted Nikon fluorescence microscope with digital camera (Eclipse TE200; Nikon Inc., Tokyo, Japan). The number of vessels was counted in 10 randomly selected cross-sections under fluorescent microscopy (200×).
Real-Time Quantitative PCR
Gene expression was determined by TaqMan® real-time quantitative PCR on the 7500 Real Time PCR System (Life Technologies) using TaqMan® PCR Master Mix (Life Technologies) (27). Total RNA was extracted from tissue or cells with RNeasy Plus Mini Kit (Qiagen, Venlo, Limburg, Netherlands) according to the manufacturer's instructions. First-strand cDNA was generated using the TaqMan® Multiscribe Reverse Transcription Kit (Life Technologies) primed with a mix of oligo dT and random hexamers. Relative mRNA expression of target gene normalized to GAPDH was calculated by using the formula relative expression level = 2−ΔCt, where ΔCt = Ct gene of interest – Ct GAPDH. The primers and probes were designed using Primer Express 3.0 (Life Technologies) (Table 1).
Table 1.
Primers and Probes for TaqMan® qRT-PCR
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Probe (5′-3′) |
|---|---|---|---|
| Akt1 | ACGACCGCCTCTGCTTTG | ACACGCGCTCACGAGACA | CCAATGGAGGCGAGCTCTTCTTCCA |
| Angpt1 | CAGATACAACAGAATGCGGTTCA | TGAGACAAGAGGCTGGTTCCTAT | AACCACACGGCCACCATGCTGG |
| Angpt2 | CTACAGGATTCACCTTACAGGACTCA | CTTCCTGGTTGGCTGATGCT | TGATTTTGCCCGCCGTGCCT |
| Ccl2(MCP-1) | TGTCTCAGCCAGATGCAGTTAAT | CCGACTCATTGGGATCATCTT | CCCCACTCACCTGCTGCTACTCATTCA |
| Cntf | TGAAGACAGAAGCAAACCAGC | AGAACGGCTACAGAGGTCCC | TGGCTTTCGCAGAGCAAACACCT |
| Cxc12(SDF-1) | CTGGGCACAGTTACAGGTGGT | TGCTCTGGTGGAAGGTTGCT | CTCCCCAGGGCTCCCAGCAAA |
| Egr2 (Krox20) | TCGCCAGAAGGAGAGGAAGA | GAAGACTGGGCGGATGCA | AGTGCCCCCTCGTCAT |
| Fgf2 (bFGF) | AAGGATCCCAAGCGGCTCTA | CGGCCGTCTGGATGGA | ACGGCGGCTTCTTCCTGCGC |
| Gapdh | CCGAGGGCCCACTAAAGG | TGCTGTTGAAGTCACAGGAGACA | CATCCTGGGCTACACTGAGGACCAGG |
| Gdnf | TTGTAGCGGTTCCTGTGAAG | CCTGGCCTACCTTGTCACTT | CGGCCGAGACAATGTACGACAAA |
| Hgf | CTCAGTGTTCAGAAGTTGAATGCAT | TGCCTGATTCTGTGTGATCCA | CTGCAACGGTGAAAGCTACAGAGGTCCC |
| Igf1 | AGACGGGCATTGTGGATGA | ACATCTCCAGCCTCCTCAGATC | TGTTGCTTCCGGAGCT |
| Mag | CCTGGCCACGGTCATCTA | ACACCAGTACTCCCCATCGT | AGCTGGAACTCCCTGC |
| Mbp | CGAGGAGAGGCTGAAAAAAAGAG | TGTCTTCTGAGGCGGTCTGA | CGTTGGCAAGCTTT |
| Mmp2 | GCAAGGTGTGGTGTGCAAC | ATCCTTGGTCAGGACAGAAGC | CAACCAACTACGATGATGACCGGAAGTG |
| Mpz (P0) | CCTGAAGGAGGCCGAGAT | TTGAAGGTCCCCACCTCATC | CACTATGCCAAGGGTC |
| Ngfb | CACTGTGGACCCCAAACTGTTT | GGTGGAGGCTGGGTGCTA | AAACGGAGACTCCGTTCACCCCG |
| Ngfr (p75NTR) | TGGGCCTTGTGGCCTATATT | CGCCTTGTTTATTTTGTTTGCA | CTTTCAAGAGGTGGAACAG |
| Nos3 (eNos) | CACAAGAGTTACAAAATCCGATTCA | TCTGTGTTACTGGATTCCTTCCTTT | CCACTGGTATCCTCTTGGCGGCG |
| Ntrk3 (TrkC) | CGTCCTTCTGGTGGTCCTCTT | CAGCCACAGGACCCTTCATT | TCAACAAGTACGGTCGACGG |
| Pgf (Plgf) | ATGCTGGCCATGAAGCTGTT | GCCCCCTGGGAGTGTACAG | ACCCAGCTAGGACCTGCAAGAAGCAAG |
| Pmp22 | GTCCTGTCCCTGTTCCTGTT | GCTGCACTCATCACACACAG | ACTCTCACCAAAGGCGGCCG |
| S100b | GAGCAGGAAGTGGTGGACAAA | CACTCCCCATCCCCATCTT | TGATGGAGACGCTGGAC |
| Vegfa | GCGGGCTGCTGCAATG | CATAGTGACGTTGCTCTCCGAC | AGCCCTGGAGTGCGTGCCCA |
Histochemistry of Nerves
The structure of the sciatic nerve was determined at 4 weeks after MSC or PBS (Cellgro®; Corning, Corning, NY, USA) treatment as previously described by our group (47). Briefly, the nerve was fixed in 0.1 M sodium cacodylate (Sigma-Aldrich) buffer containing 2% glutaraldehyde (Sigma-Aldrich), 2% paraformaldehyde, and 3.5% sucrose for 2 h at 4°C with agitation. After three washes in 0.1 M sodium cacodylate buffer (10 min each), the rings were postfixed with 1% osmium tetroxide (Sigma-Aldrich) on ice for 2 h and washed three times (10 min each), all in 0.1 M sodium cacodylate buffer. The rings were then embedded in Epon 812 (Electron Microscopy Sciences, Hatfield, PA, USA) mixture and polymerized in an oven at 60°C for 24 h after dehydration in increasing concentrations of ethanol (50%, 70%, 80%, 90%, 95%, and 100%) and propylene oxide (Sigma-Aldrich) series (20 min each). Semithin (0.5–0.8 μm in thickness) sections were collected on the slide glass and subjected to toluidine blue (Sigma-Aldrich) followed by light microscopy. For the transmission electron microscope (TEM) study, embedded blocks were sectioned on an ultramicrotome with a diamond knife, and ultrathin sections were collected on copper grids. The grids were stained with 2.5% uranyl acetate (7 min; Sigma-Aldrich) and Reynolds lead citrate (2 min; Sigma-Aldrich) and were viewed with a Phillips 300 electron microscope.
