Abstract
Transcranial alternating current stimulation (tACS) is used to modulate brain oscillations to measure changes in cognitive function. It is only since recently that brain activity in human subjects during tACS can be investigated. The present study aims to investigate the phase relationship between the external tACS signal and concurrent brain activity. Subjects were stimulated with tACS at individual alpha frequency during eyes open and eyes closed resting states. Electrodes were placed at Cz and Oz, which should affect parieto-occipital areas most strongly. Source space magnetoencephalography (MEG) data were used to estimate phase coherence between tACS and brain activity. Phase coherence was significantly increased in areas in the occipital pole in eyes open resting state only. The lag between tACS and brain responses showed considerable inter-individual variability. In conclusion, tACS at individual alpha frequency entrains brain activity in visual cortices. Interestingly, this effect is state dependent and is clearly observed with eyes open but only to a lesser extent with eyes closed.
Transcranial alternating current stimulation (tACS) is a revived and strongly used tool to investigate the causal role of brain oscillations on cognition and behavior. Many recent reviews have elucidated the importance of this tool in cognitive neuroscience1,2,3.
A substantial amount of evidence points to a direct influence of tACS on brain oscillations, mostly in the form of behavioral effects showing modulations of, for instance, detection ability of weak stimuli4, content in working memory5, memory task performance6, or crossmodal illusions7. All these studies applied tACS frequencies that had been linked to the investigated cognitive processes. Thus, it has been argued that tACS can show a causal relationship to cognitive function. However, this argument relies on the assumption that input waves and brain waves will align during tACS. Considering this strong assumption it is noteworthy that no proof in humans exists so far (but see8 for in vitro animal data).
Possibilities of investigating brain oscillations during tACS stimulation have been provided recently9,10,11. In a previous study10 we focused on the proof of principle aspect of recovering a well established alpha modulation (power increase when the eyes are closed vs. open) during tACS. Here, we use the same source space data to investigate the phase relationship of tACS signal and brain oscillations during different brain states. We stimulate subjects at their individual alpha frequency (IAF, ~10 Hz). Frequencies in the alpha range have been used as stimulation frequencies in many previous tACS studies4,7,12,13 because their dynamics are easily observable in most subjects in parieto-occipital areas while at rest.
In this work, we aim to investigate five major assumptions about the effects of tACS: 1) It is assumed that tACS leads to phasic entrainment, i.e. an alignment of brain activity and tACS phase14,15 and there is evidence for entrainment of neuronal activity from animal research8,16. Here, we estimate phase coherence of tACS signal and brain signal to investigate whether and how brain activity entrains online to tACS. 2) Strongest entrainment is suggested to happen in areas showing a ‘preference’ for the entraining frequency15,17. Thus, alpha frequency stimulation should be largest in visual cortex areas, the generators for visual cortical alpha. 3) Brain state dependent efficiency of tACS has been demonstrated for after-effects14, such that alpha-tACS affected endogenous alpha oscillations only when the subjects’ alpha activity was weak (eyes open), but not so when alpha activity was strong (eyes closed). A proof for state dependent effects during tACS is still missing, thus, we investigated data from eyes open and eyes closed resting states to follow up on that question. 4) Considerable between-subject variation has been found in behavioral effects of tACS4,7,18. To uncover the true tACS effects, phases have to be aligned across subjects to reveal group effects. Considering that electrical current travels through tissue instantaneously, this is surprising; however, it is likely a result of anatomical differences. We test whether entrainment of neural oscillations in visual areas during stimulation occurs with a phase offset (tACS to brain activity) in our data. This could provide a basis to explain between-subject differences in optimal phase.
Material and Methods
Subjects
Seventeen subjects (age M 28, SD 4; 8 female) were analyzed for the present study. All subjects gave written informed consent prior to the experiment. The study protocol was approved by the local ethics committee of the University of Trento, and the study was carried out in accordance with the approved guidelines.
