Abstract
Estrogen is related with the low morbidity associated with obstructive sleep apnea hypopnea syndrome (OSAS) in women, but the underlying mechanisms remain largely unknown. In this study, we examined the relationship between OSAS and estrogen related receptor-α (ERR-α). We found that the expression levels of ERR-α and Myh7 were both downregulated in palatopharyngeal tissues from OSAS patients. In addition, we report that ERR-α is dynamically expressed during differentiation of C2C12 myoblasts. Knockdown of ERR-α via instant siRNA resulted in reduced expression of Myh7, but not Myh4. Furthermore, differentiation of C2C12 cells under 3% chronic intermittent hypoxia, a model resembling human OSAS, was impaired and accompanied by a obvious reduction in Myh7 expression levels. Moreover, activation of ERR-α with 17β-estradiol (E2) increased the expression of Myh7, whereas pretreatment with the ERR-α antagonist XCT790 reversed the E2-induced slow fiber-type switch. A rat ovariectomy model also demonstrated the switch to fast fiber type. Collectively, our findings suggest that ERR-α is involved in estrogen-mediated OSAS by regulating Myhc-slow expression. The present study illustrates an important role of the estrogen/ERR-α axis in the pathogenesis of OSAS, and may represent an attractive therapeutic target, especially in postmenopausal women.
The prevalence of obstructive sleep apnea hypopnea syndrome (OSAS) in middle-aged individuals is 4% in males and 2% in females, with a strong male predominance1. Epidemiological studies have also shown that menopause is a significant risk factor for sleep apnea in women and that hormone replacement appears to be associated with reduced risk2,3. Estrogen may therefore play a protective role during OSAS pathogenesis, but the mechanisms by which estrogen contributes to OSAS remains largely unknown.
The most common pathogenic factors contributing to OSAS include anatomic abnormalities, obesity, and muscle dysfunction of the UA4. The function of mammalian skeletal muscle is determined by different muscle fiber types, which have variable actions in regards to the rate of force production, resistance to fatigue, and energy metabolism, and range from aerobic slow-twitch to anaerobic fast-twich physiology5,6. Our previous study demonstrated that the rate of type I slow-twitch myofiber (Myh7) was decreased in the palatopharyngeal muscles of OSAS patients7. Reduced rate of Myh7 can lead to fatigue and greater susceptibility to pharyngeal collapse during sleep8. In addition, skeletal muscle shows enormous plasticity, even in adults, in response to a variety of stimuli, such as exercise9, environmental factors10, nerve signals, hypoxia, and hormones11.
As a transcription factor, ERR-α plays roles in mitochondrial metabolism, energy substrate uptake, and biogenesis12. Moreover, ERR-α appears to be required for normal skeletal myocyte differentiation and may exert its effects via modulation of MAP kinase signaling13. However, it is unclear whether ERR-α mediates the conversion of fiber types of UA muscle in OSAS patients or contributes to pharyngeal collapse.
In this study, we investigated the relationship between ERR-α and OSAS by examining the expression of ERR-α and Myh7 in male patients with OSAS. Furthermore, we also explored the the possible mechanisms underlying fiber-type switch in OSAS patients. A clearer understanding of how sex hormones influence the morbidity associated with OSAS could enable therapeutic interventions for high-risk female patients experiencing postmenopause.
Results
Expression levels of ERR-α and Myh7 are decreased in palatopharyngeal tissues from OSAS patients
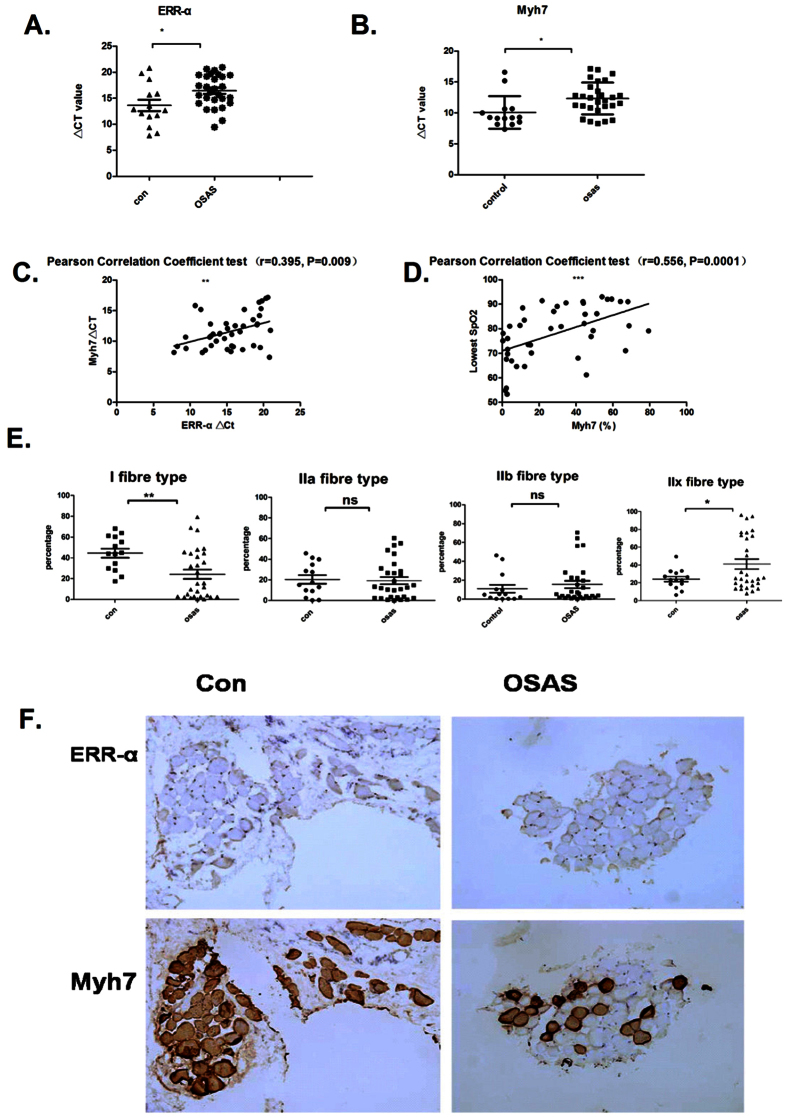
We performed real-time PCR (qRT-PCR) to assess mRNA levels of ERR-α and Myh7 in palatopharyngeal tissues isolated from patients with OSAS or patients with chronic tonsillitis (control). We examined polysomnographic data, ERR-α and Myh7 expression levels, and the percentages of Myh7 (fibre type I), Myh2 (fibre type IIa), Myh1(fibre type IIx), and Myh4 (fibre type IIb) in palatopharyngeal tissues from control and OSAS patients. All of the factors except age, shown in Table 1, have significant differences between OSAS and control group. We found that ERR-α and Myh7 were both downregulated in tissues from OSAS patients compared to tissues from control patients (Fig. 1a,b). We investigated the relationship between expression levels of ERR-α and Myh7 using Pearson’s correlation coefficient and found a significant positive association (r = 0.395, p < 0.01; Fig. 1c). Interestingly, the lowest Spo2 (oxygen saturation) was also positively correlated with Myh7 (r = 0.556, p < 0.01; Fig. 1d). Moreover, we observed that fiber type IIx was increased, whereas fiber type I was decreased in tissues from OSAS patients compared with those from control patients (Fig. 1e). Further immunohistochemical staining for ERR-α and Myh7 in the palatopharyngeal tissues confirmed a similar trend in protein levels (Fig. 1f). Taken together, these data suggest that ERR-α expression is decreased in OSAS and is closely associated with the expression of Myh7. Therefore, ER/ERR-α signaling may be involved in the regulation of fiber-type conversion in OSAS patients.