Isolation and Culture of Schwann Cells
SCs were isolated from the sciatic nerve of Wistar rats (Harlan) through a series of enzymatic digestion, trituration, and cultivation steps on laminin (Sigma-Aldrich)-coated plates, concluding with fluorescence-activated cell sorting (FACS) with an antibody against NGFR (p75NTR, 1 μg/ml; STEMCELL Technologies Inc., Vancouver, BC, Canada). Isolated SCs and S16, a rat Schwann cell line, were cultured in high glucose (~900 mg/dl; Life Technologies) media to study whether neurotrophic factors and myelin-related genes are reduced in SCs. Normal MSCs and SCs isolated from diabetic rats were cocultured in MSC culture media at a 1:1 ratio for 1–10 days, and medium was changed every 48 h. At day 7, the mixture of MSCs and SCs was detached by trypsinization (trypsin; Life Technologies), and SCs were sorted again by magnetic-activated cell sorting (MACS) with anti-NGFR (p75NTR) antibody (STEMCELL Technologies Inc.). qRT-PCR on isolated SCs was used to examine whether MSCs affect gene expression of SCs.
Statistical Analysis
All results were expressed as a mean value ± SEM. Statistical analysis was performed by Student's t-test for comparisons between two groups. A value of p < 0.05 was considered to be statistically significant.
RESULTS
Local Transplantation of MSCs Improves NCVs in Diabetic Neuropathy
To investigate the therapeutic effects of local transplantation of MSCs on diabetic nerves, diabetic rats were randomly assigned to MSC (DM + MSC)- or saline-treated (DM + PBS) groups. After intramuscular injection of 5 million MSCs or an equal volume of PBS around the sciatic nerves, we measured both motor and sensory nerve conduction velocities (NCVs) at 0, 2, 4, and 8 weeks (n = 4–6, each group) after treatment. We selected this dose based on our previous study with the same rat DN model and studies by others in which MSCs were used for hindlimb ischemia or infarcted heart (17,26,38). Nondiabetic rats that received PBS (NDM + PBS) or MSCs (NDM + MSCs) were used as controls. Electrophysiological study showed about 38% decrease in both motor and sensory NCVs in diabetic rats (DM) at 12 weeks of diabetes compared to nondiabetic rats (NDM), indicating development of severe peripheral neuropathy (NDM + PBS vs. DM + PBS: motor NCV, 78.4 ± 8.7 m/s vs. 49.2 ± 3.5 m/s; sensory NCV, 71.0 ± 2.4 m/s vs. 43.5 ± 3.0 m/s; p < 0.05 for both) (Fig. 1A, B). After local injection of MSCs, both motor and sensory NCVs of diabetic rats (DM + MSC) were restored to normal levels over 8 weeks (76.8 ± 3.3 m/s and 75.1 ± 3.0 m/s, both p < 0.05 vs. DM + PBS; not significantly different from NDM + PBS or NDM + MSC). These data indicate that MSCs could effectively improve reduced NCVs in diabetic rats.
Figure 1.
Local transplantation of MSCs improved nerve conduction velocities (NCVs) in diabetic nerves. Twelve weeks after streptozotocin injection, both motor NCVs (A) and sensory NCVs (B) in diabetic rats were significantly lower than in age-matched non-diabetic rats (NDM + PBS vs. DM + PBS: **p < 0.05; n = 6–8, each group). MSC injection significantly improved both motor (A) and sensory NCVs (B) in diabetic rats over 8 weeks (DM + PBS vs. DM + MSC: *p < 0.05; n = 6–8, each group). Abbreviations: MSC, bone marrow-derived mesenchymal stem cells; DM + MSC, diabetic rats treated with MSCs; DM + PBS, diabetic rats treated with PBS; NCV, nerve conduction velocity; NDM + MSC, nondiabetic rats treated with MSCs; NDM + PBS, nondiabetic rats treated with PBS.
MSCs Increase Vascularization in Diabetic Nerves
To examine changes in the functional blood vessels at the histological level, we harvested sciatic nerves 4 and 8 weeks after treatment, following systemic injection of FITC-conjugated BS-1 lectin to visualize functional blood vessels. Whole mount longitudinal images demonstrated that the nerves from nondiabetic rats (NDM + PBS) were well vascularized and had clear epineurial longitudinal networks and penetrating branches running from epineurial to endoneurial vessels (Fig. 2). However, in diabetic rats (DM + PBS), both the epineurial and endoneurial vessels, and especially small branches from the epineurial vessels, were markedly decreased, leaving focal areas very poorly vascularized. In contrast, MSC-injected diabetic rats (DM + MSC) showed increased vascularity in sciatic nerves, particularly small branches of epineurial blood vessels (Fig. 2A). The area of vessels in the sciatic nerves was significantly higher in the MSC-transplanted rats compared to the PBS-injected ones (Fig. 2B).