Procedure
Subjects sat in a comfortable chair in a dimly lit booth (AK3b, Vacuumschmelze, Germany) and were asked to fixate on a centrally presented cross without any further task (i.e. resting state) for two minutes with their eyes open and two minutes with their eyes closed. Resting state data were acquired in a sham condition and two individual alpha frequency (IAF) tACS blocks at different intensities (see below). The IAF was estimated from an eyes open resting state block, recorded before the start of the actual experiment, by identifying the peak frequency in the alpha range over occipital sensors (see Table S1 for details). The strong tACS condition was always after the sham and weak tACS blocks (counterbalanced) to avoid aftereffects.
TACS parameters
We used a battery-operated stimulator system (DC-Stimulator Plus, NeuroConn GmbH, Ilmenau, Germany), which was placed outside the magnetically shielded room. A magnetic resonance imaging (MRI) module (NeuroConn GmbH, Ilmenau, Germany) connected the stimulator system and two conductive rubber stimulation electrodes (7 by 5 cm) centered at Cz and Oz, applied with a conductive paste (Ten20, D.O. Weaver, Aurora, CO, USA). The positions were chosen to maximally affect the parieto-occipital cortex19. Impedance values were kept below 15 kΩ (M 6.1 SD 0.8 kΩ). The stimulator delivered a sinusoidal current, without a DC offset, at the IAF. To keep subjects naive about the tACS stimulation, we stimulated below each subject’s sensation/phosphene threshold10. Average stimulation intensity was 0.65 mA (SD 0.45). In the weak tACS condition we stimulated at 0.05 mA, a stimulation intensity that should not affect neural activity20.
MEG recording and analysis
Magnetoencephalography (MEG) was recorded from 306 sensors (102 magnetometers, 204 planar gradiometers) from 102 positions above the participants’ heads (Neuromag Vectorview, Elekta Oy, Helsinki, Finnland). The continuous data in each condition was band-pass filtered (1–200 Hz, 4th order Butterworth), down-sampled to 512 Hz and then cut into 2 s long, non-overlapping epochs. For the tACS conditions, the epochs were aligned to a trough of the ongoing tACS under the occipital electrode.
To overcome the sensor level tACS artifact, the data were projected into source space by means of linearly constrained minimum variance (LCMV) beamforming21, applied to 889 equally-spaced (1.5 cm) points covering the whole brain, resulting in 889 virtual sensors. By using single-shell headmodels22 together with the lead field matrix and the covariance matrix (obtained from individual trials and averaged), we estimated beamformer filters which we subsequently multiplied with the epochs to obtain source level epochs. The used grid was in Montreal Neurological Institute (MNI) space and warped to the individual head space.
All analysis was accomplished with the open-source toolbox FieldTrip23 embedded in Matlab (Mathworks, Natick, USA).
Phase coherence
First, epochs were averaged separately for the resting state (eyes open vs. closed) and tACS conditions (strong tACS, weak tACS, sham) for each subject. Then we obtained complex wavelet coefficients at the stimulation frequency via continuous Morlet wavelet transform between 0.5 to 1.5 s (in steps of 0.005 s) relative to epoch onset. Wavelets had a fixed width of 7 cycles. Phase (in radians) was then calculated with the Matlab function angle.
We computed phase coherence24,25 between the tACS reference signal (captured at a magnetometer under the occipital electrode, MEG2121) and all virtual sensors (889 grid points) for the tACS conditions, and between an artificial cosine (at tACS frequency) and all virtual sensors for the sham condition. Phase coherence (PC) is calculated as follows:
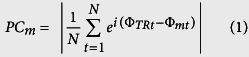 |
where N is the number of time points, ΦTRt the phase of the tACS reference signal and Φmt the phase at virtual sensor m at time point t. Thus PC is coherence of the phase differences between tACS artifact and any virtual sensor over time. The same measure has recently been shown to be able to capture entrainment by tACS in the motor cortex11. To estimate the temporal lag between tACS and virtual sensors, we transformed the mean phase angle differences (tACS reference signal to virtual sensors) to millisecond values by multiplying them with 1000 and dividing them by 2π * f, where f is the IAF.
