Abstract
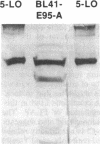
The expression of arachidonate 5-lipoxygenase (arachidonate:oxygen 5-oxidoreductase, EC 1.13.11.34) and the 5-lipoxygenase-activating protein (FLAP) genes in human tonsillar B cells and lymphoblastoid B-cell lines was demonstrated at the transcriptional level by reverse transcription-PCR analysis. Also, five lymphoblastoid T-cell lines were investigated and found to express the FLAP gene but not the 5-lipoxygenase gene, suggesting that the transcriptional regulation of these two genes is different. Western blot analysis of the cytosolic proteins from a lymphoblastoid B-cell line with an antiserum raised against purified human leukocyte 5-lipoxygenase revealed an immunoreactive band that comigrated with recombinant human 5-lipoxygenase. Intact B cells produced very low amounts of leukotriene B4 and 5-hydroxyeicosatetraenoic acid upon stimulation with the calcium ionophore A23187 and arachidonic acid, in comparison to the amounts formed by sonicates of these cells. However, preincubation of intact lymphoblastoid B cells with the glutathione-depleting agents azodicarboxylic acid bis(dimethylamide) or 1-chloro-2,4-dinitrobenzene prior to the addition of the calcium ionophore A23187 and arachidonic acid led to similar amounts of leukotriene B4 as were formed by sonicated cells. In contrast, the glutathione synthesis inhibitor buthionine sulfoximine diminished the cellular level of glutathione by greater than 90% but did not influence the production of leukotriene B4 or 5-hydroxyeicosatetraenoic acid in intact cells. These results demonstrate that certain drugs affecting the redox status can stimulate the cryptic 5-lipoxygenase activity in intact lymphoblastoid B cells but that the mechanism of this activation is unclear and appears not to be directly related to intracellular glutathione levels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. A., Pothier L., Hellerstein E. E., Boileau G. Malignant immunoblastoma: immunoglobulin synthesis and the progression to leukemia in heterotransplanted acute lymphoblastic leukemia, chronic lymphatic leukemia, lymphoma, and infectious mononucleosis. Cancer. 1973 Jun;31(6):1397–1407. doi: 10.1002/1097-0142(197306)31:6<1397::aid-cncr2820310615>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Avila-Cariño J., Torsteinsdottir S., Ehlin-Henriksson B., Lenoir G., Klein G., Klein E., Masucci M. G. Paired Epstein-Barr virus (EBV)-negative and EBV-converted Burkitt lymphoma lines: stimulatory capacity in allogeneic mixed lymphocyte cultures. Int J Cancer. 1987 Nov 15;40(5):691–697. doi: 10.1002/ijc.2910400521. [DOI] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Claesson H. E., Haeggström J. Human endothelial cells stimulate leukotriene synthesis and convert granulocyte released leukotriene A4 into leukotrienes B4, C4, D4 and E4. Eur J Biochem. 1988 Apr 5;173(1):93–100. doi: 10.1111/j.1432-1033.1988.tb13971.x. [DOI] [PubMed] [Google Scholar]
- Claesson H. E., Odlander B., Jakobsson P. J. Leukotriene B4 in the immune system. Int J Immunopharmacol. 1992 Apr;14(3):441–449. doi: 10.1016/0192-0561(92)90174-j. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Diehl R. E., Opas E., Rands E., Vickers P. J., Evans J. F., Gillard J. W., Miller D. K. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990 Jan 18;343(6255):282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fu J. Y., Medina J. F., Funk C. D., Wetterholm A., Rådmark O. Leukotriene A4, conversion to leukotriene B4 in human T-cell lines. Prostaglandins. 1988 Aug;36(2):241–248. doi: 10.1016/0090-6980(88)90310-3. [DOI] [PubMed] [Google Scholar]
- Funk C. D., Hoshiko S., Matsumoto T., Rdmark O., Samuelsson B. Characterization of the human 5-lipoxygenase gene. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2587–2591. doi: 10.1073/pnas.86.8.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzelmann A., Schatz M., Ullrich V. Involvement of glutathione peroxidase activity in the stimulation of 5-lipoxygenase activity by glutathione-depleting agents in human polymorphonuclear leukocytes. Eur J Biochem. 1989 Apr 1;180(3):527–533. doi: 10.1111/j.1432-1033.1989.tb14678.x. [DOI] [PubMed] [Google Scholar]
