Abstract
The kinetics of dengue virus (DENV)-specific IgA antibody in urine and the potential correlation with disease severity remain elusive. In this study, 262 serial urine samples from 78 laboratory-confirmed patients were assayed by a commercial immunoglobulin A (IgA) kit against DENV. All cases were classified into dengue fever (DF) and severe dengue (SD) according to the 2009 WHO/TDR guideline. The total positive rate of IgA in urine was 59%. DENV-specific IgA was detected in urine from day 2 to day 13 after the onset of illness in DF patients; While for SD patients, anti-DENV IgA could be detected till day 14. The positive rate of IgA in patients with secondary infection was higher than that in patients with primary infection. Importantly, during 4–7 days after the onset of illness, the IgA positive rate of SD patients was significantly higher than that of DF patients. Especially, the intensity of IgA signal in SD patients was obviously stronger than that in DF patient at the recovery stage. Overall, our results suggested that the existence of DENV-specific IgA antibodies in urine might be a warning sign for the severity of disease and its measurement might provide valuable guidance for proper patient management.
Dengue has constituted a public health emergency of international concern due to the rapid spread and domination in the world. Dengue has a wide spectrum of clinical presentations, often with unpredictable clinical evolution and outcome1. The four serotypes (1~4) of dengue virus (DENV) can cause either mild or severe clinical manifestations, and the latter is generally associated with sequential secondary heterotypic DENV infection. According to the 2009 WHO/TDR dengue guidelines for diagnosis, treatment, prevention and control, dengue can be further classified with dengue fever (DF) and severe dengue (SD)2. Recently, several countries have licensed Dengvaxia from Sanofi Pateur for the prevention of dengue. Early laboratory diagnosis should be of high value because some patients progress from mild to severe disease and sometimes to death over a short period3.
Serological assays are now widely used for dengue diagnosis. Dengue-specific IgM and IgG capture enzyme linked immunosorbent assays (ELISAs) are the most common serological assays and dozens of commercial kits have been approved for clinical use in various countries4. Recently, DENV-specific IgA antibodies were detected in serum and saliva of dengue patients5,6. Moreover, several studies have reported a higher IgA response in serum in secondary infection cases7,8,9, and the levels of IgA in serum might be correlated with the severity of disease10,11. In addition, a commercial dengue IgA rapid test has been developed based on immunochromatographic technology, which was more sensitive and specific as compared to reference ELISA12,13,14,15.
Recently, urine is becoming increasingly popular as a noninvasive approach for the laboratory diagnosis of dengue cases when blood samples are difficult to obtain16,17,18,19. However, it has not been evaluated whether the detection of urinary IgA is an alternative method used for dengue diagnosis. Vasquez et al. has reported the kinetics of IgA in serum, saliva and urine samples from adult patients with dengue infection20. However, the kinetics and role of DENV-specific IgA in urine samples from patients with different clinical manifestations and infection type remain largely unknown. The aim of this study is to characterize the DENV-IgA antibody profiles in urine samples from DF and SD patients, and to clarify the potential relationship between IgA and the severity of disease.
Results
Characteristics of the enrolled patients
Table 1 summarizes the characteristics of all enrolled patients. Of all patients, 53 were classified as DF and 25 as SD according to the criteria defined by 2009 WHO/TDR guidelines2. The proportion of dengue for males was similar to females (P = 0.321). The median age of patients with DF was 38.8 ± 16.7 years old, while 61.4 ± 20.7 for SD, which implied that the most affected cases were old people in SD cases (P < 0.001). Among the SD patients, 56.0% were diagnosed as secondary infections. In contrast, only 15.1% of the DF patients were identified as secondary infections. The ratio of secondary infection in patients with SD was significantly higher than patients with DF (P < 0.001). In addition, the ratio of acute kidney injury (AKI) in patients with SD was significantly higher than patients with DF (P < 0.001). DENV-specific real-time RT-PCR assay revealed that all four DENV serotypes were found in the acute sera from enrolled patients, and 62 patients were infected with DENV serotype 1. Two DF cases and three SD cases, that were negative for DENV nucleic acid detection, were confirmed with NS1 antigen examination for DENV.