Statistical analysis
To test whether there was increased PC during tACS compared to sham we compared values across the whole brain with Student’s t-tests (weak tACS vs. sham; strong tACS vs. sham) in the eyes open and eyes closed conditions. We used a permutation approach using cluster statistics that controls for the multiple comparison problem26. We ran 5000 randomizations, used the maximum sum per cluster as test statistic and set the alpha level to 5%.
To compare PC across all conditions in the visual cortex (mean over voxels along the Calcarine sulcus as selected using the Automated Anatomical Labeling [AAL] atlas) we computed a repeated measures analysis of variance (ANOVA) comprised of the factors resting state (eyes open; eyes closed) and tACS condition (sham, weak, strong). The Greenhouse-Geisser correction was applied to account for sphericity violations27. We report the corrected p-values and epsilon coefficient where appropriate. Post-hoc test were corrected using the false-discovery-rate (FDR) procedure28.
Results
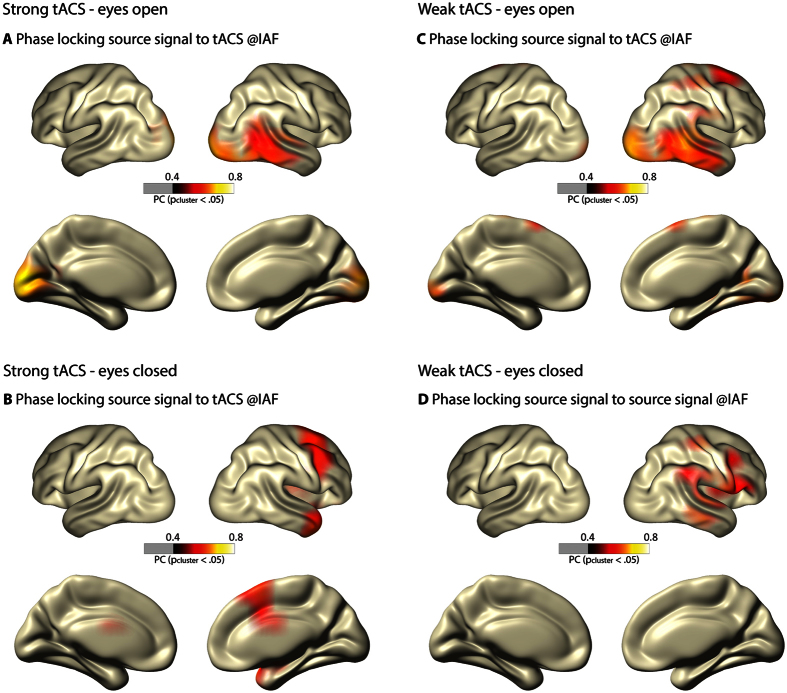
With eyes open, weak and strong tACS resulted in increased PC compared to sham in the occipital pole including some right inferior temporal areas (both pcluster < 2*10−4, Fig. 1A,C). Coherence values were largest in visual areas.
Figure 1. Phase coherence (PC) in the eyes open and eyes closed resting state conditions for weak and strong tACS.
(A,B) show PC during strong tACS, (C,D) show PC during weak tACS. (A,C) show the eyes open state and (B,D) the eyes closed state. Brain activity shows increased PC in visual cortex areas with eyes open but not with eyes closed. All cortex maps are thresholded for significant differences (cluster corrected) in PC between tACS conditions and sham. An MNI template brain was used for visualization purposes.
With eyes closed in the strong tACS condition, only superior frontal regions (pcluster = 0.003, Fig. 1B) showed significant PC increases. With eyes closed in the weak tACS condition, regions covering right inferior frontal, pre- and post central areas as well as superior temporal cortex showed significant PC increases (pcluster = 0.001, Fig. 1D).
