Abstract
Biohydrogen production has received widespread attention from researchers in industry and academic fields. Response surface methodology (RSM) was applied to evaluate the effects of several key variables in anaerobic fermentation of glucose with Clostridium butyrium, and achieved the highest production rate and yield of hydrogen. Highest H2 yield of 2.02 mol H2/mol-glucose was achieved from 24 h bottle fermentation of glucose at 35 °C, while the composition of medium was (g/L): 15.66 glucose, 6.04 yeast extract, 4 tryptone, 3 K2HPO4, 3 KH2PO4, 0.05 L-cysteine, 0.05 MgSO4·7H2O, 0.1 MnSO4·H2O and 0.3 FeSO4·7H2O, which was very different from that for cell growth. Sugarcane bagasse and Jatropha hulls were selected as typical tropical biomass wastes to produce sugars via a two-step acid hydrolysis for hydrogen production. Under the optimized fermentation conditions, H2 yield (mol H2/mol-total reducing sugar) was 2.15 for glucose, 2.06 for bagasse hydrolysate and 1.95 for Jatropha hull hydrolysate in a 3L fermenter for 24 h at 35 °C, with H2 purity of 49.7–64.34%. The results provide useful information and basic data for practical use of tropical plant wastes to produce hydrogen.
Hydrogen is a clean fuel that only produces water as its environmentally benign product in combustion, and therefore attracts attentions from researchers in the world1. The specific energy of hydrogen (122 kJ/g) is 2.75 times that of regular hydrocarbon fuels2. Annual global production of lignocellulosic biomass is about 2.20 × 1012 Kg (dry weight) from agriculture and forestry residuals, energy crops, aquatic plants and algae3. These rich and sustainable lignocellulosic resources potentially produced H2 with low cost from biomass via hydrolysis and fermentation4.
South China is located in subtropical and tropical regions that were suitable to grow energy or economic crops, such as sugarcane and Jatropha curcas L5. In China, sugarcane production increased from 21.12 in 1978 to 128.2 million tons in 2013, which produced large amount of bagasse as waste in sugar industry6. Large amount of bagasse was produced as industrial waste, which could be used to product biofuels such as bioethanol and biohydrogen7,8. On the other hand, Jatropha is a promising energy crop for biodiesel industry. More than 2,000 biodiesel production plants had been built up in China by 2007 with many residual hulls produced9,10. Jatropha hulls were previously studied to produce 2.3-butanediol and biogas11,12.
Lignocellulosic biomass has complex structure composing of cellulose, hemicellulose and lignin, which should be disrupted before hydrolyzing cellulose and hemicellulose to soluble sugars13. Various types of agricultural residues (such as rice straw and corn stover) were hydrolyzed with dilute-acid for the production of hydrogen14,15. Compared with hydrochloric acid, nitric acid and phosphoric acid, sulfuric acid was more effective in producing fermentable sugars16. Most hemicellulose was hydrolyzed in the first step. The second step was then performed at severer conditions to open up the cellulose structure for improving the accessibility of enzymes for hydrolysis, or to hydrolyze cellulose with acid17. Therefore, two-step dilute acid hydrolysis process was one of the effective pretreatment methods.
Clostridium butyricum as a model bacterium with clear metabolic pathways, was widely used in hydrogen production18,19,20. Preliminary results suggested that the optimized fermentation medium by response surface methodology (RSM) improved the growth of C. butyricum effectively21. However, the most optimal culture medium for the strain growth was not optimal for H2 yield. To the best of our knowledge, the hydrolysate of Jatropha hulls for biohydrogen production has not yet been reported in any previous work. This study aims to (i) optimize the fermentation media for biohydrogen production using glucose as carbon source by RSM, and (ii) the results was further used to efficiently produce biohydrogen from the hydrolysates of bagasse and Jatropha hulls by two-step acid hydrolysis.
Results and Discussion
Glucose and hydrolysates were fermented with C. butyrium in both bottles (batch) and a fermenter. Single-factor bottle experiments were conducted to find best culture medium for hydrogen production from glucose with results given in supplementary materials (Figure S1). Linear relation between dry cell weight (DCW) and optical density at 650 nm (OD650) was plotted in supplementary materials (Figure S2). Both H2 yield and DCW versus different variables were illustrated in supplementary materials (Figure S3). Plackett-Burman (PB) design, experimental results and analysis with the help of software JMP package (version 10, SAS Institute Inc., Raleigh, NC) were demonstrated in Tables 1, 2, 3, , respectively. Study on the path of steepest ascent could be observed in Table 4. Central composite design (CCD), experimental results and analysis were summarized in Tables 5 and 6. Response surface plot and corresponding contour, canonical analysis for RSM were given in Fig. 1 and Table 7, respectively. Products versus time in bottle fermentation of hydrolysates of bagasse and Jatropha hulls were showed in Figs 2 and 3. Composition analysis of bagasse and Jatropha hulls was given in Table 8. Products produced from glucose and hydrolysates in both bottles and fermenter were presented in Table 9. Table 10 Compares hydrogen yield in this study with reported data. All the experiments were repeated more than two times and the standard deviation (σ) was within the range of 0.002 to 0.11 (mol/mol) for H2 production, and 0.008 to 0.09 (g/L) for microbial growth. Detailed results were presented and discussed below.
Table 1. Variables and their levels employed in Plackett-Burman design.
| Factor (g/L) | Variables | Levelsa | |
|---|---|---|---|
| −1 | 1 | ||
| Glucose | X1 | 15 | 25 |
| Yeast extract | X2 | 3.5 | 6.5 |
| Tryptone | X3 | 3 | 5 |
| K2HPO4 | X4 | 2 | 4 |
| KH2PO4 | X5 | 2 | 4 |
| L-Cysteine | X6 | 0.05 | 0.15 |
| MgSO4·7H2O | X7 | 0.0 | 0.1 |
| FeSO4·7H2O | X8 | 0.2 | 0.4 |
ax1 = (X1 − 20)/5; x2 = (X2 − 5)/1.5; x3 = (X3 − 4)/1; x4 = (X4 − 3)/1; x5 = (X5 − 3)/1; x6 = (X6 − 0.1)/0.05; x7 = (X7 − 0.05)/0.05; x8 = (X8 − 0.3)/0.1.
Table 2. Plackett-Burman design variables (in code levels) with H2 yield as response for 24 h bottle fermentation at 35 °C with 130 rpm shaking.
| Run | Variable level |
H2 yield (mol/mol)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | ||
| 1 | −1 | −1 | −1 | 1 | −1 | −1 | 1 | −1 | 1.39 ± 0.08 |
| 2 | −1 | 1 | −1 | −1 | 1 | −1 | 1 | 1 | 1.50 ± 0.02 |
| 3 | −1 | −1 | 1 | −1 | −1 | 1 | 1 | 1 | 1.41 ± 0.07 |
| 4 | −1 | −1 | −1 | 1 | −1 | −1 | 1 | −1 | 1.36 ± 0.05 |
| 5 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 1.44 ± 0.03 |
| 6 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | −1 | 1.30 ± 0.09 |
| 7 | −1 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 1.41 ± 0.05 |
| 8 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 1.28 ± 0.05 |
| 9 | 1 | −1 | −1 | −1 | 1 | −1 | −1 | 1 | 1.32 ± 0.05 |
| 10 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1.35 ± 0.01 |
| 11 | 1 | −1 | −1 | 1 | −1 | 1 | 1 | 1 | 1.23 ± 0.04 |
| 12 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 1.34 ± 0.05 |
aValues were given by mean ± standard deviation (n = 3).