Table 1. Comparison of polysomnography data, ERR-α and Myh7 relative expression, and percentage of Myh7 in palatopharyngeal tissues between control and OSAS groups (two independent t-test).
| Parameter | Con (n = 14) | OSAS (n = 28) | 95% CI | P |
|---|---|---|---|---|
| Age | 37.43 ± 8.13 | 40.32 ± 6.78 | −7.69--- 1.90 | 0.23 |
| AHI | 1.46 ± 1.41 | 59.21 ± 22.47 | −66.48--- −48.99 | 0.00* |
| ERR-α△CT | 13.62 ± 4.08 | 16.42 ± 3.06 | −5.07--- −0.53 | 0.017* |
| Myh7△CT | 10.23 ± 2.56 | 12.33 ± 2.55 | −3.79--- −0.41 | 0.016* |
| Myh7% | 43.68 ± 16.85 | 21.97 ± 23.88 | 36.16--- 7.26 | 0.004* |
| BMI | 22.89 ± 2.20 | 28.23 ± 3.00 | −7.17--- −3.51 | <0.005* |
| Lowest Spo2 | 89.93 ± 2.27 | 72.16 ± 8.67 | 14.22–21.33 | <0.005* |
| Average Spo2 | 95.79 ± 0.65 | 92.68 ± 3.71 | 1.64–4.59 | <0.005* |
Figure 1. ERR-α and Myh7 levels are decreased in OSAS palatopharyngeal tissues.
(a) qRT-PCR analysis of ERR-α mRNA levels. The ΔCT value of ERR-α was significantly different between OSAS and control tissues (t = 2.494, p < 0.05). (b) qRT-PCR analysis of Myh7 mRNA levels. The ΔCT value of Myh7 was significantly different between OSAS and control tissues (t = 2.658, p < 0.05). (c) Pearson’s correlation coefficient was calculated to test the correlation between ERR-α and Myh7 expression. A significant positive correlation was observed (n = 42 specimens, r = 0.395, p < 0.01). (d) Pearson’s correlation coefficient was calculated to test the correlation between Spo2 and Myh7 expression. A significant positive correlation was observed between the percentage of Myh7 and the lowest SPO2 (n = 42 specimens, r = 0.556, p = 0.0001). (e) qRT-PCR analysis of the expression levels of different muscle fiber types. Significant differences were observed between the OSAS and control groups for type I (p < 0.05) and type IIx fibers (p < 0.05). (f) Representative images of immunohistochemical staining for ERR-α and Myh7 in palatopharyngeal tissues. Magnification = 200x. Dark blue-brown staining represents positive ERR-αantigen. Statistical analysis were performed using independent t tests (A, B, E) and Pearson Correlation test (C, D). *p < 0.05, **p < 0.01, ***p < 0.001, *p < 0.05, **p < 0.01, ***p < 0.001.
ERR-α is required for proper C2C12 differentiation and expression of Myh7 in myoblasts and myotubes
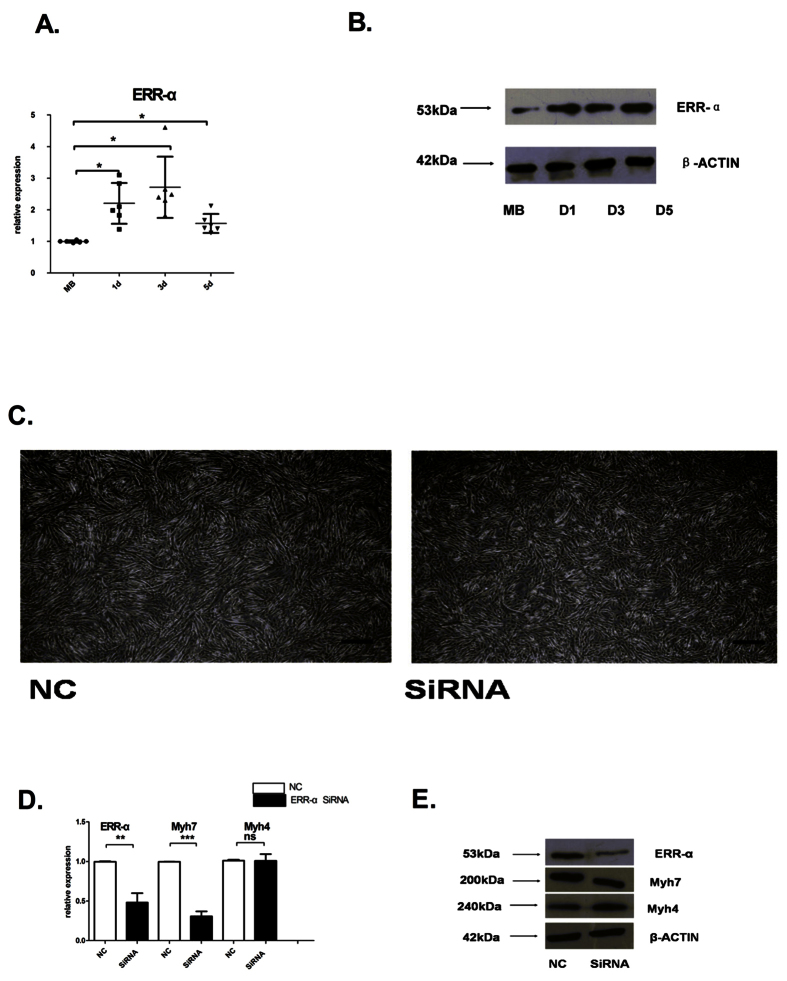
Recently, many studies have focused on the role of ERR-α and its coactivator PGC-1α in the direct transcriptional regulation of genes involved in energy metabolism14. We compared the relative expression of ERR-α in day (d) 0 myoblast (MB) and d1, d3, and d5 myotube (MT) by qRT-PCR (Fig. 2a). We found that ERR-α was dynamically expressed (1.57-fold to 2.71-fold in MT) during C2C12 differentiation. A similar trend was observed for ERR-α protein levels (Fig. 2b). Our findings are consistent with a recently reported transcript expression profilefor ERR-α in C2C12 cells13.