Table 1. Characteristics of patients infected by DENV*.
| DF (n = 53)No. (%) | SD (n = 25)No. (%) | TotalNo. (%) | P valuea | |
|---|---|---|---|---|
| Gender | ||||
| Male | 36 (67.9) | 14 (56) | 50 (64.1) | 0.321 |
| Female | 17 (32.1) | 11 (44) | 28 (35.9) | |
| Age, years, mean ± SD | 38.8 ± 16.7 | 61.4 ± 20.7 | 46.0 ± 20.9 | <0.001 |
| Infection type | ||||
| primary | 45 (84.9) | 11 (44) | 56 (71.8) | <0.001 |
| secondary | 8 (15.1) | 14 (56) | 22 (28.2) | |
| AKIb, No. (%) | 11 (20.8) | 16 (64.0) | 27 (34.6) | <0.001 |
| Serotype | ||||
| DENV-1 | 41 (77.4) | 21 (84.0) | 62 (79.5) | |
| DENV-2 | 3 (5.7) | 1 (4.0) | 4 (5.1) | |
| DENV-3 | 3 (5.7) | 0 (0) | 3 (3.8) | |
| DENV-4 | 4 (7.5) | 0 (0) | 4 (5.1) |
*Data are from 78 patients recruited from Guangzhou Eighth People’s Hospital.
aP values are for the comparison of dengue fever (DF) and severe dengue (SD).
bAKI denotes acute kidney injury.
Detection of DENV-IgA in urine samples
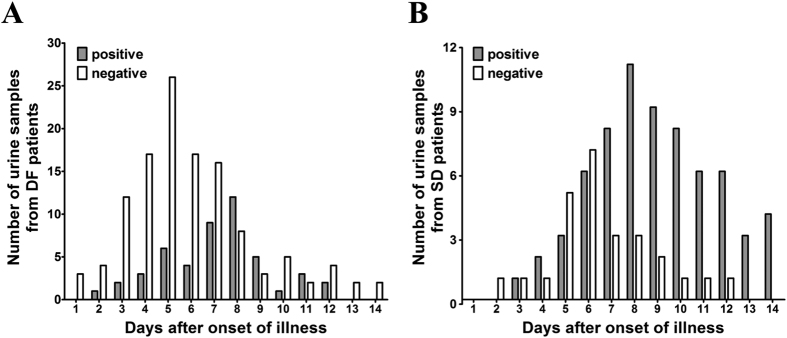
None of the 20 urine samples collected from healthy donors was reactive for DENV-specific IgA. Of the 78 patients, only 46 (59.0%) were IgA positive, including 24 DF patients and 22 SD patients (Table 2). The kinetics of DENV-specific IgA antibodies in urine was depicted in Fig. 1. Of DF patients (Fig. 1A), anti-DENV IgA was detected from day 2 till day 13 after the onset of the illness. While for SD patients (Fig. 1B), anti-DENV IgA was positive from day 3 to day 14. The detailed information of each patient was listed in Table S1. Of the 22 patients with secondary infection, 59.1% was positive for DENV-specific IgA in urine. In contrast, for patients with primary infection, only 23.2% (13/56) was positive. The positive rate of IgA in patients with secondary infection was significantly higher than patients with primary infection (P = 0.0038) (Table 3).
Table 2. Summary of the detection of IgA among DF and SD patients at different time points of the disease.
| No. of days of fever | DF | SD |
|---|---|---|
| Positive/Total no. (%) | Positive/Total no. (%) | |
| 1–3 | 3/16 (18.8) | 1/3 (33.3) |
| 4–7 | 15/46 (32.6) | 13/19 (68.4) |
| >7 | 16/24 (66.7) | 18/18 (100) |
| Total | 24/53 (45.3) | 22/25 (88) |
Figure 1.
Distribution of IgA-positive and IgA-negative urine samples from DF (A) and SD (B) patients over the 14-days period.
Table 3. Comparison between IgA detection in the urine of patients with primary infection and secondary infection*.
| IgA detection | Infection type |
|
|---|---|---|
| Primary | Secondary | |
| Positive | 13 | 13 |
| Negative | 43 | 9 |
*The chi square analysis showed that there was a significant increase of IgA detection rate in the urine from patients with secondary infection compared with primary infection (P = 0.0038).
Comparison of the positive rate of DENV-IgA detection between DF and SD groups
Table 2 shows the positive rate of DENV-IgA detection among DF and SD patients recruited in the study. There were 53 DF and 25 SD cases totally. Only 24 (45.3%) DF cases were positive for IgA, whereas 22 (88%) SD cases were positive. During the different phase of the illness, the positive rate of IgA in the urine of SD patients was higher than that in DF patients. The proportion of patients distributed across different time limits was not well balanced (Table S2), the data during 4–7 days after the onset of the illness were used to analyze to evaluate the association between IgA positive rate and disease severity. Statistical analysis using Fisher exact tests demonstrated that there was a significant increase of IgA positive rate in the urine from SD patients compared with DF patients (P = 0.017). These data implied that urinary IgA might be a significant marker for the diagnosis of SD.