Table 3. Effects and statistical analysis of variablesa.
| Variable | Coefficient | Std. Error | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 1.3644 | 0.0054 | 251.54 | <0.0001b |
| X1 | −0.0611 | 0.0054 | −11.27 | 0.0015b |
| X2 | 0.0256 | 0.0054 | 4.71 | 0.0181b |
| X3 | 0.0078 | 0.0053 | 1.45 | 0.2439 |
| X4 | −0.0161 | 0.0053 | −3.00 | 0.0579 |
| X5 | 0.0167 | 0.0056 | 2.96 | 0.0593 |
| X6 | −0.0122 | 0.0053 | −2.27 | 0.1077 |
| X7 | 0.0067 | 0.0055 | 1.22 | 0.3112 |
| X8 | 0.0083 | 0.0055 | 1.52 | 0.2260 |
aR2 = 0.9858, R2 (Adj) = 0.9478. bStatistical signification at 95% of confidence level (p < 0.05).
Table 4. Experimental results along the path of the steepest ascent for 24 h bottle fermentation at 35 oC with 130 rpm shaking.
| Test | Glucose (g/L) | Yeast extract (g/L) | H2 yield (mol/mol) a |
|---|---|---|---|
| 1 | 20 | 5.0 | 1.62 ± 0.12 |
| 2 | 18 | 5.5 | 1.63 ± 0.03 |
| 3 | 16 | 6.0 | 1.78 ± 0.02 |
| 4 | 14 | 6.5 | 1.51 ± 0.03 |
| 5 | 12 | 7.0 | 1.33 ± 0.02 |
| 6 | 10 | 7.5 | 1.31 ± 0.28 |
| 7 | 8 | 8.0 | 1.20 ± 0.01 |
aValues were given by mean ± standard deviation (n = 3).
Table 5. Levels of the factors, experimental design and the results of the central composite design.
| Run | Coded variable level | Real variable level | H2 yield (mol/mol-TRS)a | |||
|---|---|---|---|---|---|---|
| X1 | X2 | X1 | X2 | Observed | Predicted | |
| 1 | 1 | −1 | 17 | 5.5 | 1.62 ± 0.03 | 1.61 |
| 2 | 0 | 0 | 16 | 6.0 | 2.02 ± 0.05 | 2.00 |
| 3 | −1.41 | 0 | 14.59 | 6.0 | 1.84 ± 0.01 | 1.86 |
| 4 | −1 | −1 | 15 | 5.5 | 1.79 ± 0.08 | 1.78 |
| 5 | 0 | 0 | 16 | 6.0 | 1.99 ± 0.02 | 2.00 |
| 6 | 0 | 0 | 16 | 6.0 | 1.98 ± 0.01 | 2.00 |
| 7 | 0 | 0 | 16 | 6.0 | 2.01 ± 0.02 | 2.00 |
| 8 | 0 | −1.41 | 16 | 5.29 | 1.64 ± 0.03 | 1.66 |
| 9 | 0 | 1.41 | 16 | 6.71 | 1.71 ± 0.07 | 1.72 |
| 10 | −1 | 1 | 15 | 6.5 | 1.85 ± 0.09 | 1.83 |
| 11 | 0 | 0 | 16 | 6.0 | 2.01 ± 0.02 | 2.00 |
| 12 | 1.41 | 0 | 17.41 | 6.0 | 1.61 ± 0.02 | 1.61 |
| 13 | 1 | 1 | 16 | 6.5 | 1.63 ± 0.04 | 1.63 |
aValues were given by mean ± standard deviation (n = 3).
Table 6. Significance test of regression coefficienta.
| Variable | Coefficient | Std. Error | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 2.002 | 0.0088 | 227.04 | <0.0001b |
| X1 | −0.0894 | 0.0070 | −12.83 | <0.0001b |
| X2 | 0.0211 | 0.0070 | 3.03 | 0.0191b |
| X1X2 | −0.0125 | 0.0098 | −1.27 | 0.2454 |
| X1X1 | −0.1329 | 0.0075 | −17.77 | <0.0001b |
| X2X2 | −0.1579 | 0.0075 | −21.12 | <0.0001b |
aR2 = 0.9918, R2 (Adj) = 0.986. bStatistical signification at 95% of confidence level (p < 0.05).
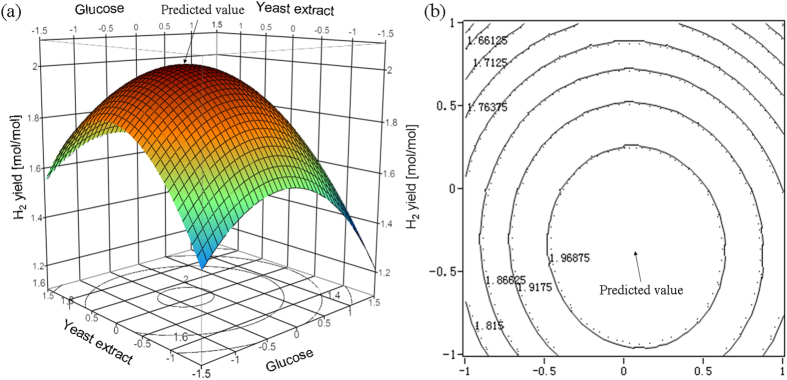
Figure 1.
(a) Response surface plot and (b) corresponding contour of the mutual effects of glucose and yeast extract on H2 yield (24 h bottle fermentation at 35 °C with 130 rpm shaking).
Table 7. Analysis of variance and canonical analysis for the parameters of RSM.
| Source | Degree of freedom | Sum of square | Mean square | F-value | Probability > F |
|---|---|---|---|---|---|
| Model | 5 | 0.330755 | 0.066151 | 170.1574 | <0.0001 |
| Error | 7 | 0.002722 | 0.000389 | ||
| C. total | 12 | 0.333477 | |||
| Lack of fit | 3 | 0.001641 | 0.000547 | 2.0264 | 0.2528 |
| Pure error | 4 | 0.001088 | 0.000270 | ||
| Canonical analysis | |||||
| X1 | X2 | Predicted H2 yield: 2.02 (mol/mol) |
|||
| Eigen value | −0.3402 | 0.0804 | |||
| Actual value | 15.6598 | 6.0402 | |||
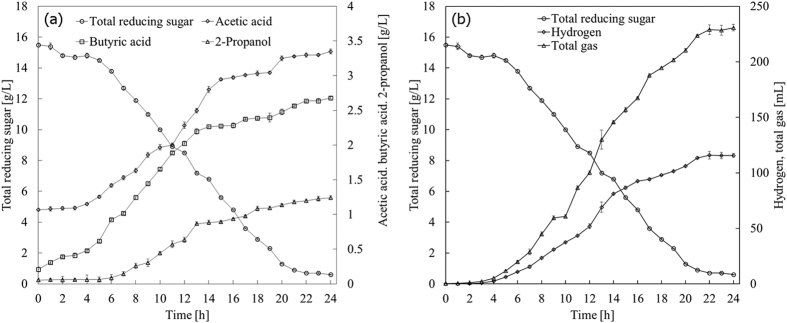
Figure 2.