Figure 2. ERR-α and Myh7 expression patterns during C2C12 differentiation.
(a) qRT-PCR analysis of ERR-α levels relative to 18S levels at indicated time points during MT differentiation. Graphs represent fold change over the expression level at MB stage. Results shown are representative of at least six independent experiments. (b) Western blot analysis of ERR-α protein levels during C2C12 differentiation. β-actin was used as a loading control. MB represented the control group. (c) Representative images at 4 h C2C12 differentiation after siRNA transfection. Magnification = 40x, scale bar = 500 μm. (d) qRT-PCR analysis of ERR-α, Myh7, and Myh4 levels relative to 18S levels in C2C12 cells transfected with ERR-α siRNA compared with control siRNA (NC). Graph represents fold change over the expression level of NC. Results shown are representative of at least three independent experiments. (e) Western blot analysis of ERR-α, Myh7, and Myh4 protein levels. β-actin was used as a loading control. Statistical analysis in (a) was performed using one-way ANOVA and Dunnett’s T3 test and in (d) using independent t tests. *p < 0.05, **p < 0.01, ***p < 0.001.
To examine the contribution of ERR-α to MB differentiation, we used small interfering RNA (siRNA) to knockdown ERR-α in C2C12 cells. SiRNA-mediated downregulation of ERR-α (0.48-fold) resulted in reduced MT formation and was associated with significantly decreased mRNA (0.31-fold) and protein levels of Myh7 (Fig. 2c–e). However, ERR-α-depletion had no effect on the expression of Myh4. These results suggest that ERR-α affects the onset and maintenance of Myh7 expression during skeletal myocyte differentiation.
Chronic intermittent hypoxia inhibits the expression of Myh7at all stages of C2C12 differentiation, but inhibits ERR-α only during early stages
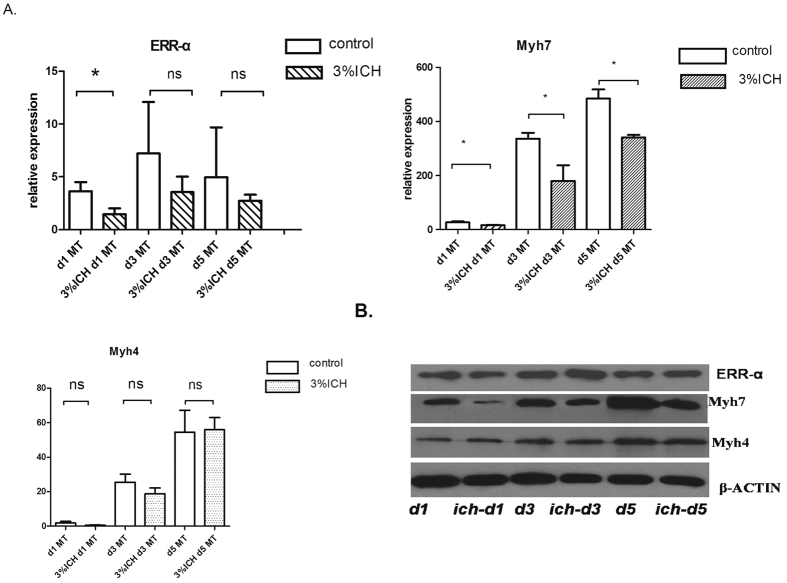
Chronic intermittent hypoxia (CIH) is a characteristic pathological component of OSAS15. To recapitulate human OSAS pathology in vitro, we exposed C2C12 cells to 3% CIH16. C2C12 cells exposed to CIH only moderately underwent MT fusion and displayed reduced Myh7 levels during all stages of differentiation compared to cells under normoxia (d1MT, 16.573 ± 1.407 vs. 26.852 ± 3.881; d3MT, 180.14 ± 57.75 vs. 335.72 ± 21.93; d5MT, 341.18 ± 8.97 vs. 484.33 ± 33.39). Myh4 protein levels increased over the course of C2C12 differentiation, but were not significantly affected by CIH (Fig. 3a,b). Furthermore, comparing with normoxia, ERR-α levels decreased only during the early stages (d1MT) of differentiation under CIH (Fig. 3a,b).
Figure 3. Myh7 and ERR-α expression levels are suppressed by CIH.
(a) qRT-PCR analysis of ERR-α,Myh7, and Myh4 levels relative to 18S in C2C12 cells exposed to 3% chronic intermittence hypoxia (CIH) or 21% O2(control) over the course of differentiation. Results shown are representative of at least three independent experiments. (b) Western blot analysis of ERR-α, Myh7, and Myh4 protein levels in C2C12 cells exposed to 3% CIH or 21% O2. Normal differentiation cell were as control group. β-actin was used as a loading control. Statistical analysis in (a) was performed using independent t tests. *p < 0.05, **p < 0.01, ***p < 0.001.