The ANOVA on PC amplitude in Calcarine areas (Fig. 2) showed a main effect of resting state (F(1, 16) = 4.77, p = 0.044) and a main effect of tACS condition (F(2, 32) = 4.50, pGG = 0.021, ε = 0.952). These main effects were further explained by an interaction (F(2, 32) = 3.56, pGG = 0.042,, ε = 0.961). Post hoc tests showed no differences in PC between the eyes open and eyes closed state in the sham condition (F(1, 16) = 0.47, pFDR > 0.050) while PC was larger during eyes open compared to eyes closed in the weak tACS condition (F(1, 16) = 14.28, pFDR < 0.010). The strong tACS condition showed a trend for the same effect (eyes open > eyes closed; F(1, 16) = 4.21, pFDR < 0.100). Furthermore, the weak and strong tACS conditions yielded larger PC than sham in the eyes open state (F(1, 16) > 6.53, pFDR < 0.05), however, no differences between tACS conditions (sham; weak; strong) were observed in the eyes closed resting state (all F(1, 16) < 3.09, pFDR > 0.05).
Figure 2. Phase coherence (PC) in visual cortex.
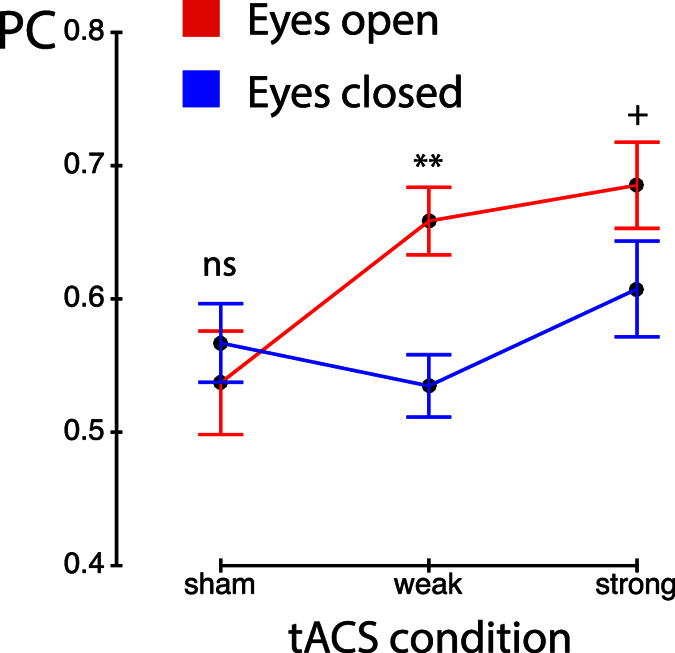
Voxels in the Calcarine cortex were selected using the Automated Anatomical Labeling (AAL) atlas. PC increases with eyes open from sham to both tACS conditions (pFDR < 0.01) but no tACS condition difference was found with eyes closed (pFDR > 0.05). Error bars represent the standard error of the mean. ns – not significant, + - pFDR < 0.1, **pFDR < 0.01.
We calculated the temporal lag in both weak and strong tACS conditions for the eyes open states, the conditions that showed significant PC in the visual cortex (Fig. 3). With strong tACS, the temporal lag in occipital areas as measured from the virtual sensor with highest PC was on average 6.0 ms, however, the variation across subjects was quite considerable (SD 28 ms, see also supplementary Figure S1). With weak tACS, the average temporal lag was −8.8 ms with again considerable variation (SD 25 ms). In both tACS conditions the temporal lag was different along visual cortex areas and not one constant value.
Figure 3. Phase lag of brain activity to tACS signal in the entrained regions in the occipital pole.