H2 and by-products production versus time in bottle fermentation of bagasse hydrolysates under the optimized medium at 35 °C with 130 rpm shaking (a) liquid products (b) gas products.
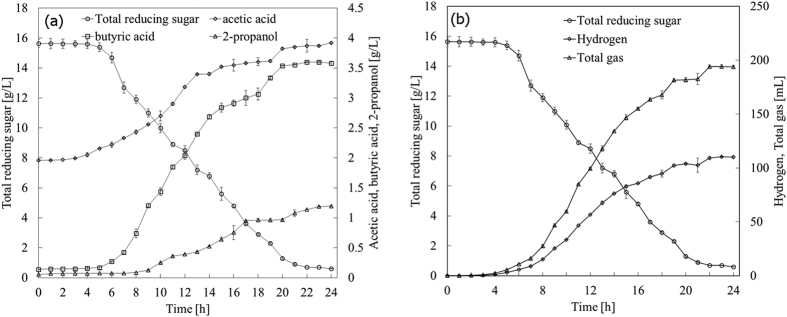
Figure 3.
H2 and by-products production versus time in bottle fermentation of Jatropha hulls hydrolysates under the optimized medium at 35 °C with 130 rpm shaking (a) liquid products (b) gas products.
Table 8. Structural carbohydrates and lignin in bagasse and Jatropha hullsa.
| Biomass | Glucan (%) | Xylan (%) | Galactan (%) | Arabinan (%) | Mannan (%) | Lignin (%) | Ash (%) | Extractives (%) |
|---|---|---|---|---|---|---|---|---|
| Bagasse | 42.05 ± 0.62 | 19.79 ± 2.81 | 2.47 ± 0.29 | 1.79 ± 0.02 | 1.12 ± 0.05 | 20.82 ± 0.12 | 1.98 ± 0.25 | 9.29 ± 0.01 |
| Jatropha hulls | 36.95 ± 1.35 | 9.78 ± 1.35 | 0.82 ± 0.03 | 0.30 ± 0.00 | 2.47 ± 0.53 | 27.9 ± 0.1 | 1.19 ± 0.19 | 18.61 ± 0.03 |
aAll data were given by mean ± standard deviation (n = 3).
Table 9. Comparisons of product yields from glucose, hydrolysates of bagasse and Jatropha hulls for H2 production for 24 h fermentation at 35 °C with 130 rpm stirring/shaking in both reactorsa.
| Substrate | Reactor | H2 yield (mol/mol) | Acetic acid (AC) (g/L) | Butyric acid (BC) (g/L) | 2-propanol (g/L) | AC/BC Ratio | Gas volume (mL) | Composition (%): H2 | CO2 |
|---|---|---|---|---|---|---|---|---|---|
| Glucose | Fermenter | 2.15 ± 0.03 | 3.87 ± 0.12 | 3.35 ± 0.09 | 0.84 ± 0.01 | 1.15 | 8120 ± 37.98 | 64.34 ± 0.47 | 35.66 ± 0.40 |
| Bottle | 2.03 ± 0.02 | 3.14 ± 0.06 | 2.87 ± 0.04 | 1.27 ± 0.02 | 1.09 | 233 ± 3.79 | 52.69 ± 0.14 | 47.31 ± 0.81 | |
| Bagasse hydrolysate | Fermenter | 2.06 ± 0.02 | 3.46 ± 0.09 | 3.34 ± 0.07 | 1.36 ± 0.03 | 1.03 | 8532 ± 33.51 | 53.92 ± 0.61 | 46.08 ± 0.09 |
| Bottle | 1.99 ± 0.03 | 2.28 ± 0.03 | 2.47 ± 0.02 | 1.24 ± 0.03 | 0.9 | 230 ± 5.69 | 50.16 ± 0.09 | 49.84 ± 0.04 | |
| Jatropha hulls hydrolysate | Fermenter | 1.95 ± 0.06 | 2.37 ± 0.01 | 3.54 ± 0.03 | 1.15 ± 0.01 | 0.67 | 8767 ± 88.77 | 49.70 ± 0.49 | 50.29 ± 0.20 |
| Bottle | 1.89 ± 0.02 | 1.97 ± 0.01 | 3.43 ± 0.03 | 1.20 ± 0.01 | 0.57 | 195 ± 1.49 | 56.62 ± 1.30 | 43.38 ± 0.24 |
aAll data were given by mean ± standard deviation (n = 3).
Table 10. Comparison of biohydrogen yield in this study with reported data.
| Raw biomass | Carbon resource | Microorganism | Reducing Sugar (g/L) | H2 yield (mol/mol) |
|---|---|---|---|---|
| Bagasse | Glucose Xylose | C. butyricum | 20 | 1.7318 |
| Bagasse | Glucose Xylose | Clostridium sp. | 10 | 0.819 |
| Starch | Hexose | C. butyricum | 5 | 2.020 |
| Glucose | Glucose | C. butyricum ATCC19398 | 3 | 1.837 |
| Glucose | Glucose | C. butyricum | 2.5 | 1.4–2.338 |
| Glucose | Glucose | Clostridium sp. | 20 | 0.8536 |
| Glucose | Glucose | C. butyricum | 15.64 | 2.15a |
| Bagasse | Glucose Xylose | C. butyricum | 15.64 | 2.06a |
| Jatropha hulls | Glucose Xylose | C. butyricum | 15.64 | 1.95a |
aData in this study in 3L fermenter.
Single-factor experiments
Single-factor experiments were performed to verify factors in fermentation medium that affected H2 yield, and to provide basic data for following experiments. Batch (bottle) fermentation was performed at 35 °C for 24 h with the culture medium conditions given in Method section and shaking rate of 130 rpm. It can be concluded from Figure S1 that fermentation medium producing the highest H2 yield of 1.67 mol H2/mol-glucose contained (g/L): 15 glucose, 5 yeast extract, 3 K2HPO4, 3 KH2PO4, 0.05 L-cysteine, 0.05 MgSO4·7H2O, 0.1 MnSO4·H2O and 0.3 FeSO4·7H2O, as compared to the yield of 1.28 mol H2/mol-glucose (increased by 30.47%) with the initial medium. Microbial growth was also studied under different variable concentrations in culture medium. Regression curve presents well-linear relationship between DCW and OD650 (Figure S2). It was found that the optimal concentrations of variables for microbial growth and H2 yield were different (Figure S3). Obviously, the optimal concentrations of glucose, L-cysteine, MgSO4·7H2O and FeSO4·7H2O were 30, 0.1, 0.15 and 0.1 g/L for DCW, respectively, while they were 15, 0.05, 0.05 and 0.3 g/L for H2 production (Figure S3). The most optimal culture medium for the strain growth was not the maximum for hydrogen production.