Estrogen stimulation promotes Myh7 expression, but is counter acted by an ERR-α antagonist
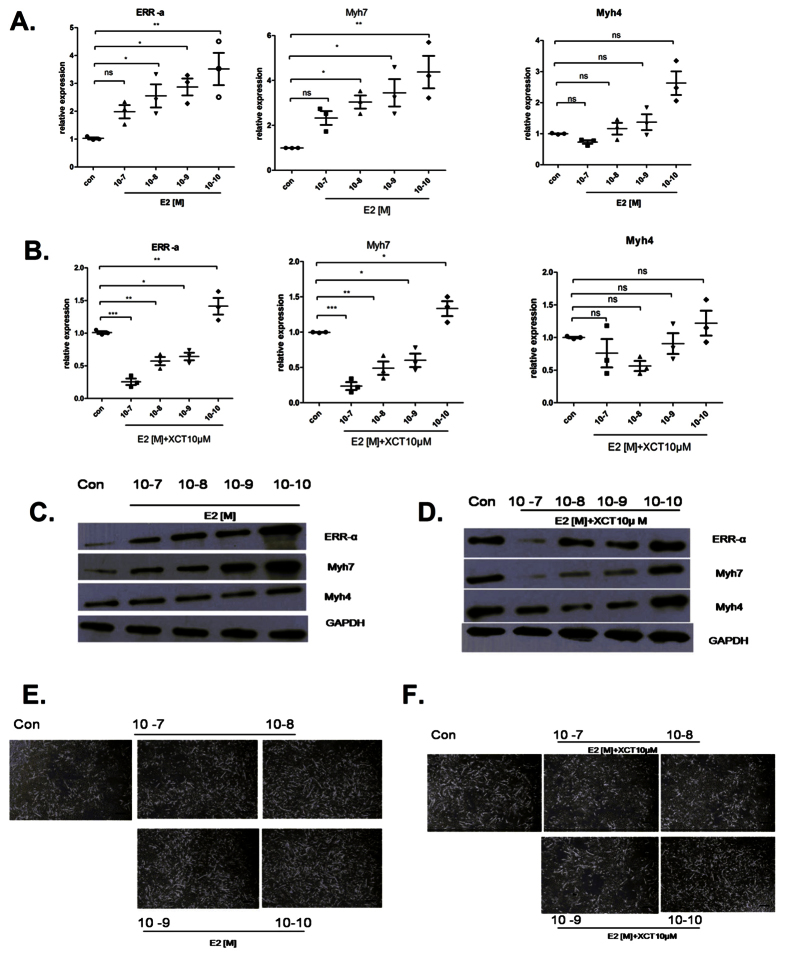
We found that endogenous ERR-α expression is induced during myogenesis. Therefore, we assessed whether ERR-α overexpression, stimulated by estrogen, affected MT formation. The addition of 17β-estradiol (E2) during C2C12 differentiation significantly induced MT formation (Fig. 4e). The qRT-PCR analysis showed that ERR-α obviously increased (1.98–3.52-fold) in response to E2. The expression of Myh7 also increased (2.33–4.38-fold; Fig. 4a) in response to E2, but there was not statistically significant for Myh4 (0.73—2.63-fold; Fig. 4a). We also observed a similar trend in protein expression changes (Fig. 4a).
Figure 4. Effects of estrogen and ERR-α antagonists on ERR-α and Myh7 expression levels.
(a) qRT-PCR analysis of ERR-α, Myh7, and Myh4 levels relative to 18S in C2C12 cells pretreated with 10−7–10−10 M estrogen (E2) for 48 h (d2MT) compared with control cells (con). (b) qRT-PCR analysis of ERR-α, Myh7, and Myh4 levels relative to 18S in C2C12 cells 48 h (d2MT) after differentiation pretreated with 10−7–10−10 M estrogen (E2) and 10 μM XCT790 compared with control cells (con). Results shown are representative of at least three independent experiments. (c) Western blot analysis of ERR-α, Myh7, and Myh4 protein levels in d2 C2C12 cells pretreated with 10−7–10−10 M estrogen (E2) compared with control cells (con). GAPDH was used as a loading control. (d) Western blot analysis of ERR-α,Myh7, and Myh4 protein levels in d2MT C2C12 cells pretreated with 10−7–10−10 M estrogen (E2) and 10 μM XCT790 compared with control cells (con). GAPDH was used as a loading control. Statistical analysis was performed using one-way ANOVA and Dunnett’s T3 test. *p < 0.05, **p < 0.01, ***p < 0.001. (e) Representative images of C2C12 cells pretreated with 10−7–10−10 M estrogen (E2) at d2MT of differentiation. Magnification = 40x, scale bar = 500 μm. (f) Representative images of C2C12 cells pretreated with 10−7–10−10 M E2 and 10 μM XCT790 at d2MT of differentiation. Magnification = 40x, scale bar = 500 μm.
We evaluated whether the effect of E2 could be blocked by XCT790, a specific antagonist of ERR-α13. Pretreatment of C2C12 cells with XCT790 and E2 caused a delay in MT formation (Fig. 4f). Moreover, XCT790 blocked the E2-stimulated increase in ERR-α and Myh7 mRNA (Fig. 4b) and protein levels (Fig. 4d) observed in d2 MT. Importantly, Myh4 expression was unaffected by E2 or XCT790. Collectively, these results suggest that the ER/ERR-α axis regulates Myh7 expression during myogenesis.