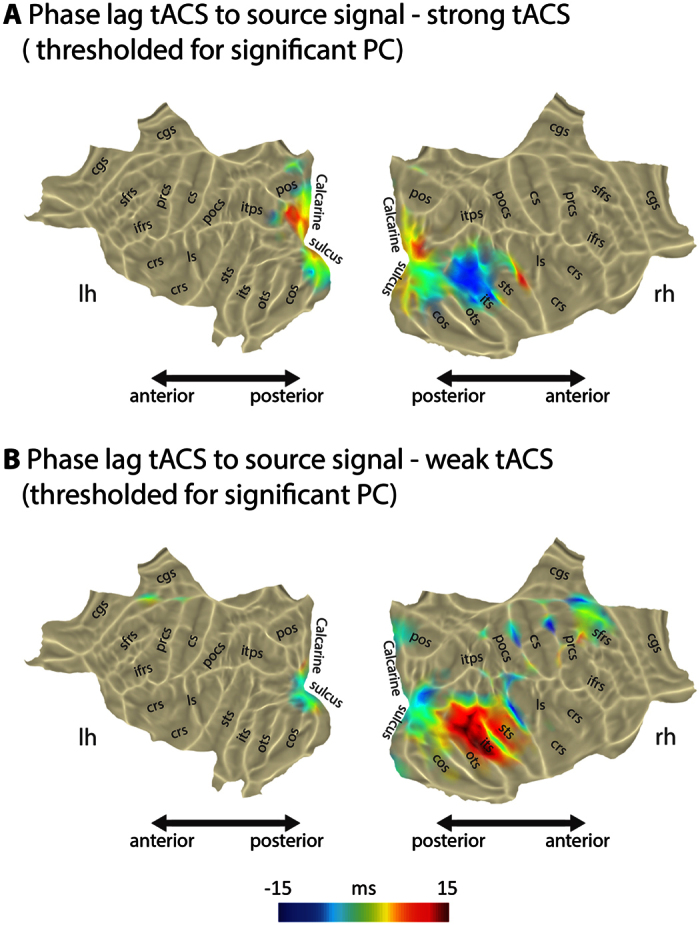
The phase lag shifts along the visual cortex; there seem to be no instantaneous effects in the areas that are strongly phase locked. Individual subjects’ phase lag shows a substantial variation. The abbreviations for cortical sulci are based on the Mindboggle-101 dataset31.
Discussion
The present study proves that phase locking of neural oscillations to external alpha frequency tACS emerges in the visual system. Thus our first aim - to show entrainment of brain activity to tACS - was successful.
Our second goal was to test entrainment in regions showing a ‘preference’ for the entraining frequency15,17. Phase locking was strongest in visual cortex areas, where endogenous alpha activity can be localized. Considering that the current flow should affect broad regions along occipital and parietal areas19, this regional specific entrainment is evidence for entrainment in a region preferring the alpha rhythm.
Our third goal was to show whether brain state dependent effects that have been shown for after-effects of tACS14,29 can be found online. Indeed, we see robust tACS entrainment compared to sham in visual cortex areas when subjects had their eyes open (interestingly even with weak tACS stimulation, but see below), yet, no such increase was observed in visual cortex in eyes closed resting state data. We estimated the IAF based on eyes open resting state data at the beginning of the experiment10, thus, it seems natural that tACS at that frequency affects brain activity during a similar brain state. In reverse, this also means that alpha with eyes open represents a different state than eyes closed alpha, the latter less prone to perturbation by tACS. This finding might also provide an explanation under which circumstances tACS does not show effects. In a recent study30 no after-effects following multiple short tACS intervals (1 s) were found. The authors argue that these short intervals do not allow for neural plasticity to emerge, underlying tACS after-effects (see also15). Alternatively, they could be observing a state dependency effect. Namely they estimate the IAF in an eyes closed resting state block, however, tACS is applied while the subject is performing a visual task, thus the subject is in a different state, possibly not as susceptible to tACS. Likely, this interpretation is overly simplistic, yet, it allows for creating hypotheses that need to be tested in future experiments.