Furthermore, we analyzed the difference in terms of the intensity of IgA signal between DF and SD cases. As shown in Fig. 2, there was no obvious difference between SD and DF patients at the early stage of disease (Day 2 to day 8). However, the IgA signal intensity in SD patients was obviously higher than that in DF group since day 8 post disease onset. Moreover, detail analysis for serial samples from the same patients revealed high consistence and strong signal intensity (>1). These observations confirmed that the higher level of IgA signal is relevant to progress to serious dengue disease.
Figure 2. Kinetics of the intensity of IgA signal in urine samples from DF and SD patients over the 14-days period.
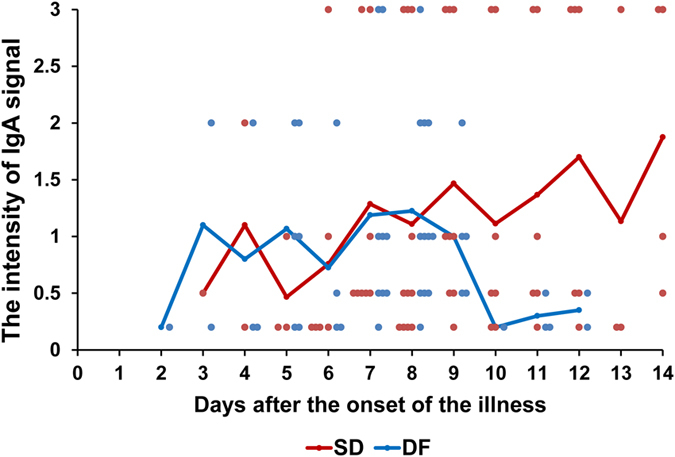
The intensity of IgA signal in the urine over time was shown for 24 patients with DF and 22 with SD. 48 data points were from the DF group and 68 data points from the SD group.
Discussion
We reported here the first evidence of correlation between urinary IgA and the severity of dengue disease. Our results showed the IgA positive rate of SD patients was significantly higher than that of DF patients during 4–7 days after the onset of illness. Furthermore, the intensity of IgA signal in SD patients was obviously stronger than that in DF patients at the recovery stage. This finding showed that the presence of IgA in urine may be used as a warning sign for the development of SD, especially when detected during 4–7 days of disease onset. Urine is a body fluid with low concentration of proteins derived from plasma. However, abundant proteins occur in the urine due to the enhancement of glomerular filtration membrane permeability when kidney is damaged. It has been known that AKI is a potential complication of SD21. The prevalence of AKI was 4.9% among 81 adults with dengue hemorrhagic fever (DHF)22, and 60% in fatal DHF in Taiwan15. In India, AKI was observed in 10.8% of 223 patients with DF, and was associated with an increased mortality23. A recent report showed that DHF-related AKI caused a high mortality rate of 64.0% in Thailand24. In the present study, 64% of SD patients had AKI, which was a possible factor resulting in a significant increase of IgA antibody in the urine, compared to that in DF patients (21% with AKI). Very recently, Upadhaya reported a dengue case with AKI, and renal biopsy demonstrated IgA deposits in the mesangium25.
Generally, secondary heterotypic infection is known as a risk factor for SD26. In our study, it was also found that the ratio of secondary infection in patients with SD was significantly higher than patients with DF. Our findings confirmed that the positive detection rate of IgA in patients with secondary infection was significantly higher than patients with primary infection. This indirect evidence also supported the association of IgA and disease severity. In our work, the total ratio of secondary infection among the enrolled patients was high (28.2%), and it is a little unexpected in a non-endemic country, which may be related to co-circulation of multiple DENV serotypes in Guangdong province in recent years27.
Our study has some limitations. Firstly, the sample distribution was incomparable (at patient level) during days 1–3 and >7 days (Table S2). The percentage of DF patients during days 1–3 and >7 days were both less than 50%, and the percentage of SD patients during days 1–3 was only 12%. So, only the data during 4–7 days after the onset of the illness were used to analyze the association between occurrence of DENV-IgA and the severity of disease. Secondly, the IgA positive rate in urine for all patients is 59%, and nearly half of the patients were IgA negative throughout the course of the illness. Moreover, the IgA detection results for the same patients varied at different times, and this inconsistency may be accountable for sampling time, fluid therapy and some other uncertain factors. So, urinary IgA alone may not be a good diagnostic marker for severe dengue infections. In addition, the majority of the patients were infected with DENV serotype 1, and only a limited number of samples were collected from patients infected with other serotypes.