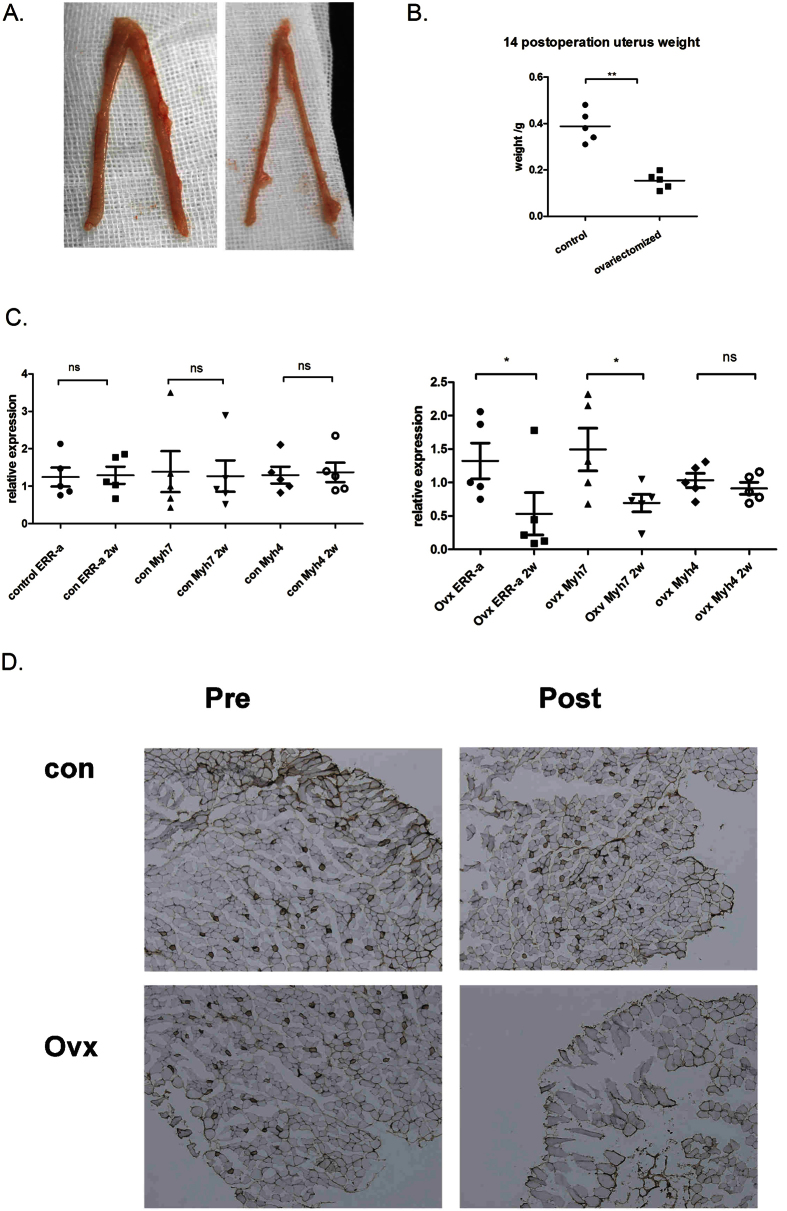
ERR-α expression and Myh7 are decreased in sternohyoid muscles of ovariectomized rats
E2-induced regulation of ERR-α and Myh7 expression in C2C12 cells prompted us to investigate the existence of a similar mechanism in vivo. To address this question, we used ovariectomized (OVX) rats, which recapitulate the changes observed during human postmenopause. Marked hypoplasia in rats that underwent OVX was confirmed by visual inspection (Fig. 5a) and by measuring the weight of the dissected uterus two weeks after surgery (OVX, 0.15 g vs. sham, 0.39 g; Fig. 5b). RNA was extracted from the sternohyoid muscle before and two weeks after surgery. The qRT-PCR analysis revealed that ERR-α (0.40-fold) and Myh7 (0.46-fold) expression levels were significantly decreased two weeks after OVX (Fig. 5c). But Myh4 (0.83-fold) expression had no significantly change 2wk after OVX (Fig. 5c). Immunohistochemical staining of sternohyoideus muscle revealed obviously decreased Myh7 protein levels two weeks after OVX comparing with pre-operation (Fig. 5d). These data suggest that OVX is associated with decreased Myh7 and ERR-α expression levels in vivo.
Figure 5. Effects of ovariectomy on ERR-α and Myh7 expression levels.
(a) Marked uterine hypoplasia was obvious in ovariectomized (OVX) but not sham rats 2 weeks after surgery. (b) Weight of uteri 2weeks after OVX or sham surgery. (c) qRT-PCR analysis of ERR-α, Myh7, and Myh4 levels relative to 18S in sternohyoid muscles before and 2 weeks after surgery. (d) Representative images of immunohistochemical staining for Myh7 in sternohyoid muscle before and 2 weeks after surgery. Magnification = 100x. Data are presented as means ± SEM. Statistical analysis was performed by independent t test or paired t test. *p < 0.05, **p < 0.01, ***p < 0.001, OVX vs. sham. Similar results were observed in three independent experiments.
Discussion
The clinical data presented in this study (Fig. 1) strongly suggest that ERR-α plays a role in the regulation of fiber-type conversion in OSAS patients, a characteristic of OSAS pathophysiology8. Additionally, differences in the expression of Myhc-slow (Myh7) did not appear to be attributed to differences in age. Alterations in fibre type and MyHC composition are suggestive of a neuromuscular disorder of the soft palate in SDB patients17. These changes may ultimately impair the ability of the muscles to maintain pharyngeal patency. For the first time, we report that Myh7 was positively correlated with ERR-α and some parameters in the PSG report. Most studies suggest that OSAS is associated with increased type II and decreased type I fibers. However, our findings show that OSAS is associated with increased type IIx (Myh1), rather than the whole type II fibers, and decreased type I fibers in palatopharyngeal tissues. It remains uncertain if such alterations in fiber type directly cause OSAS17. The actual fiber-type changes underlying OSAS are far from clear and are likely to be associated with many factors, such as age, gender, and biopsy position.
ERR-α is an important transcription factor that regulates oxidative stress and metabolic homeostasis. The ERR-α gene is stimulated by estrogen in the mouse uterus and heart, but not in the liver18. The ERR-α/PGC-1α axis regulates the expression of genes involved in glucose oxidation, left ventricular systolic function, and heart failure19. PGC-1α is a principal factor that regulates muscle fiber-type determination20. Therefore, estrogen may increase the endurance of muscle by regulating muscle fiber type through the ERR-α pathway. In particular, special attention should be given to functional muscle alterations of the airways, as these may influence the evolution and treatment of the OSAS21.
We also observed that ERR-α was dynamically expressed during C2C12 differentiation and affected the expression levels of Myhc-slow (Myh7), but not Myhc-fast (Myh4). Furthermore, 3% CIH inhibited the expression of Myhc-slow, but not Myhc-fast, during the entire course of differentiation, and inhibited ERR-α only at early stages of differentiation. CIH is a hallmark of OSAS thatinitiates a cascade of events involving oxidative stress and inflammatory processes22. Episodic hypoxia, a consequence of periodic airway occlusion, is responsible for OSAS progression through the impairment of the neural control systems and through altered respiratory muscle contractile function, leading to a vicious cycle of further airway obstruction23. Many studies utilize CIH as the exposure factor underlying OSAS16,24. CIH increased the diaphragm muscle type IIb (Myh4) areal density in adult male Wistar rats25. Our CIH experiments demonstrate that ERR-α may participate in the early stages of fiber-type switch. Moreover, estrogen improved the endurance of the genioglossus muscle in OVX rats exposed to CIH26.