PB design
PB design was used to identify variables that had significant effects on H2 production. The medium compositions were: glucose, yeast extract, tryptone, K2HPO4, KH2PO4, L-cysteine, MgSO4·7H2O and FeSO4·7H2O (Table 1). Both high and low levels (1 and −1) of each variable were chosen based on the above preliminary single-factor experiments. Table 2 gave the PB experiment results in 24 h batch fermentation at 35 °C with 130 rpm, while the statistical analysis of PB experiment data was summarized in Table 3. The significance of each variable was determined by their corresponding p-values. Factors evidencing p-values of less than 0.05 were considered to be significant effects on the response22, and were subsequently studied in further optimization studies. In this case, X1 (glucose; p = 0.0015) and X2 (yeast extract; p = 0.0181) were statistically significant in the production of H2 (p < 0.05). X1 had the greatest negative impact on H2 yield. On the other side, X2 had positive effect on H2 yield. X3 (tryptone), X5 (KH2PO4), X7 (MgSO4·7H2O) and X8 (FeSO4·7H2O) were set at their high levels according to the positive effects although they were insignificant to H2 yield. Factors such as X4 (K2HPO4) and X6 (L-cysteine) with negative effects were selected on their low levels. To approach the optimum response, a fitted first-order model equation for H2 yield (Y) was obtained from the PB design experiments:
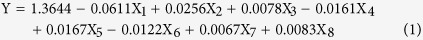 |
The coefficient of each variable in Eq. (1) represents the effect weight of the variable on H2 yield. The quality of the fitted polynomial model equation was expressed by the coefficient of determination (R2). The obtained R2 was 0.9858, indicating that 98.58% variability in the response could be explained by the model, but only 1.42% variability was not explained. Meanwhile, the value of adjusted determination coefficient (Adj R2 = 0.9478) was very high, which advocates a high significance of the model23. These results show that the response equation provides a suitable model for the PB design experiments.
Path of the steepest ascent
Experiments were started from zero level in PB design. If t-value was positive, climbing direction would increase and vice versa24. Overall, step size was determined by the above t-values (Table 3) and single-factor experiments. According to t-values (Table 3) and single-factor results, step size of 2 and 0.5 g/L was set for glucose and yeast extract, respectively. Glucose shows decreasing trend but an increasing trend for yeast extract. The concentrations of other factors were steadfast in all trials at their optimal levels. It was observed that H2 yield increased along the path from test 1 to 3, reached the peak of 1.78, and decreased from test 4 to 7. This means that the optimal level was close to that in test 3 (16.0 g/L glucose and 6.0 g/L yeast extract). In Table 4, the highest H2 yield was 1.78 mol H2/mol-glucose for test 3. In these experiments, H2 yield was remarkably improved. This suggests that the steepest ascent method was an effective technique to determine an optimal level. However, the optimal values for these two variables need to be determined via the following CCD.
RSM and CCD
A series of experiments were carried out via RSM to obtain an optimal combination of glucose and yeast extract. Table 5 gave five different coding levels, full experimental design and the results of CCD, with the center point set based on the results in steepest ascent test (Table 4).
Table 6 summarizes the significance test of regression coefficients. Using multivariate regression analysis of the observed data (Table 6), the obtained model illustrates the relationship between glucose (X1) and yeast extract (X2) corresponding to H2 yield (Y) as below:
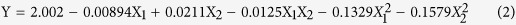 |
where Y was the predicted H2 yield, X1 and X2 were the coded values of glucose and yeast extract. The regression model (Eq. 2) can be used to predict the range of H2 production for various levels of the selected variables.
Determination and adjusted determination coefficients (R2 and Adj R2) were calculated as 91.72% and 85.8% for the regression, demonstrating the agreement between the experimental and predicted H2 yields that provides a good estimation of the response within the range of process conditions.
Analysis of variance (ANOVA) is essential to test the significance and adequacy of model. High t-value reveals an adequate explanation of the variation of data regarding to their mean value. The t-test and p-values served to examine the significance of each coefficient, which also illustrated the interaction strength among independent variables25. The t-test of each significant variable level was given in Table 6. In this model term, the linear and quadratic of X1, X1X1 and X2X2 (p < 0.05) were recognized as the significant factors26, suggesting that H2 yield was directly related to these two main factors (glucose and yeast extract). However, the interaction between glucose and yeast extract (X1X2, p > 0.05) seemed to be less significant on H2 production. F-values of the model and lack of fit were 170.16 and 2.03, respectively (Table 7), while model p-values and p-values of lack of fit were <0.0001 and 0.2528, suggesting that the model was good fit to the experimental data.
Three dimensional (3D) response surface and corresponding contour of glucose and yeast extract were presented in Fig. 1. The response surface (Fig. 1a) displays an obvious convex, revealing the optimum conditions were well-defined. The contour plot was almost circular, suggesting that the interaction of glucose and yeast extract had a less significant effect on H2 production. In the surface plot, H2 yield achieved the highest value within the studied region. Canonical correlation analysis (CCA) is a multivariate technique focused on determining the relationship among groups of variables in a datum set27. CCA was further conducted (Table 7), at X1 = −0.3402 (glucose, 15.66 g/L) and X2 = 0.0804 (yeast extract, 6.04 g/L), the predicted maximum H2 yield corresponding to these values was 2.02 mol H2/mol-glucose.
Experimental validation of the optimized medium
In order to verify the model (Eq. 2) adequacy in predicting the maximum yield of H2, experiments were performed under optimum medium composition with 3 repetitions. H2 yield was 2.03 ± 0.02 mol H2/mol-glucose after 24 h bottle fermentation that agrees excellently with the predicted value (2.02 mol H2/mol-glucose).
In summary, RSM is an efficient tool to optimize the medium composition for hydrogen production by C. butyrium. The optimized medium was (g/L): 15.66 glucose, 6.04 yeast extract, 4 tryptone, 3 K2HPO4, 3 KH2PO4, 0.05 L-cysteine, 0.05 MgSO4·7H2O, 0.1 MnSO4·H2O and 0.3 FeSO4·7H2O with 2.03 mol H2/mol-glucose after 24 h. On the other hand, previous study gives the optimum fermentation medium for the growth of C. butyricum was composed of (w/v): 2% glucose, 0.5% pectin, 0.2% casein, 3.98% soyabean cake extract, 0.1% (NH4)2SO4, 0.124% NaHCO3, 0.37% corn steep flour, 0.02% MnSO4·H2O, 0.02% MgSO4·7H2O and 0.002% CaCl2 at pH 7.521. The optimized media for growth and H2 production were different. All the above experiments were conducted in sealed 100 mL bottles. The obtained optimized results were further used for the production of H2 from actual biomass wastes below.