In conclusion, our results demonstrate that IgA is detected more frequently in the urine samples from patients with SD than patients with DF and its presence may be a warning sign for clinical management.
Methods
Ethical approval
The study was approved and supervised by the ethics committee of Guangzhou Eighth People’s Hospital (Reference Number 20100835). All experiments were performed in accordance with approved relevant guidelines and regulations. The informed consent was obtained from all subjects.
Study design
A total of 262 serial urine samples were obtained from 78 hospitalized patients in Guangzhou Eighth People’s Hospital in China. All patients were laboratory confirmed and grouped into DF or SD according to the criteria defined by the 2009 WHO/TDR guidelines2. Meanwhile, serum samples were collected from all patients during the acute phrase of illness (within 7 days after the onset of fever) for DENV genome detection by using the DENV serotyping real-time PCR kit (DaAn, China). DENV infection was confirmed by the presence of NS1 antigen in acute sera using the enzyme-linked immunosorbent assay (Diagnostic Kit for Dengue Virus NS1 Antigen, WANTAI, China). Dengue-specific IgM and IgG antibodies in acute sera were determined by capture ELISA (Panbio, Australia), and the infection type (primary or secondary infection) was defined as previous described28. In addition, 20 urine samples collected in 2013 from healthy donors were also included as negative control.
Detection of DENV-specific IgA antibodies
The IgA antibody detection in urine samples was carried out using ASSURE® Dengue IgA Rapid test (MP Diagnostics) according to the recommended protocol. Urine samples were centrifuged at 400 g for 5 min and the supernatant was used for IgA detection. The intensity of IgA signal was recorded as 0.2, 0.5, 1, 2, 3, respectively using an intensity scale provided with the kit.
Statistical analysis
The OpenEpi version 2.3.1 software was used for statistical analysis. Continuous variables were compared by Student’s t-test, and categorical variables were assessed using the Fisher exact test. Differences were considered significant at p < 0.05.
Additional Information
How to cite this article: Zhao, H. et al. Dengue Specific Immunoglobulin A Antibody is Present in Urine and Associated with Disease Severity. Sci. Rep. 6, 27298; doi: 10.1038/srep27298 (2016).
Supplementary Material
Acknowledgments
We are grateful for the patients and clinical care providers who participated in the study. This work was supported by the National Natural Science Foundation (NSFC) of China (Nos 81301491 and 31270974), Guangzhou Science and Technology Program for Public Wellbeing (Nos 2014Y2-00185 and 201508020263), and the Special Program of National Science and Technology of China (Nos 2013ZX10004-805 and 2012ZX10004301-003). CF Qin was supported by the Excellent Young Scientist Program of the NSFC of China (No. 81522025) and and the Newton Advanced Fellowship from the Academy of Medical Sciences, UK and the NSFC of China (No. 81661130162).
Footnotes
Author Contributions H.Z., C.-F.Q. and F.-C.Z. designed the study; H.Z. and S.Q. performed the experiments; H.Z., S.Q., W.-X.H., K.-Y.S., F.-C.Z. and C.-F.Q. analyzed the data; J.W., H.-Q.Y., Y.-Q.D. and S.-Y.Z. contributed reagents/materials/analysis tools; H.Z. and C.-F.Q. wrote the paper.