Female patients experiencing postmenopause or exhibiting a higher BMI present with OSAS more frequently than their male counterparts27. Estrogen could influence exercise metabolism28 and pharyngeal dilator muscle activity29. A significant increase in genioglossus electromyogram (EMGgg) was found in the postmenopausal group re-examined after hormone therapy29,30. Denies and colleagues investigated the long-term effects of a high-fat diet and subsequent obesity on muscle fiber type composition and found that the high-fat diet was associated with a significantly lower proportion of slow, type I fibers in the soleus muscle of male, but not female, mice31. The critical pathophysiological feature of OSAS is sleep-related pharyngeal collapse of the UA, which is partly determined by the properties of different muscle fiber types. The underlying mechanisms of low morbidity associated with OSAS in female patients are not completely understood. Hormone replacement therapy is still not widely used for the prevention and treatment to these postmenopausal women with high risk for OSAS. Therefore, further investigation is urgently needed.
Lastly, our data suggest that E2 regulates Myhc-slow expression in a concentration-dependent manner. The inhibition of estrogen signaling by the ERR-α antagonist XCT790 was able to completely abolish E2-induced expression of Myhc-slow. Furthermore, animal experiments also demonstrated that ERR-α and Myhc-slow were decreased in the OVX group. These findings suggest that ERR-α expression is regulated by estrogen in C2C12 cells in vitro and in muscles in vivo. Taken together, the data imply that ERR-α intersects with the estrogen axis in muscle to promote MT formation and shift fiber types from Myhc-fast to Myhc-slow.
Conclusions
We have demonstrated for the first time that ERR-α expression is markedly reduced in OSAS patients. E2 can upregulate Myhc-slow expression in a concentration-dependent manner through ERR-α, which maybe contribute to improve UA muscle fatigue. Multiple lines of evidence suggest that ERR-α is involved in E2-mediated low morbidity by regulating Myhc-slow expression. These findings highlight ERR-α/Myhc-slow as an important transcriptional regulatory cascade contributing to E2-mediated muscle protection, thus providing a potentially novel therapeutic target for the treatment of postmenopausal OSAS.
Methods
Clinical specimens
Primary biopsy specimens and normal biopsies of palatopharyngeal muscle were obtained from the Otolaryngological Department in Nanfang Hospital (Southern Medical University, Guangzhou, China). Twenty-eight adult male patients with severe OSAS and 14 adult male patients with chronic tonsillitis, but not OSAS (control group), were recruited between February 2011 and July 2013. Both tissue types were histologically confirmed by hematoxylin and eosin (H&E) staining. No patient reported a history of local operation, radiotherapy, or neuromuscular disease. Informed consent was obtained from each patient, and the research protocols were approved by the Ethics Committee of Nanfang Hospital. Methods were carried out in accordance with the guidelines of Ethics Committee of Nanfang Hospital. The project was approved by Chinese ClinicalTrials. gov (ChiCTR-CCC-13003415) on May 31, 2013. Diagnoses and surgical treatments for all OSAS patients were performed according to the guidelines established by the Chinese Medical Association. Disease severity was assessed by polysomnography(PSG). Sleep apnea was defined as airflow being reduced to less than 10% of baseline for more than 10 s. Hypopnoea was defined as airflow being reduced to less than 30% of baseline for more than 10 s in association with a 4% oxygen desaturation or less than 50% of baseline for more than 10 s in association with a 3% oxygen desaturation or micro-arousal. When AHI was >30, we defined the condition as severe.
Cell culture and reagents
Mouse C2C12 myoblasts (ATCC) were cultured in high glucose DMEM containing10% fetal bovine serum (FBS) and 1% penicillin-streptomycin antibiotic solution. The differentiation media contained 2% horse serum instead of 10% FBS. Cells were passaged by trypsinization (0.5% trypsin in 0.5 mM EDTA, Gibco BRL) upon reaching 70% confluency. Normoxic cells were cultured under a humidified atmosphere of 95% air and 5% CO2 at 37 °C. For hypoxia experiments, cells were exposed to 3% O2 in an incubated chamber (Thermo 3111; USA) adjusted to the desired O2 concentration. Episodic cycles were set as follows: 8 h per day of 3% O2, 5% CO2, and balanced N2 for 35 min, then 21% O2 for 25 min, followed by 16 h of 21% O2 over the course of 5 days16. To investigate the effects of 17β-estradiol (E2) on differentiation, cells were seeded in 6-well plates (Corning, Falcon) in the presence or absence of E2 (Sigma, USA). E2 diluted in ethanol was added to cells to a final concentration of 10−7 to 10−10 M at the beginning of differentiation and cells were collected after 48 h. XCT790 (Sigma, USA) was diluted in DMSO and added to cells to a final concentration of 10 μM, the same volume of DMSO as blank control.
siRNA transient transfection of C2C12 cells
We purchased siRNA oligonucleotides for ERR-α from Invitrogen™ Life Technologies. Cells were transfected with negative control (NC) oligos or ERR-α-specific oligos for 48 h. Transfections were performed withLipofectamine 2000 (Invitrogen, Carlsbad, USA), according to the manufacturer’s directions, using 100 nMsiRNA per well of a 6-well plate. Cells were cultured in complete media without antibiotics until they reached 50–60% confluency 24 h after plating and were then incubated with siRNA-liposome complexes. Cells were harvested 48 h and 72 h later for Western blot and qRT-PCR assays.