Hydrogen from hydrolysates of bagasse and Jatropha hulls
Bagasse and Jatropha hulls mainly consist of cellulose and hemicellulose and lignin11,18. The chemical compositions of bagasse and Jatropha hulls were analyzed according to the technical report from US National Renewable Energy Laboratory (NREL)28,29 (Table 8). Bagasse consisted of 25.17 wt% hemicellulose and 42.05 wt% cellulose, but 13.37 wt% hemicellulose and 36.95 wt% cellulose for Jatropha hulls. Bagasse and Jatropha hulls were hydrolyzed by two-step dilute acid hydrolysis in an autoclave. After reaction, the hydrolysates were neutralized, concentrated, detoxificated, and fermented under the optimized medium obtained from glucose. Figures 2 and 3 illustrated liquid and gas products vs. time in the bottle fermentation of hydrolysates from bagasse and Jatropha hulls, respectively. For the fermentation of bagasse hydrolysate (Fig. 2), as time increased, yields of H2, acetic acid and butyric acid increased with the decreasing TRS. These liquid products rose very slowly before 4 h as the strain was in the lag phase for a short adaptation to the new environment30. In Fig. 2a, after 5 h, acetic acid and butyric acid grew rapidly to 3.25 and 2.48 g/L at 20 h (vs. 1.26 and 0.62 g/L at 5 h), and slightly rose further to 3.35 and 2.68 g/L at 24 h, respectively. Acetate and butyrate rose slightly after 15 h and stopped increasing after 20 h with the consumption of TRS because the accumulation of acetate and butyrate caused a sharp drop in culture pH and inhibited the growth of bacteria. 2-Propanol also presented similar rising trend. Similarly, H2 production (Fig. 2b) rose sharply from 3.05 to 115.87 mL at time from 4 to 22 h, and changed little until 24 h. At the same time, total gas rose much higher from 5.29 at 4 h to 228.9 mL at 22 h because CO2 was produced. For the fermentation of hydrolysate of Jatropha hulls (Fig. 3a), acetic acid and butyric acid increased rapidly to 3.87 and 3.59 g/L at 22 h from 2.16 and 0.17 g/L at 5 h. However, more butyric acid (3.52 g/L) produced at 24 h from the hydrolysate of Jatropha hulls than that of bagasse (2.68 g/L). The initial acetic acid concentration from the hydrolysate of Jatropha hulls (1.96 g/L) was higher than that of bagasse (1.06 g/L) that may inhibit the further production of acetic acid. More acetic acid was produced from the hydrolysate of bagasse (2.28 g/L) than Jatropha hulls (1.97 g/L) at 24 h (Table 9). Contrary, the production of butyric acid from the hydrolysate Jatropha hulls (3.43 g/L) was more than that of bagasse (2.47 g/L). The growth of strains was likely to be inhibited by the acetate produced when grown on media containing glucose31. So, less H2 was produced from hydrolysate of Jatropha hulls (110.4 vs. 115.87 mL for bagasse hydrolysate at 24 h) via acetic route discussed below.
Sugars were also fermented in a 3L fermenter for 24 h at 35 °C with 130 rpm stirring (Table 9). H2 yield from glucose is 2.15 (mol H2/mol-glucose), which is slightly higher than the experiment in bottle fermentation (2.02 mol H2/mol-glucose) because hydrogen pressure in bottle fermentation increased as gas accumulated in the fixed 100-mL bottle that may prevent hydrogen production. But, no pressure rose for the fermenter since it was connected on-line to an empty gas bag. Previous study shown that partial pressure of H2 (pH2) was an extremely important factor for continuous H2 synthesis32. The hydrogen evolution rate and yield were improved by 10% and 15%, respectively, by reducing the H2 partial pressure through pumping out produced gas in a fermentor with silicone rubber33. So, high pressure prevented hydrogen production in bottle fermentation. The hydrogen production also affected by its concentration. As hydrogen concentration increased, H2 synthesis decreased and metabolic pathways shifted towards the production of more reduced substrates, such as lactate, ethanol, acetone, butanol or alanine32. On the other hand, acids produced in the bottles without neutralization may also hamper the production of H2. Acetate accumulation caused a sharp drop of culture pH and subsequent inhibition of bacterial hydrogen production, that were reported previously34. For fermenter experiments, H2 yield from hydrolysates of bagasse and Jatropha hulls is 2.06 and 1.95 (mol H2/mol-TRS), which reached 95.8% and 90.7% of that from pure glucose, respectively.
Generally, glucose or other reducing sugars (e.g., xylose) were the preferred carbon source for fermentation with overall reactions as below35:
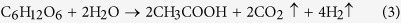 |
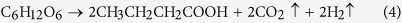 |
According to Eq. (3), 4 mol H2/mol-glucose was obtained with acetic acid and CO2 as by-products. However, if butyric acid was formed as by-product, only 2 mol H2/mol-glucose was produced (Eq. 4). The ratio of acetic/butyric acids obtained could be related to the production of H2. Previous studies showed that the increase of the ratio was accompanied by increased production of H235. In Table 9, it was well-confirmed in fermenter that acetic/butyric acid ratio was 0.67, 1.03 and 1.15 (g/g) for Jatropha hulls, bagasse and glucose, corresponding to their H2 yield (mol/mol-TRS) of 1.95, 2.06 and 2.15, respectively. As comparison, bottle experiments with lower acetic/butyric acid ratio (g/g) of 0.57, 0.9 and 1.09 for hydrolysates of Jatropha hulls, bagasse and glucose had lower H2 yield (mol/mol-TRS) of 1.89, 1.99 and 2.03 (Table 9). Gas is composed of 49.7–64.34% H2 and 35.66–50.29% CO2 from anaerobic fermentation in bottles and fermenter. H2 from glucose (64.34%) had much higher concentration than those from hydrolysates of bagasse (53.92%) and Jatropha hulls (49.7%) in fermenter. The concentrations of H2 produced from glucose and bagasse in fermenters were higher than those in bottles (64.34% and 53.92% vs. 52.69% and 50.16%). However, H2 produced from Jatropha hulls (49.7%) in fermenter was less pure than that in bottles (56.62%).
Table 10 compares biohydrogen production in this work with other previous studies from various types of biomass with different microorganisms. In those previous studies, the yield of H2 fermented by bagasse hydrolysate was 0.8–1.73 mol H2/mol-TRS18,19, and 0.85–2.3 mol H2/mol-glucose36,37,38. This study produced comparable biohydrogen yields to the reported studies (1.95–2.15 vs. 0.8–2.3 mol H2/mol-TRS).
Conclusions
Fermentation medium for H2 production from glucose with Clostridium butyricum was optimized by response surface methodology with the highest H2 yield of 2.02 mol H2/mol-glucose for batch fermentation at 35 °C for 24 h. The optimized medium was composed of (g/L): 15.66 glucose, 6.04 yeast extract, 4 tryptone, 3 K2HPO4, 3 KH2PO4, 0.05 L-cysteine, 0.05 MgSO4·7H2O, 0.3 FeSO4·7H2O and 0.1 MnSO4·H2O. Higher H2 yield of 2.15 mol H2/mol-glucose was achieved for 24 h in a well-controlled fermenter. The hydrolysates of bagasse and Jatropha hulls from two-step dilute acid hydrolysis were further successfully fermented to hydrogen with yields of 2.06 and 1.95 (mol H2/mol-total reducing sugars) in fermenter, respectively. Higher H2 yield from glucose and bagasse hydrolysate was due to their high acetic/butyric acid ratio via acetic route for hydrogen production. It is also found that optimized condition for H2 production was not the best condition for microbial growth. Tropical biomass wastes as inexpensive raw materials can be effectively produce biohydrogen.
Methods
Materials
Substrates [glucose (99.8%), xylose (99.9%), fructose (99.5%), galactose (99.8%) and mannose (99.7%)], yeast extract, L-cysteine and tryptone were bought from Bomei Biotech Co., Ltd. (Heifei, Anhui). Calcium carbonate (99.8%), K2HPO4 (99%), KH2PO4 (99.8%), Na2HPO4 (99%), NaCl (99.5%), MnSO4·H2O (99%), MgSO4·7H2O (99%, FeSO4·7H2O (99%), glycerol (99%), ethanol (99.7%) and sulfuric acid (98%) were bought from Xilong Chemical Factory Co., Ltd. (Shantou, Guangdong). Standard sugars of glucose, xylose, arabinose, mannose and galactose (purity > 99%) were bought from Sigma-Aldrich (Shanghai). Acetic acid (99.5%), butyric acid (99.7%) and 2-propanol (99.9%) were from Aladdin Industrial Corporation (Shanghai). Activated carbon (powder, 97%) was purchased from Fengchuan Chemical Reagent Co., Ltd (Tianjin).