Figure 2.
MSC transplantation improved neural vascularity in diabetic nerves. Rat hindlimbs were perfused with FITC-conjugated BS-1 lectin 4 and 8 weeks after MSC transplantation to visualize functional blood vessels. In longitudinal whole-mount images of the sciatic nerves, vasa nervora, represented by green fluorescence (A), and quantification of vascular area at 8 weeks after MSC transplantation (B) indicated that control diabetic nerves (DM + PBS) showed a marked decrease in small branches of large epineurial blood vessels and percent vascular area. This reduced neural vascularity in diabetic control rats (DM + PBS) compared to nondiabetic rats (NDM + PBS) was significantly increased after MSC transplantation (DM-MSC). n = 4. Abbreviations: MSC, bone marrow-derived mesenchymal stem cells; DM + MSC, diabetic rats treated with MSCs; DM + PBS, diabetic rats treated with PBS; NDM + PBS, non-diabetic rats treated with PBS. Scale bar: 100 μm.
Transplanted MSCs Are Preferentially Localized to Nerves and Vasa Nervora
To explore the mechanisms underlying the improvement in nerve function following MSC treatment, we examined MSC distribution 4 and 8 weeks after cell injection. To track the injected MSCs, we labeled them with a red fluorescent dye, CM-DiI. Because we injected cells directly into the thigh muscles, we examined the cell fate in both muscles and sciatic nerves. Before sacrifice, FITC-conjugated BS-1 lectin was injected into the abdominal aorta to visualize blood vessels in hindlimbs. Whole mount longitudinal images under fluorescent microscopy showed that a large number of the injected MSCs were engrafted in nerves (Fig. 3A), and many of them were observed along the vasa nervora (Fig. 3B). These findings clearly support the tropism of MSCs toward nerves in diabetes. This close spatial relationship of MSCs with vasa nervora suggests that neurally engrafted MSCs were likely to have induced angiogenesis in the vasa nervora by secreting cytokines and by physical interaction.
Figure 3.
Intramuscularly injected MSCs migrated and engrafted in nerves. (A) A representative whole-mount image of a sciatic nerve from a diabetic rat after perfusion with FITC-conjugated BS-1 lectin (green) demonstrated preferential and sustained engraftment of DiI-labeled MSCs (red) into sciatic nerves at 4 and 8 weeks after MSC transplantation. (B) Higher magnification images showed alignment of MSCs mainly along the vasa nervosa at 8 weeks after MSC transplantation. Scale bars: 100 μm (A) and 50 μm (B).
MSC Transplantation Increases Expression of Angiogenic and Neurotrophic Factors Reduced in Diabetic Nerves
Accordingly, we next determined whether transplantation of MSCs can increase gene expression of angiogenic and neurotrophic factors in diabetic sciatic nerves harvested at 2 weeks after treatment. Quantitative real-time RT-PCR (qRT-PCR) analysis showed that mRNA expression levels of angiogenic and neurotrophic genes in diabetic nerves were significantly lower than in nondiabetic control nerves (DM + PBS vs. NDM + PBS, Vegfa: 0.4 ± 0.1-fold, Hgf: 0.2 ± 0.1-fold, Mmp2: 0.3 ± 0.1-fold, Cxcl12: 0.5 ± 0.1-fold, p < 0.001; Fgf2: 0.5 ± 0.2-fold, Angpt1: 0.1 ± 0.03-fold, Angpt2: 0.4 ± 0.1-fold, Nos3: 0.4 ± 0.1-fold, Igf1, 0.2 ± 0.1-fold, Ngfb: 0.4 ± 0.2-fold, Ccl2: 0.4 ± 0.1-fold, p < 0.01; Pgf: 0.4 ± 0.2-fold, p < 0.05) but were significantly increased by MSC transplantation (DM + MSCs vs. DM + PBS, Vegfa: 4.4 ± 1.0-fold, Igf1: 4.4 ± 1.0-fold, Mmp2: 5.3 ± 1.7-fold, Ccl2: 5.6 ± 1.3-fold, Cxcl12: 3.5 ± 0.7-fold, p < 0.001; Angpt1: 19.6 ± 8.2-fold, Angpt2: 2.9 ± 0.8-fold, Nos3: 3.2 ± 1.1-fold, p < 0.01; Fgf2: 3.2 ± 1.3-fold, Pgf: 2.9 ± 1.2-fold, Hgf: 6.6 ± 3.7-fold, Ngfb: 3.0 ± 1.3-fold, p < 0.05) (Fig. 4). These findings indicate that intramuscular injection of MSCs restored the defective expression of multiple humoral factors associated with angiogenesis and neuronal survival in diabetic nerves.
Figure 4.
Upregulation of various humoral factors in diabetic sciatic nerves injected with MSCs. Two weeks after intramuscular injection of MSCs, total RNA was isolated from the sciatic nerves. The levels of mRNA of multiple humoral factors were determined by qRT-PCR and normalized to Gapdh × 1,000. The levels of angiogenic and neurotrophic factors were significantly higher in diabetic nerves injected with MSCs (DM + MSC) than in those treated with PBS (DM + PBS) (*p < 0.05; **p < 0.01; ***p < 0.001; n = 4–6).