RNA extraction and qRT-PCR
Total RNA was extracted from the samples using RNAiso Plus (TAKARA, Shiga, Japan), according to the manufacturer’s instructions. RNA concentration was measured by spectrophotometry. After cDNA synthesis (All-in-One First-Strand cDNA Synthesis kit, GeneCopoeia Inc., USA), qRT-PCR was performed using All-in-One qPCR Mix (GeneCopoeia Inc., USA) on the ABI 7500HT System (Applied Biosystems) with primers described in Table 2. PCR conditions were as follows: 95 °C for 10 min followed by 40 cycles of 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 34 s. The specificity of each qRT-PCR reaction was verified by melting curve analysis. Duplicate reactions were run for each sample. All samples were normalized based on the expression levels of 18S ribosomal RNA and fold changes were calculated using the 2ΔΔCT method. The percentage of MyhX in the sample was calculated as follows (where X = 1, 2, 4, or 7):
Table 2. Primers for qRT-PC.
| Primer | Description |
|---|---|
| Human Myh7 | Accession No: NM000257.2, product length: 112 |
| Sense 5′-TGCCACATCTTGATCTGCTC-3′; | |
| Antisense 5′-CTCGGCTTCAAGGAAAATTG-3' | |
| Human ERR-α | Accession No: NM-004451.3, product length 116 |
| Sense 5′-CACTATGGTGTGGCATCCTGT-3' | |
| Antisense 5′-CGTCTCCGCTTGGTGATCTC-3' | |
| Human Myh4 | Accession No: NM-017533.2, product length: 86 |
| Sense 5′- CCCGCAATGCAGAGGAGAA -3' | |
| Antisense 5′- GCTGGTGTCCTGTTCCTTCTTC -3' | |
| Human Myh2 | |
| Accession No: NM017534.5, product length: 109 | |
| Sense 5′-CTGATGCCATGGAATGACTG-3' | |
| Antisense 5′-CCCTATGCTTTATTTCCTTTGC-3 | |
| Human Myh1 | Accession No: NM-005963.3, product length: 98 |
| Sense 5′- GGAGGAACAATCCAACGTCAA -3' | |
| Antisense 5′- TGACCTGGGACTCAGCAATG -3' | |
| Human 18S | |
| Sense 5′-GTAACCCGTTGAACCCCATT-3' | |
| Antisense 5′-CCATCCAATCGGTAGTAGCG-3' | |
| Rat Myh7 | |
| Accession No: NM-17240.2, product length: 159 | |
| Sense 5′-TACAATGCGCAAGTGGTAGC-3' | |
| Antisense 5′- GACGGTCTTACCAGCTCCG-3' | |
| Rat Myh4 | Accession No: NM-019325.1, product length:136 |
| Sense 5′-CAG GTC AAC AAG CTG -3' | |
| Antisense 5′-GAT ATA CAG GAC AGT GAC AAA GAA CG-3' | |
| Rat ERR-α | Accession No: Nm-001008511.2, product length: 301 |
| Sense 5′-AGTACAGCTGTCCGGCCTCCAAC-3′ | |
| Antisense 5′-GGCATGGCATACAGCTTCTCAGG -3' | |
| Rat 18S | |
| Sense 5′- GGGAGGTAGTGACGAAAAATAACAAT-3' | |
| Antisense 5′- TTGCCCTCCAATGGATCCT-3 |
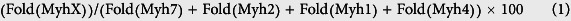 |
Western blot analysis
C2C12 cells were harvested and extracted with lysis buffer (Genechem, Shanghai, China) at 4 °C. Nuclear protein extracts were prepared using a protein fractionation kit (Genechem, Shanghai, China), according to the manufacturer’s directions. Total protein was isolated and quantified using the BCA assay. The protein lysates were separated on a 10% SDS-PAGE gel and then transferred to polyvinylidenedifluoride (PVDF) membranes (Millipore, USA). Primary antibodies were diluted in 5% non-fat milk prepared in phosphate-buffered saline (PBS) containing 0.05% Tween-20 and applied to the membranes with gentle shaking overnight at 4 °C. The next day, membranes were incubated with horseradish peroxidase (HRP)-labeled goat-anti mouse IgG (1:5000; Santa Cruz Biotechnology) for 60 min at room temperature and then washed in 0.05% Tween-20/PBS for 4 min. Finally, X-ray film was exposed to the PVDF membranes for 1 min. The intensity of the chemiluminescent signals was quantified using Image J software. The ERR-α antibody was purchased from Millipore (USA), the Myh4 antibody was purchased from Proteintech Group Inc (USA), and the Myh7 antibody was purchased from Abcam (USA). Beta-actin or GAPDH were used as internal standards to control for protein loading.
Immunohistochemistry
Formalin-fixed, paraffin-embedded muscle tissue samples were sectioned to 4 μm thickness and stained with ERR-α antibody (1:125; Millipore, USA) and Myh7 antibody (1:400; Abcam, USA).
Ovariectomy of rats
Age-matched female Sprague-Dawley(SD) rats (permission number: (SCXK(Yue)2011–0029) weighing 180–200 g at the time of arrival were used for all experiments. Each group of five SD rats was kept under standard laboratory conditions for up to 2 weeks. Ovariectomy (OVX) or sham operations (sham) were conducted after successful general paralysis. Sternohyoid muscle biopsies were collected before and 2 weeks after OVX or sham surgery. Animals were sacrificed and the uteri weighed to ensure efficacy of the OVX surgery. Total RNA from sternohyoid muscle samples, which had been snapfrozen in liquid nitrogen, was isolated using Trizol reagent (Life Technologies). Samples were then analyzed by qRT-PCR and immune histochemistry as described above. All experiments were performed in accordance with the Animal Facility Institutional Animal Care and Use Committee regulations. All experimental protocol on the animals were approved by Nanfang Hospital Animal Care Committee (NFHACC 2011-CZ102).
Statistical analysis
SPSS 13.0 for windows software was used for statistical analysis. Data are presented as mean ± SEM. All experiments were performed at least three independent times. The onesample Kolmogorov-Smirnov test was used to analyzenormally distributed data. Levene’stest was used to assess homogeneityofvariance. The two-tailed Student’s t test was used for comparisons between two independent groups. One-way ANOVA and Dunnett’s T3 tests were used for comparisons between multiple independent groups. P values of <0.05 were considered statistically significant.
Additional Information
How to cite this article: Chen, H. H. et al. Estrogen/ERR-α signaling axis is associated with fiber-type conversion of upper airway muscles in patients with obstructive sleep apnea hypopnea syndrome. Sci. Rep. 6, 27088; doi: 10.1038/srep27088 (2016).
Acknowledgments
The project was supported by the Guangdong Natural Science Foundation of China (Grant No. 2015A030310049 to Chen HH) and Medical Scientific Research Foundation of Guangdong Province of China (Grant No. A2014411 to Chen HH).
Footnotes
The authors declare no competing financial interests.