Our fourth goal, investigating individual phase entrainment differences, was based on previous behavioral findings on between subject variance when investigating neural phase during tACS4,7,18. Thus, we investigated the temporal lag between tACS and brain activity in visual cortex regions in those conditions that showed significant phase coherence in visual cortex (Fig. 3 and Figure S1). The entrained areas show an average temporal lag from tACS to brain oscillations of around 6 ms with strong and −9 ms with weak tACS; however, in both cases with large variation (Figure S1). This challenges the rather naive view that the tACS input wave can be directly translated into brain responses. Unfortunately, in the current experiment we did not record behavioral data. In future experiments, it is crucial to investigate behavioral and neural response together to see how well the temporal lag can predict the behavioral response within and across subjects. Here, we used individually adjusted tACS intensities, which could additionally explain temporal lag variation (even though in our study intensity did not correlate with temporal lag across subjects, see Table S1). However, further investigations are needed to explore how and if intensities can influence the tACS-brain activity lag.
Surprisingly, in data from a weak intensity tACS block (0.05 mA) also recorded during eyes open and eyes closed resting states, we found very similar patterns as with strong tACS. Specifically, we found increased entrainment in visual regions with eyes open but not with eyes closed. Such effects were not expected, considering that these intensities should not affect neural activity20 - the condition was planned as control condition. However, our results clearly raise the question of how low tACS intensities can be and still have an influence on brain activity. Modelling studies with higher intensities (1 mA) suggest that low intensity transcranial electrical stimulation reaches cortical areas because the effects scale linearly19. A preliminary correlation analysis on the tACS intensities across subjects (Figure S2) shows a trend to an increase in phase coherence with increasing intensity. However, our study was not designed for this question and more modelling studies are needed as well as a thorough follow-up that varies tACS intensities within subjects to investigate this further.
In conclusion, our results provide evidence for topographically specific entrainment of brain activity to alpha frequency tACS. In particular, entrainment was observed in a state dependent manner, mainly during eyes open resting state. Interestingly, entrainment occurs with subject specific phase lags between tACS and brain activity and also different lags within the visual system. Finally, our results challenge existing ideas that low intensity tACS will not affect neural activity.
Additional Information
How to cite this article: Ruhnau, P. et al. Eyes wide shut: Transcranial alternating current stimulation drives alpha rhythm in a state dependent manner. Sci. Rep. 6, 27138; doi: 10.1038/srep27138 (2016).
Supplementary Material
Acknowledgments
P.R., T.N., M.F. and N.W. were supported by the European Research Council (ERC StG 283404, WIN2CON awarded to N.W.). C.S.H. was supported by the German Research Foundation (grants SFB/TRR31 and HE 3353/8-1, special priority program 1665). We thank Dr. Gianpiero Monittola for technical assistance and Miss Hayley Prins for proof reading an earlier version of the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions P.R., T.N., M.F., C.S.H., G.D. and N.W. conceived the experiment. T.N. and M.F. collected the data. P.R. and T.N. analyzed the data. P.R. created the figures. P.R., T.N., M.F., C.S.H., G.D. and N.W. wrote the paper and approved the final version of the manuscript.