MSC Transplantation Restores Ultrastructural Morphology of Myelinated Fibers Altered in Diabetic Nerves
We then examined whether MSC transplantation could affect the ultrastructure of diabetic nerves, which is characterized by morphological alterations in the myelin sheath, a hallmark of DN. Accordingly, we performed transmission electron microscopy for sciatic nerves obtained at 4 weeks after MSC or PBS injection. Ultrastructure of the sciatic nerve in the NDM + PBS group was normal (Fig. 5A). In the DM + PBS group, degeneration of myelinated axons was apparent with splitting and infolding of the lamellae in the myelin sheath. Axonal atrophy, illustrated by noticeable shrinkage of the axon detached from the myelin sheath, was also observed (Fig. 5B). However, the diabetic animals treated with MSCs (DM + MSC group) showed markedly fewer morphologic alterations in ultrastructural features of myelin sheath and axons. Lamellar separation of the myelin sheath and axonal atrophy were less obvious. The fine structure of SCs in the DM + MSC group was seemingly normal, as in the NDM + PBS group (Fig. 5C). These data suggest that MSC transplantation restored the ultrastructure of the myelin sheath and axons in diabetic sciatic nerves.
Figure 5.
Degenerative structure of myelin in diabetic nerves restored by MSC transplantation. (A) Electron microscopy of sciatic nerve cross-sections from nondiabetic rats injected with PBS (NDM + PBS), diabetic rats injected with PBS (DM + PBS), and diabetic rats injected with MSCs (DM + MSC). (B) Diabetic nerves (DM + PBS) showed splitting and infolding lamellar layers in myelin sheaths and separation of shrunken axon from myelin sheath (arrows). (C) MSC-treated diabetic nerves (DM + MSC) showed restored ultrastructure of myelinated fibers and axons similar to normal. Scale bars: 5 μm (A) and 2 μm (B, C).
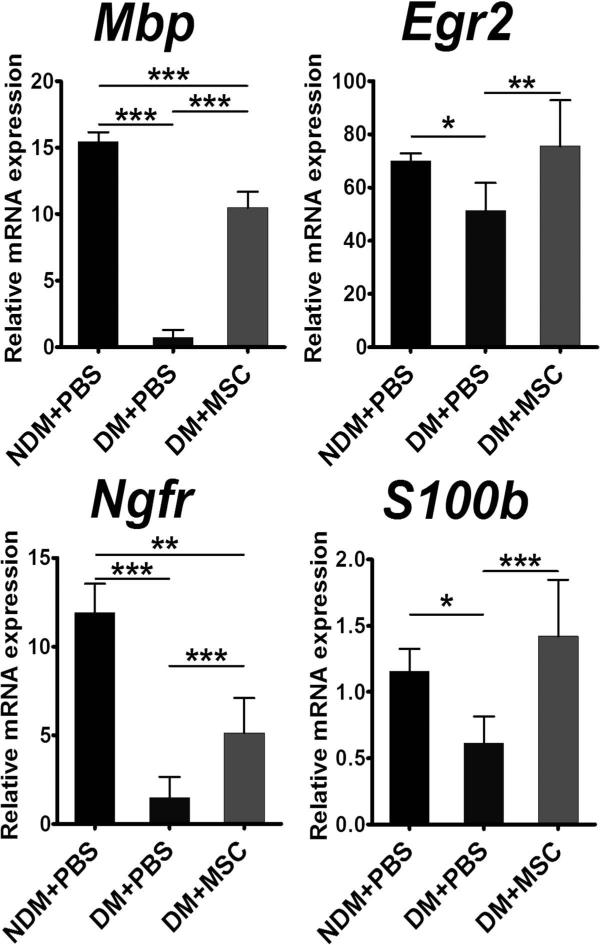
Gene Expression Related to Myelination Is Reduced in Diabetic Nerves and Is Increased by MSC Transplantation
We next explored whether MSC transplantation could also affect the myelination-related genes that are exclusively expressed in SCs. We harvested sciatic nerves 2 weeks after intramuscular injection of MSCs or PBS and conducted qRT-PCR. We found that mRNA expression of genes related to myelination was in general decreased in diabetic nerves, showing statistically significant reduction in myelin basic protein (Mbp) and nerve growth factor receptor (Ngfr, also known as p75NTR) (DM + PBS vs. NDM + PBS, Mbp: 0.1 ± 0.04-fold, Ngfr: 0.1 ± 0.1-fold, p < 0.001). After MSC transplantation, expression of all these genes clearly increased, with significant statistical difference shown for Mbp (DM + PBS vs. DM + MSC, 14.3 ± 11.2-fold, p < 0.001) (Fig. 6). Expression of early growth response 2 (Egr2, also known as Krox20), S100 calcium-binding protein B (S100b), and Ngfr was not significant due to large individual variations (Fig. 6). These data suggest that MSC injection paracrinely modulated gene function related to SCs and myelination.
Figure 6.
Effect of MSC injection on the levels of myelination-related genes. Two weeks after intramuscular injection of MSCs, total RNA was isolated from the sciatic nerves. The levels of mRNA of myelination-related genes were determined using qRT-PCR and normalized to Gapdh ×1,000. The levels of Mpb, a major myelination protein for peripheral nerve, were significantly higher in diabetic nerves treated with MSC (DM + MSC) than in those treated with PBS (DM + PBS). *p < 0.05, **p < 0.0, ***p < 0.001 (n = 4–6).