Jatropha hulls were purchased from Yunnan Shenyu New Energy Co., Ltd. (Chuxiong, Yunnan). Bagasse was bought from Dehong (Yunnan). Biomass samples were dried at 45 °C until constant weight (WFO-710, EYELA, Tokyo Rikakikai Co., Ltd.), ground in a pulverizer (9FC-15, Xudong Machinery Manufacturing Co., Ltd., Leshan, Sichuan) and sieved through 80 mesh for analysis and hydrolysis.
Microorganism and batch (bottle) fermentation
C. butyricum (CICC 20763) used for producing H2 was purchased from China Center of Industrial Culture Collection (CICC, Beijing). The culture was maintained on corn meal agar stab at 4 °C. The seed medium composed of (g/L): 20 glucose, 5 yeast extract, 5 tryptone and 3 NaCl with pH 6.5. To make the seed cultivation, a loop of C. butyricum from a fresh slant tube was inoculated into a serum bottle (250 mL) containing 50 mL seed medium and then incubated in a rotary shaker (ZWY-2102C, Zhicheng Analytical Instrument Manufacturing Co., Ltd., Shanghai) at 35 °C with 130 rpm for 12 h.
The initial medium for anaerobic fermentation was composed of (g/L): 25 glucose, 10 tryptone, 5 Na2HPO4, 3 NaCl and 0.05 FeSO4·7H2O. A 4% (v/v) of the seed culture was inoculated into the medium for fermentation. After sterilization at 121 °C for 20 min in an autoclave (HVE-50, Hirayama Manufacturing Corp., Tokyo), substrates with C. butyricum (about 20 mL, initial pH 6.5) were loaded into 100-mL bottles in an anaerobic incubator (YQX-II, CIMO Medical Instrument Manufacturing Co., Ltd., Shanghai) to maintain anaerobic environment by nitrogen purge (99.999% N2, Meisel Gas Products Co., Ltd., Kunming, Yunnan) and sealed by rubber-aluminium cap. The bottles with samples were put in an incubator shaker (ZWY-2102C) at 130 rpm and 35 °C for batch fermentation. After fermentation (up to 24 h), gas was collected in a bag (500 mL) by a 50 mL syringe (Agilent Inc., Palo Alto, CA) and its volume (e.g., up to 233 mL at 24 h) was measured by the same syringe. Gas was analyzed by gas chromatograph (GC) and liquid sample by high performance liquid chromatograph (HPLC), ultraviolet (UV)-visible spectrophotometer and biosensor introduced in detail below.
PB design
PB design was applied to select factors that significantly influenced H2 yield, based on the first-order (linear) model as below22:
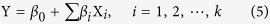 |
where Y was H2 yield as response; Xi was coded independent factor and βi was linear coefficient; β0 was intercept value. Each variable had two-levels, high and low, coded by (+1) and (−1), respectively.
Path of steepest ascent method
The method of steepest ascent given by Box and Wilson is a procedure for moving sequentially along the direction of the maximum increase in response39. The direction in which H2 yield increased most rapidly was that of steepest ascent, started from the zero level of variables (significant factors) in PB design, while the step size was decided by the estimated coefficient ratio from Eq. 5, together with practical experience. This test was disused until response no longer increased. As a result, the steepest ascent method allowed factors approaching the optimal level and gave a more limited region for RSM optimization40.
CCD and RSM
CCD was employed to optimize the two most significant factors (glucose and yeast extract) for the maximum H2 yield, screened by PB design. These two independent factors were studied at five different levels (−1.41, −1, 0, +1 and +1.41), and a set of 13 experiments were carried out (Table 5). The behavior of the experiment was explained by the following second-order polynomial equation23:
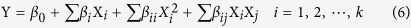 |
where Y was the predict response, β0 was intercept, Xi and Xj were input variables which influenced the response Y, βi was linear coefficient, βii was quadratic coefficient, and βij was interaction coefficient. The second-order polynomial coefficients were also calculated.
Composition analysis of bagasse and Jatropha hulls
Composition analysis of bagasse and Jatropha hulls was conducted according to the standard US NREL protocols28,29. All the raw materials were dried at 45 °C until achieving constant weight. Biomass sample (2–10 g, oven dry weight, ODW) was extracted in a Soxhlet extractor with deionized water and ethanol at boiling points for 12–24 h, respectively. The extracted solutions were dried in a flask to remove water and ethanol at 45 to 60 °C by a rotary evaporator. The extracted solid biomass (0.3 ± 0.01g, dried) was hydrolyzed in a 25-mL tube containing 4.98 ± 0.01 g concentrated sulfuric acid (72 wt%) stirred by a magnetic bar at 30 °C water bath for 1 h, and then diluted to 4 wt% sulfuric acid solution by adding 84 ± 0.01 mL deionized water. The diluted solution with biomass sample was put in a 100 mL bottle sealed by a rubber-aluminum cap and autoclaved at 121 °C for 60 ± 1min (HVE-50) for second-step hydrolysis, at the same time, a reference standard sugar mixture (glucose, xylose, galactose, arabinose and mannose) with similar concentrations to the sugars in biomass sample was also put in the autoclave to calibrate the decomposition of each sugar. The filtrated supernatant was collected for acid-soluble lignin determination by using UV-Visible spectrophotometer at 240 nm wavelength (UV-1800, Shimadzu, Kyoto). The supernatant was neutralized to pH 5.0–6.0 with calcium carbonate for sugar analysis described below. The solid residual separated from the hydrolysate by filtration (0.22 μm pore size) was oven-dried for acid-insoluble lignin determination. Crucibles containing residues were placed in a muffle furnace (4–10, Ever light medical equipment Co., Ltd, Beijing) at 575 ± 25 °C for 24 h to determine ash weight.
Hydrolysis of Jatropha hulls and bagasse
Two-step dilute acid hydrolysis was conducted in a 500-mL high-pressure autoclave (FCFD05-30, Yantai Jianbang Chemical Mechanical Co. Ltd., Shandong). In the first-step hydrolysis, bagasse or Jatropha hulls (20 g, dry weight) mixed with 2 wt% sulphuric acid solution with solid-liquid ratio (SLR) of 1:10 (g/g) was hydrolyzed at 130 °C for 1 h41. After reaction, solid residue was filtrated (0.22 μm pore size), washed and dried for second-step hydrolysis in 4 wt% sulphuric acid solution with 15 g solid residue, SLR of 1:10 (g/g) at 150 °C for 1 h11. Hydrolysates from both steps were mixed together, and neutralized to pH 6.0 with calcium hydroxide. After filtration (0.22 μm pore size), the solution was concentrated to 15.66 g/L TRS by a rotary evaporator at 60–70 °C. The concentrated solutions (100 mL) were further detoxicated with 2 wt% activated carbon at 50 °C for 2 h, and used for the following fermentations. The hydrolysates were fermented under the optimized medium obtained from glucose in bottles because they mainly contained hexose and pentose sugars with minor of acids, furfural, hybrid protein and other toxic components that were neutralized and detoxified.