Author Contributions C.H.H. wrote the main manuscript and L.S.J. prepared all figures. L.J. designed all the primers and give some valuable advise to the paper. G.Y.F. did the cellular hypoxia experiments. X.Z.S. did the Immunohistochemical staining. H.T.T. performed the animal experiment and W.F. isolated Total RNA from it. L.X. collected the clinic samples and Z.F.P. preserved the samples well. P.X.H. did the cell experiment and Y.B.L. wrote the methods section. L.X.P. provided important technical and conceptual insights during the preparation of this manuscript. All authors reviewed the final version of the manuscript.
References
- Jordan A. S., McSharry D. G. & Malhotra A. Adult obstructive sleep apnoea. Lancet. 383, 736–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixler E. O. et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 163, 608–613 (2001). [DOI] [PubMed] [Google Scholar]
- Manber R., Kuo T. F., Cataldo N. & Colrain I. M. The effects of hormone replacement therapy on sleep-disordered breathing in postmenopausal women: a pilot study. Sleep. 26, 163–168 (2003). [PubMed] [Google Scholar]
- Casale M. et al. Obstructive sleep apnea syndrome: from phenotype to genetic basis. Curr Genomics. 10, 119–126 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajsharghi H. & Oldfors A. Myosinopathies: pathology and mechanisms. Acta Neuropathol 125, 3–18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro E., Giammarioli A. M., Chiandotto S., Spoletini I. & Rosano G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: redox signaling and role of autophagy. Antioxid Redox Sign. 21, 154–176 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. H. et al. [Pathologic changes of the palatopharyngeal muscles in adult patients with obstructive sleep apnea hypopnea syndrome]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 48, 746–751 (2013). [PubMed] [Google Scholar]
- Smirne S. et al. Muscle fibre type and habitual snoring. Lancet 337, 597–599 (1991). [DOI] [PubMed] [Google Scholar]
- Sabbah H. N. et al. Decreased proportion of type I myofibers in skeletal muscle of dogs with chronic heart failure. Circulation. 87, 1729–1737 (1993). [DOI] [PubMed] [Google Scholar]
- Clanton T. L. & Klawitter P. F. Invited review: Adaptive responses of skeletal muscle to intermittent hypoxia: the known and the unknown. J Appl Physiol (1985) 90, 2476–2487 (2001). [DOI] [PubMed] [Google Scholar]
- Ianuzzo C. D., Hamilton N. & Li B. Competitive control of myosin expression: hypertrophy vs. hyperthyroidism. J Appl Physiol (1985) 70, 2328–2330 (1991). [DOI] [PubMed] [Google Scholar]
- Villena J. A. & Kralli A. ERRα: a metabolic function for the oldest orphan. Trends Endocrinol Metab. 19, 269–276 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. & Huss J. M. Estrogen-related receptor alpha regulates skeletal myocyte differentiation via modulation of the ERK MAP kinase pathway. Am J Physiol Cell Physiol. 301, C630–C645 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss J. M., Torra I. P., Staels B., Giguere V. & Kelly D. P. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 24, 9079–9091 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly J. R. et al. Tempol ameliorates pharyngeal dilator muscle dysfunction in a rodent model of chronic intermittent hypoxia. Am J Respir Cell Mol Biol. 46, 139–148 (2012). [DOI] [PubMed] [Google Scholar]
- Shan X. et al. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol Dis. 28, 206–215 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindman R. & Stal P. S. Abnormal palatopharyngeal muscle morphology in sleep-disordered breathing. J Neurological Sci. 195, 11–23 (2002). [DOI] [PubMed] [Google Scholar]
- Shi S. et al. The relationship between structural/MHC changes in upper airway palatopharyngeal muscle morphology and obstructive sleep apnea/hypopnea syndrome. Eur Arch Otorhinolaryngol. 271, 109–116 (2014). [DOI] [PubMed] [Google Scholar]
- Liu D., Zhang Z., Gladwell W. & Teng C. T. Estrogen stimulates estrogen-related receptor alpha gene expression through conserved hormone response elements. Endocrinology. 144, 4894–4904 (2003). [DOI] [PubMed] [Google Scholar]
- Shen T. et al. Tbx20 functions as an important regulator of estrogen-mediated cardiomyocyte protection during oxidative stress. Int J Cardiol. 168, 3704–3714 (2013). [DOI] [PubMed] [Google Scholar]
- Lin J. et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 418, 797–801 (2002). [DOI] [PubMed] [Google Scholar]
- Braga A. et al. Predictors of uvulopalatopharyngoplasty success in the treatment of obstructive sleep apnea syndrome. Sleep Med. 14, 1266–1271 (2013). [DOI] [PubMed] [Google Scholar]
- Lavie L. & Lavie P. Coronary collateral circulation in sleep apnea: a cardioprotective mechanism? Chest. 137, 511–512 (2010). [DOI] [PubMed] [Google Scholar]
- Bradford A., McGuire M. & O’Halloran K. D. Does episodic hypoxia affect upper airway dilator muscle function? Implications for the pathophysiology of obstructive sleep apnoea. Respir Physiol Neurobiol 147, 223–234 (2005). [DOI] [PubMed] [Google Scholar]
- Ryan S., Taylor C. T. & McNicholas W. T. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 112, 2660–2667 (2005). [DOI] [PubMed] [Google Scholar]
- Shortt C. M. et al. Reactive Oxygen Species Mediated Diaphragm Fatigue in a Rat Model of Chronic Intermittent Hypoxia. Exp Physiol. 99, 688–700 (2014). [DOI] [PubMed] [Google Scholar]
- Huang Y. & Liu Y. H. Effects of phytoestrogens on genioglossus contractile properties in ovariectomized rats exposed to chronic intermittent hypoxia may be independent of their estrogenicity. Eur J Oral Sci. 119, 128–135 (2011). [DOI] [PubMed] [Google Scholar]
- Valipour A. Gender-related differences in the obstructive sleep apnea syndrome. Pneumologie. 66, 584–588 (2012). [DOI] [PubMed] [Google Scholar]
- Oosthuyse T. & Bosch A. N. The effect of the menstrual cycle on exercise metabolism: implications for exercise performance in eumenorrhoeic women. Sports Med. 40, 207–227 (2010). [DOI] [PubMed] [Google Scholar]
- Popovic R. M. & White D. P. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. (1985) 84, 1055–1062 (1998). [DOI] [PubMed] [Google Scholar]
- Denies M. S. et al. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol Rep. 28, e00204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]