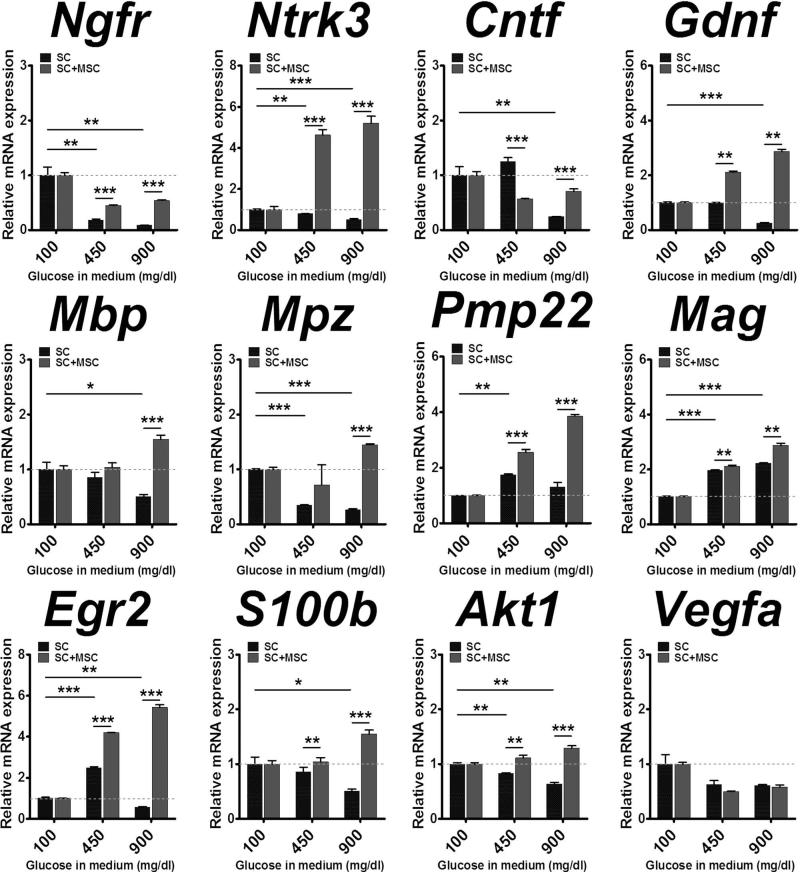
Reduced Gene Expression in Cultured Schwann Cells Are Restored by Coculture With MSCs
To further confirm paracrine effects of MSCs on the gene expression of Schwann cells in a more controlled setting, we cocultured these two cells in normal and two high-glucose conditions (450 mg/dl and 900 mg/dl). Seven days after culture, only SCs were isolated from coculture by MACS with anti-NGFR (p75NTR) antibody. As a control, SCs were cultured alone without MSCs under the same conditions. The SCs that were either cultured alone or cocultured with MSCs were subjected to qRT-PCRs. In single-cultured SCs, the expression levels of neurotrophin receptors Ngfr and Ntrk3 (also known as TrkC) were significantly decreased in high compared to normal glucose conditions. However, cocultured SCs showed significantly higher expression of these two genes compared to single-cultured SCs in high-glucose conditions (Fig. 7). Similar patterns were found in neurotrophins Cntf and Gdnf, which are key mediators for peripheral nerve myelination, myelination-related genes Mbp, Mpz, Pmp22, Mag, Egr2, and S100b, and cell survival gene Akt1 (Fig. 7). These results provide strong support that MSCs modulate the gene expression of SC identity and function in diabetic conditions.
Figure 7.
Upregulation of neurotrophic factors and myelination-related genes in diabetic Schwann cells cultured with MSCs. Schwann cells (SCs) were cultured with and without MSCs in different glucose concentrations to mimic diabetic conditions. Seven days after culture, only SCs were isolated by MACS and subjected to qRT-PCR. The expression levels of SC-specific genes were reduced at 450 mg/dl and 900 mg/dl glucose compared to the levels at 100 mg/dl (dotted lines), but were significantly increased after coculture with MSCs. *p < 0.05, **p < 0.01, ***p < 0.001 (n = 4–6).
DISCUSSION
In this study, we have demonstrated that local transplantation of BM-derived MSCs improved experimental DN through direct effects on peripheral nerves. DN is characterized by vascular insufficiency, axonal atrophy, and myelination defects. As a common pathogenetic mechanism, deficiency of both angiogenic and neurotrophic factors are suggested. Accordingly, we hypothesized that a therapy that can modulate both vascular and neural components would effectively reverse DN. In this regard, MSCs are an optimal candidate because they possess paracrine factors that affect both angiogenic and neural components. In this study, we have identified several new mechanisms by which MSCs can improve DN. While other studies have suggested the indirect effects of MSCs on DN, that is, enhanced angiogenesis in the muscle by MSC injection improved blood flow in diabetic nerve (50), our study for the first time demonstrated the direct effects of MSCs on peripheral nerves. First, our study demonstrated engraftment of numerous MSCs in the diabetic nerves. Second, a robust increase in vascularity in diabetic nerves was found after MSC transplantation. Third, this study also offers the first evidence that MSCs can directly modulate myelination and axonal regeneration through their paracrine effects in DN.
The neurotropism of BM-derived MSCs is of note. Intramuscularly injected MSCs migrated to the sciatic nerves and were heavily localized within the nerves, particularly near vasa nervora. Although the mechanism remains to be determined, such a significant magnitude of tropism of MSCs to blood vessels is unreported. These phenomena were previously noted in our studies with BM-derived EPCs and mononuclear cells and seem to be a common mechanism for BM-derived cells on diabetic nerves (19,26). The engrafted MSCs survived in the nerves long term (>8 weeks). While studies showed the short-lasting engraftment of transplanted BM cells in a myocardial infarction model (5,42), our data demonstrated that the same cells remain engrafted much longer in the peripheral nerves, suggesting the importance of host niches for survival of the transplanted cells. These common characteristics of BM-derived cells, that is, robust peripheral neurotropism, sustained engraftment, and vascular localization, could have caused robust and prolonged paracrine effects and led to the reversal of functional and histological impairment of peripheral nerves in DN.