References
- Elling R., Henneke P., Hatz C. & Hufnagel M. Dengue fever in children: where are we now? Pediatr Infect Dis J 32, 1020–1022 (2013). [DOI] [PubMed] [Google Scholar]
- WHO/TDR. Dengue - Guidelines for diagnosis, Treatment, prevention and control. http://www.who.int/tdr/publications/training-guideline-publications/dengue-diagnosis-treatment/en/index.html Date of access: 12/05/2015 (2009). [PubMed]
- Rathakrishnan A. & Sekaran S. D. New development in the diagnosis of dengue infections. Expert Opin Med Diagn 7, 99–112 (2013). [DOI] [PubMed] [Google Scholar]
- Vazquez S., Hafner G., Ruiz D., Calzada N. & Guzman M. G. Evaluation of immunoglobulin M and G capture enzyme-linked immunosorbent assay Panbio kits for diagnostic dengue infections. J Clin Virol 39, 194–198 (2007). [DOI] [PubMed] [Google Scholar]
- Balmaseda A. et al. Diagnosis of dengue virus infection by detection of specific immunoglobulin M (IgM) and IgA antibodies in serum and saliva. Clin Diagn Lab Immunol 10, 317–322 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa M. et al. Immunoglobulin A antibody responses in dengue patients: a useful marker for serodiagnosis of dengue virus infection. Clin Diagn Lab Immunol 12, 1235–1237 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen J. et al. Diagnostic value of dengue virus-specific IgA and IgM serum antibody detection. Microbes Infect 1, 1085–1090 (1999). [DOI] [PubMed] [Google Scholar]
- Vazquez S. et al. Dengue specific immunoglobulins M, A, and E in primary and secondary dengue 4 infected Salvadorian children. J Med Virol 86, 1576–1583 (2014). [DOI] [PubMed] [Google Scholar]
- Vazquez S. et al. Serological markers during dengue 3 primary and secondary infections. J Clin Virol 33, 132–137 (2005). [DOI] [PubMed] [Google Scholar]
- Koraka P. et al. Kinetics of dengue virus-specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. J Clin Microbiol 39, 4332–4338 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rivera I. L., Parham L., Murillo W., Moncada W. & Vazquez S. Humoral immune response of dengue hemorrhagic fever cases in children from Tegucigalpa, Honduras. Am J Trop Med Hyg 79, 262–266 (2008). [PubMed] [Google Scholar]
- Ahmed F. et al. Evaluation of ASSURE Dengue IgA Rapid Test using dengue-positive and dengue-negative samples. Diagn Microbiol Infect Dis 68, 339–344 (2010). [DOI] [PubMed] [Google Scholar]
- Tan Y. Y. et al. Development of ASSURE Dengue IgA Rapid Test for the Detection of Anti-dengue IgA from Dengue Infected Patients. J Glob Infect Dis 3, 233–240 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz Hernandez S. I. et al. Evaluation of a novel commercial rapid test for dengue diagnosis based on specific IgA detection. Diagn Microbiol Infect Dis 72, 150–155 (2012). [DOI] [PubMed] [Google Scholar]
- Naz A. et al. Evaluation of efficacy of various immunochromatographic rapid tests for dengue diagnosis. Pak J Med Sci 30, 166–171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T. et al. Detection of dengue virus genome in urine by real-time reverse transcriptase PCR: a laboratory diagnostic method useful after disappearance of the genome in serum. J Clin Microbiol 50, 2047–2052 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuansumrit A. et al. Dengue nonstructural protein 1 antigen in the urine as a rapid and convenient diagnostic test during the febrile stage in patients with dengue infection. Diagn Microbiol Infect Dis 71, 467–469 (2011). [DOI] [PubMed] [Google Scholar]
- Poloni T. R. et al. Detection of dengue virus in saliva and urine by real time RT-PCR. Virol J 7, 22 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. et al. First confirmation of imported dengue virus serotype 2 complete genome in urine from a Chinese traveler returning from India. Virol J 11, 56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez S. et al. Kinetics of antibodies in sera, saliva, and urine samples from adult patients with primary or secondary dengue 3 virus infections. Int J Infect Dis 11, 256–262 (2007). [DOI] [PubMed] [Google Scholar]
- K J. L. & Nayer A. Dengue-associated kidney disease. J Nephropathol 3, 57–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwanitkit V. Acute renal failure in the fatal cases of dengue hemorrhagic fever, a summary in Thai death cases. Ren Fail 27, 647 (2005). [DOI] [PubMed] [Google Scholar]
- Huy N. T. et al. Factors associated with dengue shock syndrome: a systematic review and meta-analysis. PLoS Negl Trop Dis 7, e2412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoprasopwattana K., Pruekprasert P., Dissaneewate P., Geater A. & Vachvanichsanong P. Outcome of dengue hemorrhagic fever-caused acute kidney injury in Thai children. J Pediatr 157, 303–309 (2010). [DOI] [PubMed] [Google Scholar]
- Upadhaya B. K. et al. Transient IgA nephropathy with acute kidney injury in a patient with dengue fever. Saudi J Kidney Dis Transpl 21, 521–525 (2010). [PubMed] [Google Scholar]
- Halstead S. B. & Cohen S. N. Dengue Hemorrhagic Fever at 60 Years: Early Evolution of Concepts of Causation and Treatment. Microbiol Mol Biol Rev 79, 281–291 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. et al. Isolation and characterization of dengue virus serotype 2 from the large dengue outbreak in Guangdong, China in 2014. Sci China Life Sci 57, 1149–1155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam P. K. et al. Clinical characteristics of Dengue shock syndrome in Vietnamese children: a 10-year prospective study in a single hospital. Clin Infect Dis 57, 1577–1586 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.