References
- Herrmann C. S., Rach S., Neuling T. & Strüber D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front Hum Neurosci 7, 279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A. & Paulus W. Transcranial alternating current stimulation (tACS). Front Hum Neurosci 7, 317 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf T. A. & Sack A. T. Using brain stimulation to disentangle neural correlates of conscious vision. Front Psychol 5, 1019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuling T., Rach S., Wagner S., Wolters C. H. & Herrmann C. S. Good vibrations: oscillatory phase shapes perception. NeuroImage 63, 771–778 (2012). [DOI] [PubMed] [Google Scholar]
- Vosskuhl J., Huster R. J. & Herrmann C. S. Increase in short-term memory capacity induced by down-regulating individual theta frequency via transcranial alternating current stimulation. Front Hum Neurosci 9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R., Nitsche M. A., Korman C., Batsikadze G. & Paulus W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr Biol 22, 1314–1318 (2012). [DOI] [PubMed] [Google Scholar]
- Cecere R., Rees G. & Romei V. Individual Differencesin Alpha Frequency Drive Crossmodal Illusory Perception. Curr Biol 25, 231–235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich F. & McCormick D. A. Endogenous electric fields may guide neocortical network activity. Neuron 67, 129–143 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich R. F. et al. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol 24, 333–339 (2014). [DOI] [PubMed] [Google Scholar]
- Neuling T. et al. Friends, not foes: Magnetoencephalography as a tool to uncover brain dynamics during transcranial alternating current stimulation. NeuroImage 118, 406–413 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski M. et al. Mapping entrained brain oscillations during transcranial alternating current stimulation (tACS). NeuroImage, doi: 10.1016/j.neuroimage.2015.10.024 (2015). [DOI] [PubMed] [Google Scholar]
- Zaehle T., Rach S. & Herrmann C. S. Transcranial Alternating Current Stimulation Enhances Individual Alpha Activity in Human EEG. PLoS ONE 5, e13766 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen A., Gross J. & Thut G. Alpha Power Increase After Transcranial Alternating Current Stimulation at Alpha Frequency (α-tACS) Reflects Plastic Changes Rather Than Entrainment. Brain Stimul 8, 499–508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuling T., Rach S. & Herrmann C. S. Orchestrating neuronal networks: sustained after-effects of transcranial alternating current stimulation depend upon brain states. Front Hum Neurosci 7, 161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G., Schyns P. G. & Gross J. Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front Psychol 2, 170 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reato D., Rahman A., Bikson M. & Parra L. C. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J Neurosci 30, 15067–15079 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M. et al. Natural Frequencies of Human Corticothalamic Circuits. J Neurosci 29, 7679–7685 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecke L., Formisano E., Herrmann C. S. & Sack A. T. 4-Hz Transcranial Alternating Current Stimulation Phase Modulates Hearing. Brain Stimul 8, 777–783 (2015). [DOI] [PubMed] [Google Scholar]
- Neuling T., Wagner S., Wolters C. H., Zaehle T. & Herrmann C. S. Finite-Element Model Predicts Current Density Distribution for Clinical Applications of tDCS and tACS. Front Psychiatry 3, 83 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reato D. Effects of weak transcranial alternating current stimulation on brain activity—a review of known mechanisms from animal studies. Front Hum Neurosci 7, 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen B. D., Van Drongelen W., Yuchtman M. & Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44, 867–880 (1997). [DOI] [PubMed] [Google Scholar]
- Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys Med Biol 48, 3637–3652 (2003). [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E. & Schoffelen J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011, 156869–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux J.-P. et al. Studying single-trials of phase synchronous activity in the brain. IJBC 10, 2429–2439 (2000). [Google Scholar]
- Mormann F., Lehnertz K., David P. & E Elger C. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D 144, 358–369 (2000). [Google Scholar]
- Maris E., Schoffelen J.-M. & Fries P. Nonparametric statistical testing of coherence differences. J Neurosci Methods 163, 161–175 (2007). [DOI] [PubMed] [Google Scholar]
- Geisser S. & Greenhouse S. W. An Extension of Box’s Results on the Use of the F Distribution in Multivariate Analysis. Ann Math Stat 29, 885–891 (1958). [Google Scholar]
- Benjamini Y. & Hochberg Y. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J R Stat Soc Series B Stat Methodol 57, 289–300 (1995). [Google Scholar]
- Silvanto J. & Muggleton N. G. New light through old windows: moving beyond the ‘virtual lesion’ approach to transcranial magnetic stimulation. NeuroImage 39, 549–552 (2008). [DOI] [PubMed] [Google Scholar]
- Strüber D., Rach S., Neuling T. & Herrmann C. S. On the possible role of stimulation duration for after-effects of transcranial alternating current stimulation. Front Cell Neurosci 9, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. & Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci 6, 171 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.