MSC transplantation increased anatomical and functional vascularity in diabetic nerves, which was shown by BS-1 lectin perfusion studies. Mechanistically, angio-genesis played a major role in enhanced neovascularization in nerves; we found hardly any transdifferentiation of MSCs into endothelial cells. Supporting this notion is the increased expression of representative angiogenic factors, such as Vegfa, Fgf2, Angpt1, Angpt2, Pgf, Hgf, Nos3, Igf1,and Ngfb after MSC transplantation, which were reduced in diabetic nerves. These factors also function as neurotrophic factors, which can protect neurons and recover SC function. This upregulation of dual angio-neurotrophic factors may be one of the greatest benefits of stem cell therapy over a single protein or gene therapy, as this function can induce neural and vascular recovery synergistically.
MSC transplantation also restored neural structure and function in diabetic nerves. MSCs directly upregulated the expression of myelination-related genes and neurotrophic factors, enhanced the cellular functions of SCs and neurons, and restored myelinated fiber structures in diabetic nerves. In our DN model, decreased motor and sensory NCVs indicated the presence of defects in myeli-nation. In fact, electron microscopy studies demonstrated degenerative changes in axons and defective myelination in the sciatic nerves. Moreover, expression of genes related to myelination, such as Mbp and Ngfr, as well as the above-mentioned angio-neurotrophic factors were significantly suppressed in DN. SCs synthesize myelin while providing multiple support roles for the axon, contributing to saltatory conduction along axons, nerve and axon development, and axonal regeneration. SCs also provide a microenvironment favoring neural regeneration partially due to production of several neurotrophic factors (12). Dysfunction of SCs is known to play an important role in the pathogenesis of DN where hyperglycemia is considered pathogenic (20,51). In this study, in vitro experiments with cultured SCs in high-glucose conditions elicited reduced gene expression of neurotrophin receptors Ngfr and Ntrk3; neurotrophins Cntf and Gdnf; myelination-related genes Mbp, Mpz, Pmp22, Mag, Egr2, and S100b; and cell survival factors Akt and Vegfa, indicating dysfunction of SCs. Experiments with both MSC transplantation in vivo and coculture of diabetic SCs with MSCs in vitro demonstrated restoration of expression of these genes to nondiabetic levels, suggesting powerful paracrine effects of MSCs on SCs and, potentially, neurons that can underlie improved neural function and myelination in DN. While further studies are required to fully elucidate the molecular mechanisms, these direct effects of MSCs on SCs and neurons could offer new insight into the treatment of DN. Although previous studies have reported that MSCs can be transdifferentiated into cells with a SC phenotype (7,23,35–37), we rarely found such transdifferentiation in this study, ruling out their meaningful contribution to the therapeutic effects. In addition, a study reported that the functional features of transdifferentiated cells are still questionable (43).
There are some shared features of BM-derived cells between MSCs, EPCs, and MNCs in their effects on DN (19,26). All these cell types ameliorate DN through dual vascular and neurotrophic effects. These cells improved neural function, increased the vascularity, and demonstrated neurotropism, particularly vasa nervora. However, MNCs have less long-term engraftment potential compared to EPCs and MSCs. While angio-neurotrophic genes were generally enriched in all cell types, the types of increased genes were different among cell types. In MSCs, myelin-related and neurotrophin family genes were more abundant, which could underlie therapeutic effects on myelination and axonal regeneration. From a practical aspect, MSCs are more appealing for clinical application due to their easy and inexpensive isolation and expansion characteristics as well as their lack of significant immunogenicity.
Advanced DN, which has no other therapeutic option, can be an initial optimal target for cell therapy. Since this stage of DN is commonly associated with diabetic foot ulcers and/or peripheral vascular disease for which MSCs are also reported effective, a therapeutic approach of using MSCs in this advanced DN can be more valuable and effective. As the safety of autologous BM-derived MSCs has been documented by a number of clinical trials (54,57), MSC therapy for DN can be easily translated into clinical trials. Taken together, these findings suggest that BM-derived MSC transplantation represents a novel therapeutic option for treating DN.
ACKNOWLEDGMENTS
We would like to thank Andrea Wecker for critical reading of the manuscript, and Min Young Sin, Kisoo Kim, and Young Tak Joe for excellent technical assistance. This work was supported in part by NIH grants DP3DK094346; NIH contract, HHSN268201000043C; and NSF-EBICS grant, CBET-0939511.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Ajroud-Driss S, Christiansen M, Allen JA, Kessler JA. Phase 1/2 open-label dose-escalation study of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with painful diabetic peripheral neuropathy. Mol. Ther. 2013;21(6):1279–1286. doi: 10.1038/mt.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002;39(3):229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Khaldi A, Al-Sabti H, Galipeau J, Lachapelle K. Therapeutic angiogenesis using autologous bone marrow stromal cells: Improved blood flow in a chronic limb ischemia model. Ann. Thorac. Surg. 2003;75(1):204–209. doi: 10.1016/s0003-4975(02)04291-1. [DOI] [PubMed] [Google Scholar]