- Hatzelmann A., Ullrich V. Regulation of 5-lipoxygenase activity by the glutathione status in human polymorphonuclear leukocytes. Eur J Biochem. 1987 Nov 16;169(1):175–184. doi: 10.1111/j.1432-1033.1987.tb13595.x. [DOI] [PubMed] [Google Scholar]
- Jakobsson P. J., Odlander B., Claesson H. E. Effects of monocyte-lymphocyte interaction on the synthesis of leukotriene B4. Eur J Biochem. 1991 Mar 14;196(2):395–400. doi: 10.1111/j.1432-1033.1991.tb15829.x. [DOI] [PubMed] [Google Scholar]
- Jakobsson P. J., Odlander B., Steinhilber D., Rosén A., Claesson H. E. Human B lymphocytes possess 5-lipoxygenase activity and convert arachidonic acid to leukotriene B4. Biochem Biophys Res Commun. 1991 Jul 15;178(1):302–308. doi: 10.1016/0006-291x(91)91814-s. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Giblett E. R., Hansen J. A. Phenotyping human leukemic T-cell lines: enzyme markers, surface antigens, and cytogenetics. Immunogenetics. 1982;15(4):385–398. doi: 10.1007/BF00364262. [DOI] [PubMed] [Google Scholar]
- Medina J. F., Barrios C., Funk C. D., Larsson O., Haeggström J., Rådmark O. Human fibroblasts show expression of the leukotriene-A4-hydrolase gene, which is increased after simian-virus-40 transformation. Eur J Biochem. 1990 Jul 20;191(1):27–31. doi: 10.1111/j.1432-1033.1990.tb19089.x. [DOI] [PubMed] [Google Scholar]
- Miller D. K., Gillard J. W., Vickers P. J., Sadowski S., Léveillé C., Mancini J. A., Charleson P., Dixon R. A., Ford-Hutchinson A. W., Fortin R. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990 Jan 18;343(6255):278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Nilsson K., Bennich H., Johansson S. G., Pontén J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970 Oct;7(4):477–489. [PMC free article] [PubMed] [Google Scholar]
- Odlander B., Jakobsson P. J., Rosén A., Claesson H. E. Human B and T lymphocytes convert leukotriene A4 into leukotriene B4. Biochem Biophys Res Commun. 1988 May 31;153(1):203–208. doi: 10.1016/s0006-291x(88)81209-9. [DOI] [PubMed] [Google Scholar]
- Reid G. K., Kargman S., Vickers P. J., Mancini J. A., Léveillé C., Ethier D., Miller D. K., Gillard J. W., Dixon R. A., Evans J. F. Correlation between expression of 5-lipoxygenase-activating protein, 5-lipoxygenase, and cellular leukotriene synthesis. J Biol Chem. 1990 Nov 15;265(32):19818–19823. [PubMed] [Google Scholar]
- Rosén A., Uggla C., Szigeti R., Kallin B., Lindqvist C., Zeuthen J. A T-helper cell x Molt4 human hybridoma constitutively producing B-cell stimulatory and inhibitory factors. Lymphokine Res. 1986 Summer;5(3):185–204. [PubMed] [Google Scholar]
- Rouzer C. A., Matsumoto T., Samuelsson B. Single protein from human leukocytes possesses 5-lipoxygenase and leukotriene A4 synthase activities. Proc Natl Acad Sci U S A. 1986 Feb;83(4):857–861. doi: 10.1073/pnas.83.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Rands E., Kargman S., Jones R. E., Register R. B., Dixon R. A. Characterization of cloned human leukocyte 5-lipoxygenase expressed in mammalian cells. J Biol Chem. 1988 Jul 25;263(21):10135–10140. [PubMed] [Google Scholar]
- Rouzer C. A., Samuelsson B. The importance of hydroperoxide activation for the detection and assay of mammalian 5-lipoxygenase. FEBS Lett. 1986 Aug 18;204(2):293–296. doi: 10.1016/0014-5793(86)80831-6. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Funk C. D. Enzymes involved in the biosynthesis of leukotriene B4. J Biol Chem. 1989 Nov 25;264(33):19469–19472. [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Seelig G. F., Meister A. Glutathione biosynthesis; gamma-glutamylcysteine synthetase from rat kidney. Methods Enzymol. 1985;113:379–390. doi: 10.1016/s0076-6879(85)13050-8. [DOI] [PubMed] [Google Scholar]
- Steinhilber D., Herrmann T., Roth H. J. Separation of lipoxins and leukotrienes from human granulocytes by high-performance liquid chromatography with a Radial-Pak cartridge after extraction with an octadecyl reversed-phase column. J Chromatogr. 1989 Sep 1;493(2):361–366. doi: 10.1016/s0378-4347(00)82742-5. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Y., Rådmark O., Samuelsson B. Mutagenesis of some conserved residues in human 5-lipoxygenase: effects on enzyme activity. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):485–489. doi: 10.1073/pnas.89.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]