Fermentation in a fermenter
Fermentation of glucose and hydrolysates (about 1 L) with C. butyricum was also carried out in a 3L fermenter (LiFlus GX, Hanil Science Industrial Co. Ltd., Incheon, South Korea) at 35 °C for 24 h with 130 rpm stirring. Its pH was controlled at 6.5 by adding 1 M sodium hydroxide or 1 M hydrochloric acid. Gas generated (up to 8.8 L) during the fermentation process was collected into a gas bag (10 L) on-line and its volume was measured after fermentation using a wet gas meter (LMF-1, Shanghai A.K. Instruments Co., Ltd.), where water was saturated by NaHCO3 to avoid absorption of CO2. Gas and liquid products were analyzed described below.
Analytical methods
Gas was analyzed by GC (7820A, Agilent, Palo Alto, CA) equipped with four columns [three packed columns: one Porapak Q (9 ft × 1/8 in.) and two Porapak N (3 ft × 1/8 in.), and one Molecular Sieve 5A (6 ft × 1/8 in.)] with two thermal conductivity detectors (TCD). In this work, no CH4, CO, C2H6 and C2H2 gases were detected. Produced gases (H2 and CO2) were only separated by Porapak Q (CH4 together with CO were also monitored even though their peaks were overlapped). Helium (99.999% purity, Guangruida Co. Ltd., Kunming, Yunnan) was used as carrier gas. Gas sample was calibrated with a standard gas mixture (35% H2, 2% CH4, 25% CO2; v/v) (Huate Gas Co. Ltd., Foshan, Guangdong), and diluted 1–5 times into five gradients with nitrogen (99.9999%, Guangruida Co. Ltd.). All the standard calibration curves obtained had R2 > 0.999. H2 yield was defined as below:
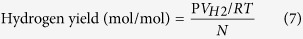 |
where P (0.8 × 105 Pa in Kunming at an altitude of 1,896 m, calculated by Barometric formula42) is atmospheric pressure; VH2 is gas volume (m3); R is perfect gas constant (8.3144, m3·Pa·mol−1 · K−1); T is gas temperature (298 K), and N is mole number of substrate (e.g., glucose, TRS, glycerol] before fermentation (mol).
Liquid samples (glucose, xylose, acetic acid and butyric acid) after fermentation were measured by HPLC (LC-20A, Shimadzu) equipped with a refractive index detector (RID, Shimadzu) setting at 55 °C and Aminex HPX-87H column (Bio-Rad, Hercules, CA). Running temperature was 60 °C and 0.005 M H2SO4 was applied as mobile phase with a flow rate of 0.6 mL/min. Each product was calibrated by its standard solutions with five different concentrations (e.g. 1, 2, 3, 4, 5 g/L). Similarly, sugars from the composition analysis of biomass were analyzed by the HPLC and Hi-Plex Pb column. The mobile phase was de-ionized water at 0.5 mL min−1. Temperatures of the detector and the column oven were 55 °C and 70 °C, respectively. Concentrations of the monomeric sugar standards were 0.05, 0.1, 0.5, 1, 1.5 and 2 g L−1. All the standard calibration curves for HPLC analysis obtained with R2 > 0.998 were used for quantitative calculation.
Microbial mass (DCW, g/L) was determined through measuring the absorbance of broth at 650 nm (OD650) by the UV-visible spectrophotometer. One unit of optical density (OD650) was estimated to be equal to 0.36 g/L DCW (y = 0.2835x + 0.0846, R2 = 0.9974, Figure S2). Besides cross-checked by HPLC, glucose in liquid samples after fermentation was mainly quickly determined using a biosensor (SBA-40, Institute of Biology, Shandong Academy of Sciences, Jinan) calibrated with glucose standard solution (100 mg/dL). TRS in the hydrolysates after two-step hydrolysis or fermentation was determined by the UV spectrophotometer using dinitrosalicylic acid (DNS) method43 calibrated with five different concentrations (0, 0.2, 0.4, 0.6, 0.8 and 1.0 g/L) with R2 > 0.9999.
Additional Information
How to cite this article: Jiang, D. et al. Biohydrogen Production from Hydrolysates of Selected Tropical Biomass Wastes with Clostridium Butyricum. Sci. Rep. 6, 27205; doi: 10.1038/srep27205 (2016).
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the funding support from Nanjing Agricultural University, Chinese Academy of Sciences [CAS 135 program (XTBG-T02) and equipment R&D grant (No. YZ201260)], and Yunnan Provincial Government (Baiming Haiwai Gaocengci Rencai Jihua). We also thank Miss Ya-ting Yang for the composition analysis of bagasse and Jatropha hulls.
Footnotes
The authors declare no competing financial interests.
Author Contributions D.J. (PhD student) designed the study, carried out the experimental work, and drafted the manuscript. Z.F. (supervisor) supervised the study and revised the manuscript. S.-x.C. helped to design CCD experiment and revised the manuscript. X.-f.T. revised the manuscript. T.-c.S. helped to perform the experiment on growth curve. All authors read and approved the final manuscript.
References
- Zeng K. & Zhang D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Progress in Energy and Combustion Science 36, 307–326 (2010). [Google Scholar]
- Sharma S. & Ghoshal S. K. Hydrogen the future transportation fuel: From production to applications. Renewable and Sustainable Energy Reviews 43, 1151–1158 (2015). [Google Scholar]
- Chandra R., Takeuchi H. & Hasegawa T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renewable and Sustainable Energy Reviews 16, 1462–1476 (2012). [Google Scholar]
- Liu C. M. et al. Biohydrogen production evaluation from rice straw hydrolysate by concentrated acid pre-treatment in both batch and continuous systems. International Journal of Hydrogen Energy 38, 15823–15829 (2013). [Google Scholar]
- Su T. C., Fang Z., Zhang F., Luo J. & Li X. K. Hydrolysis of Selected Tropical Plant Wastes Catalyzed by a Magnetic Carbonaceous Acid with Microwave. Scientific Reports 5, 17538 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Development and Reform Commission of China. Development planning of sugarcane in main production areas from 2015 to 2020. Document# 1101 (2015). in Chinese. (accessed on April 13, 2016: http://www.sdpc.gov.cn/zcfb/zcfbtz/201506/W020150604323728878521.pdf).