- 4.Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. Gdnf rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the pi3k/akt pathway. J. Clin. Invest. 2006;116(2):344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 6.Britland ST, Sharma AK, Duguid IG, Thomas PK. Ultrastructural observations on myelinated fibres in the tibial nerve of streptozotocin-diabetic rats: Effect of insulin treatment. Life Support Syst. 1985;3(Suppl. 1):524–529. [PubMed] [Google Scholar]
- 7.Caddick J, Kingham PJ, Gardiner NJ, Wiberg M, Terenghi G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006;54(8):840–849. doi: 10.1002/glia.20421. [DOI] [PubMed] [Google Scholar]
- 8.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J. Exp. Med. 2007;204(13):3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong MS, Hester J. Diabetic painful neuropathy: Current and future treatment options. Drugs. 2007;67(4):569–585. doi: 10.2165/00003495-200767040-00006. [DOI] [PubMed] [Google Scholar]
- 10.Eckersley L. Role of the Schwann cell in diabetic neuropathy. Int. Rev. Neurobiol. 2002;50:293–321. doi: 10.1016/s0074-7742(02)50081-7. [DOI] [PubMed] [Google Scholar]
- 11.Fazan VP, Salgado HC, Barreira AA. Aortic depressor nerve myelinated fibers in acute and chronic experimental diabetes. Am. J. Hypertens. 2006;19(2):153–160. doi: 10.1016/j.amjhyper.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18(7):397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 13.Gnecchi M, Danieli P, Cervio E. Mesenchymal stem cell therapy for heart disease. Vascul. Pharmacol. 2012;57(1):48–55. doi: 10.1016/j.vph.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Grandis M, Nobbio L, Abbruzzese M, Banchi L, Minuto F, Barreca A, Garrone S, Mancardi GL, Schenone A. Insulin treatment enhances expression of igf-i in sural nerves of diabetic patients. Muscle Nerve. 2001;24(5):622–629. doi: 10.1002/mus.1047. [DOI] [PubMed] [Google Scholar]
- 15.Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, Krishnamurthy S, Anthony N, Pherwani A, Majumdar AS. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J. Transl. Med. 2013;11:143. doi: 10.1186/1479-5876-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ii M, Nishimura H, Kusano KF, Qin G, Yoon YS, Wecker A, Asahara T, Losordo DW. Neuronal nitric oxide synthase mediates statin-induced restoration of vasa nervorum and reversal of diabetic neuropathy. Circulation. 2005;112(1):93–102. doi: 10.1161/CIRCULATIONAHA.104.511964. [DOI] [PubMed] [Google Scholar]
- 17.Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K, Kitamura S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc. Res. 2005;66(3):543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Jackson L, Jones DR, Scotting P, Sottile V. Adult mesenchymal stem cells: Differentiation potential and therapeutic applications. J. Postgrad. Med. 2007;53(2):121–127. doi: 10.4103/0022-3859.32215. [DOI] [PubMed] [Google Scholar]
- 19.Jeong JO, Kim MO, Kim H, Lee MY, Kim SW, Ii M, Lee JU, Lee J, Choi YJ, Cho HJ. Dual angiogenic and neurotrophic effects of bone marrow-derived endothelial progenitor cells on diabetic neuropathy. Circulation. 2009;119(5):699–708. doi: 10.1161/CIRCULATIONAHA.108.789297. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jessen KR, Mirsky R. Negative regulation of myelination: Relevance for development, injury, and demyelinating disease. Glia. 2008;56(14):1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 21.Kalichman MW, Powell HC, Mizisin AP. Reactive, degenerative, and proliferative Schwann cell responses in experimental galactose and human diabetic neuropathy. Acta Neuropathol. 1998;95(1):47–56. doi: 10.1007/s004010050764. [DOI] [PubMed] [Google Scholar]
- 22.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104(9):1046–1052. doi: 10.1161/hc3501.093817. others. [DOI] [PubMed] [Google Scholar]
- 23.Keilhoff G, Stang F, Goihl A, Wolf G, Fansa H. Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell. Mol. Neurobiol. 2006;26(7–8):1235–1252. doi: 10.1007/s10571-006-9029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR. Neurotrophins promote revascularization by local recruitment of trkb+ endothelial cells and systemic mobilization of hematopoietic progenitors. J. Clin. Invest. 2005;115(3):653–663. doi: 10.1172/JCI200522655. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H, Han JW, Lee JY, Choi YJ, Sohn YD, Song M, Yoon YS. Diabetic mesenchymal stem cells are ineffective for improving limb ischemia due to their impaired angiogenic capability. Cell Transplant. 2015;24(8):1571–1584. doi: 10.3727/096368914X682792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Park JS, Choi YJ, Kim MO, Huh YH, Kim SW, Han JW, Lee J, Kim S, Houge MA. Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem Cells. 2009;27(7):1686–1696. doi: 10.1002/stem.87. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived cd31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J. Am. Coll. Cardiol. 2010;56(7):593–607. doi: 10.1016/j.jacc.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 29.Kusano KF, Allendoerfer KL, Munger W, Pola R, Bosch-Marce M, Kirchmair R, Yoon YS, Curry C, Silver M, Kearney M. Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arterioscler. Thromb. Vasc. Biol. 2004;24(11):2102–2107. doi: 10.1161/01.ATV.0000144813.44650.75. others. [DOI] [PubMed] [Google Scholar]
- 30.Lazarovici P, Marcinkiewicz C, Lelkes PI. Cross talk between the cardiovascular and nervous systems: Neurotrophic effects of vascular endothelial growth factor (vegf) and angiogenic effects of nerve growth factor (ngf)-implications in drug development. Curr. Pharm. Des. 2006;12(21):2609–2622. doi: 10.2174/138161206777698738. [DOI] [PubMed] [Google Scholar]
- 31.Leinninger GM, Vincent AM, Feldman EL. The role of growth factors in diabetic peripheral neuropathy. J. Peripher. Nerv. Syst. 2004;9(1):26–53. doi: 10.1111/j.1085-9489.2004.09105.x. [DOI] [PubMed] [Google Scholar]
- 32.Lenhard T, Schober A, Suter-Crazzolara C, Unsicker K. Fibroblast growth factor-2 requires glial-cell-line-derived neurotrophic factor for exerting its neuroprotective actions on glutamate-lesioned hippocampal neurons. Mol. Cell. Neurosci. 2002;20(2):181–197. doi: 10.1006/mcne.2002.1134. [DOI] [PubMed] [Google Scholar]
- 33.Liu GS, Shi JY, Lai CL, Hong YR, Shin SJ, Huang HT, Lam HC, Wen ZH, Hsu KS, Chen CH. Peripheral gene transfer of glial cell-derived neurotrophic factor ameliorates neuropathic deficits in diabetic rats. Hum. Gene Ther. 2009;20(7):715–727. doi: 10.1089/hum.2009.002. others. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. USA. 2004;101(26):9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L, Chen X, Zhang CW, Yang WL, Wu YJ, Sun L, Bai LM, Gu XS, Ahmed S, Dawe GS. Morphological and functional characterization of predifferentiation of myelinating glia-like cells from human bone marrow stromal cells through activation of f3/ notch signaling in mouse retina. Stem Cells. 2008;26(2):580–590. doi: 10.1634/stemcells.2007-0106. others. [DOI] [PubMed] [Google Scholar]
- 36.Mahay D, Terenghi G, Shawcross SG. Growth factors in mesenchymal stem cells following glial-cell differentiation. Biotechnol. Appl. Biochem. 2008;51(Pt 4):167–176. doi: 10.1042/BA20070212. [DOI] [PubMed] [Google Scholar]
- 37.Mahay D, Terenghi G, Shawcross SG. Schwann cell mediated trophic effects by differentiated mesenchymal stem cells. Exp. Cell Res. 2008;314(14):2692–2701. doi: 10.1016/j.yexcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 39.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304(5671):700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 40.Mizisin AP, Shelton GD, Wagner S, Rusbridge C, Powell HC. Myelin splitting, Schwann cell injury and demyelination in feline diabetic neuropathy. Acta Neuropathol. 1998;95(2):171–174. doi: 10.1007/s004010050783. [DOI] [PubMed] [Google Scholar]
- 41.Mizisin AP, Vu Y, Shuff M, Calcutt NA. Ciliary neurotrophic factor improves nerve conduction and ameliorates regeneration deficits in diabetic rats. Diabetes. 2004;53(7):1807–1812. doi: 10.2337/diabetes.53.7.1807. [DOI] [PubMed] [Google Scholar]
- 42.Muller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J, Schwinger RH. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J. Mol. Cell. Cardiol. 2006;41(5):876–884. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 43.Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin. Pharmacol. Ther. 2007;82(3):241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 44.Ravasi M, Scuteri A, Pasini S, Bossi M, Menendez VR, Maggioni D, Tredici G. Undifferentiated MSCs are able to myelinate drg neuron processes through p75. Exp. Cell Res. 2013;319(19):2989–2999. doi: 10.1016/j.yexcr.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Said G. Diabetic neuropathy–A review. Nat. Clin. Pract. Neurol. 2007;3(6):331–340. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- 46.Salis MB, Graiani G, Desortes E, Caldwell RB, Madeddu P, Emanueli C. Nerve growth factor supplementation reverses the impairment, induced by type 1 diabetes, of hindlimb post-ischaemic recovery in mice. Diabetologia. 2004;47(6):1055–1063. doi: 10.1007/s00125-004-1424-5. [DOI] [PubMed] [Google Scholar]
- 47.Schratzberger P, Walter DH, Rittig K, Bahlmann FH, Pola R, Curry C, Silver M, Krainin JG, Weinberg DH, Ropper AH. Reversal of experimental diabetic neuropathy by vegf gene transfer. J. Clin. Invest. 2001;107(9):1083–1092. doi: 10.1172/JCI12188. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma AK, Thomas PK. Peripheral nerve structure and function in experimental diabetes. J. Neurol. Sci. 1974;23(1):1–15. doi: 10.1016/0022-510x(74)90136-1. [DOI] [PubMed] [Google Scholar]
- 49.Shi X, Chen Y, Nadeem L, Xu G. Beneficial effect of tnf-alpha inhibition on diabetic peripheral neuropathy. J. Neuroinflammation. 2013;10:69. doi: 10.1186/1742-2094-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibata T, Naruse K, Kamiya H, Kozakae M, Kondo M, Yasuda Y, Nakamura N, Ota K, Tosaki T, Matsuki T. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes. 2008;57(11):3099–3107. doi: 10.2337/db08-0031. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shy ME. Biology of peripheral inherited neuropathies: Schwann cell axonal interactions. Adv. Exp. Med. Biol. 2009;652:171–181. doi: 10.1007/978-90-481-2813-6_11. [DOI] [PubMed] [Google Scholar]
- 52.Simovic D, Isner JM, Ropper AH, Pieczek A, Weinberg DH. Improvement in chronic ischemic neuropathy after intramuscular phvegf165 gene transfer in patients with critical limb ischemia. Arch. Neurol. 2001;58(5):761–768. doi: 10.1001/archneur.58.5.761. [DOI] [PubMed] [Google Scholar]
- 53.Wellmer A, Misra VP, Sharief MK, Kopelman PG, Anand P. A double-blind placebo-controlled clinical trial of recombinant human brain-derived neurotrophic factor (rhbdnf) in diabetic polyneuropathy. J. Peripher. Nerv. Syst. 2001;6(4):204–210. doi: 10.1046/j.1529-8027.2001.01019.x. [DOI] [PubMed] [Google Scholar]
- 54.Wen Y, Meng L, Xie J, Ouyang J. Direct autologous bone marrow-derived stem cell transplantation for ischemic heart disease: A meta-analysis. Expert Opin. Biol. Ther. 2011;11(5):559–567. doi: 10.1517/14712598.2011.560567. [DOI] [PubMed] [Google Scholar]
- 55.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 2011;109(8):923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 2008;9(3):169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 57.Zhang C, Sun A, Zhang S, Yao K, Wu C, Fu M, Wang K, Zou Y, Ge J. Efficacy and safety of intracoro-nary autologous bone marrow-derived cell transplantation in patients with acute myocardial infarction: Insights from randomized controlled trials with 12 or more months follow-up. Clin. Cardiol. 2010;33(6):353–360. doi: 10.1002/clc.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]