- Cardona C., Quintero J. & Paz I. Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour Technol 101, 4754–4766 (2010). [DOI] [PubMed] [Google Scholar]
- Cheng J. & Zhu M. A novel anaerobic co-culture system for bio-hydrogen production from sugarcane bagasse. Bioresour Technol 144, 623–631 (2013). [DOI] [PubMed] [Google Scholar]
- Yang C. Y., Fang Z., Li B. & Long Y.f. Review and prospects of Jatropha biodiesel industry in China. Renewable and Sustainable Energy Reviews 16, 2178–2190 (2012). [Google Scholar]
- Sharma D. K., Pandey A. K. & Lata. Use of Jatropha curcas hull biomass for bioactive compost production. Biomass and Bioenergy 33, 159–162 (2009). [Google Scholar]
- Jiang L. Q., Fang Z., Guo F. & Yang L. B. Production of 2,3-butanediol from acid hydrolysates of Jatropha hulls with Klebsiella oxytoca. Bioresource Technology 107, 405–410 (2012). [DOI] [PubMed] [Google Scholar]
- Deeba F., Kumar V., Gautam K., Saxena R. K. & Sharma D. K. Bioprocessing of Jatropha curcas seed oil and deoiled seed hulls for the production of biodiesel and biogas. Biomass and Bioenergy 40, 13–18 (2012). [Google Scholar]
- Torget R., Walter P., Himmel M. & Grohmann K. Dilute-acid pretreatment of corn residues and short-rotation woody crops. Applied Biochemistry and Biotechnology 28–29, 75–86 (1991). [Google Scholar]
- Nguyen T. A. D., Kim K. R., Kim M. S. & Sim S. J. Thermophilic hydrogen fermentation from Korean rice straw by Thermotoga neapolitana. International Journal of Hydrogen Energy 35, 13392–13398 (2010). [Google Scholar]
- Cao G. et al. Acid hydrolysis of corn stover for biohydrogen production using Thermoanaerobacterium thermosaccharolyticum W16. International Journal of Hydrogen Energy 34, 7182–7188 (2009). [Google Scholar]
- Panagiotopoulos I. A., Bakker R. R., de Vrije T., Claassen P. A. M. & Koukios E. G. Dilute-acid pretreatment of barley straw for biological hydrogen production using Caldicellulosiruptor saccharolyticus. International Journal of Hydrogen Energy 37, 11727–11734 (2012). [Google Scholar]
- Monavari S., Galbe M. & Zacchi G. The influence of solid/liquid separation techniques on the sugar yield in two-step dilute acid hydrolysis of softwood followed by enzymatic hydrolysis. Biotechnology for Biofuels 2, 1–9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattra S., Sangyoka S., Boonmee M. & Reungsang A. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. International Journal of Hydrogen Energy 33, 5256–5265 (2008). [Google Scholar]
- Fangkum A. & Reungsang A. Biohydrogen production from sugarcane bagasse hydrolysate by elephant dung: Effects of initial pH and substrate concentration. International Journal of Hydrogen Energy 36, 8687–8696 (2011). [Google Scholar]
- Chong M. L. et al. Effects of pH, glucose and iron sulfate concentration on the yield of biohydrogen by Clostridium butyricum EB6. International Journal of Hydrogen Energy 34, 8859–8865 (2009). [Google Scholar]
- He G. Q., Kong Q. & Ding L. X. Response surface methodology for optimizing the fermentation medium of Clostridium butyricum. Letters in Applied Microbiology 39, 363–368 (2004). [DOI] [PubMed] [Google Scholar]
- Reddy L. V. A., Wee Y. J., Yun J. S. & Ryu H. W. Optimization of alkaline protease production by batch culture of Bacillus sp. RKY3 through Plackett–Burman and response surface methodological approaches. Bioresource Technology 99, 2242–2249 (2008). [DOI] [PubMed] [Google Scholar]
- Ji X. J. et al. Development of an industrial medium for economical 2,3-butanediol production through co-fermentation of glucose and xylose by Klebsiella oxytoca. Bioresource Technology 100, 5214–5218 (2009). [DOI] [PubMed] [Google Scholar]
- Jiang L. et al. Optimization of thermophilic fermentative hydrogen production by the newly isolated Caloranaerobacter azorensis H53214 from deep-sea hydrothermal vent environment. International Journal of Hydrogen Energy 39, 14154–14160 (2014). [Google Scholar]
- Mishra P. & Das D. Biohydrogen production from Enterobacter cloacae IIT-BT 08 using distillery effluent. International Journal of Hydrogen Energy 39, 7496–7507 (2014). [Google Scholar]
- Sittijunda S. & Reungsang A. Media optimization for biohydrogen production from waste glycerol by anaerobic thermophilic mixed cultures. International Journal of Hydrogen Energy 37, 15473–15482 (2012). [Google Scholar]
- Chin S. X., Chia C. H., Zakaria S., Fang Z. & Ahmad S. Ball milling pretreatment and diluted acid hydrolysis of oil palm empty fruit bunch (EFB) fibres for the production of levulinic acid. Journal of the Taiwan Institute of Chemical Engineers 52, 85–92 (2015). [Google Scholar]
- Sluiter A., Ruiz R., Scarlata C., Sluiter J. & Templeton D. Technical report No. NREL/TP-510-42619, National Renewable Energy Laboratory: Golden, CO. (2008).
- Sluiter A., Hames B., Ruiz R., Scarlata C., Sluiter J., Templeton D., et al. Technical Report No. NREL/TP-510-42618, National Renewable Energy Laboratory: Golden, CO. (2011).
- Corbet A. S. The bacterial growth curve and the history of species. Nature 131, 61–62 (1933). [Google Scholar]
- Luli G. W. & Strohl W. R. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl Environ Microbiol 56, 1004–11 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. B., Pitt L. & Love M. Biohydrogen production: prospects and limitations to practical application. International Journal of Hydrogen Energy 29, 173–185 (2004). [Google Scholar]
- Liang T. M., Cheng S. S. & Wu K. L. Behavioral study on hydrogen fermentation reactor installed with silicone rubber membrane. International Journal of Hydrogen Energy 27, 1157–1165 (2002). [Google Scholar]
- Fabiano B. & Perego P. Thermodynamic study and optimization of hydrogen production by Enterobacter aerogenes. International Journal of Hydrogen Energy 27, 149–156 (2002). [Google Scholar]
- Hafez H., Nakhla G., El. Naggar M. H., Elbeshbishy E. & Baghchehsaraee B. Effect of organic loading on a novel hydrogen bioreactor. International Journal of Hydrogen Energy 35, 81–92 (2010). [Google Scholar]
- Mizuno O., Dinsdale R., Hawkes F. R., Hawkes D. L. & Noike T. Enhancement of hydrogen production from glucose by nitrogen gas sparging. Bioresource Technology 73, 59–65 (2000). [Google Scholar]
- Lin P. Y. et al. Biological hydrogen production of the genus Clostridium: metabolic study and mathematical model simulation. International Journal of Hydrogen Energy 32, 1728–1735 (2007). [Google Scholar]
- Kataoka N., Miya A. & Kiriyama K. Studies on hydrogen production by continuous culture system of hydrogen-producing anaerobic bacteria. Water Science and Technology 36, 41–47 (1997). [Google Scholar]
- Box G. E., Hunter J. S. & Hunter W. G. Statistics for Experimenters. New York: Wiley. 1978. [Google Scholar]
- Joyce A. P. & Leung S. S. Use of response surface methods and path of steepest ascent to optimize ligand-binding assay sensitivity. Journal of Immunological Methods 392, 12–23 (2013). [DOI] [PubMed] [Google Scholar]
- Rafiqul I. S. M. & Mimi Sakinah A. M. Kinetic studies on acid hydrolysis of Meranti wood sawdust for xylose production. Chemical Engineering Science 71, 431–437 (2012). [Google Scholar]
- U.S. Standard Atmosphere, 1976. U.S. Government Printing Office, Washington, D.C., 1976.
- Miller G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31, 426–428 (1959). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.