Introduction
Recently there has been a rapid evolution in the treatment of hepatitis C heralded by development of the direct-acting antiviral agents, which target specific aspects of the hepatitis C virus viral cycle. These agents have shown improved sustained virologic response rates compared with traditional therapy with combined pegylated interferon and ribavirin.1, 2 Simeprevir, a direct-acting antiviral agent, is a second-generation viral protease inhibitor that has proven highly effective and safe for the treatment of genotype I chronic hepatitis C and was approved by the US Food and Drug Administration in November 2013.2 In clinical trials, simeprevir was initially used in combination with ribavirin and pegylated interferon alfa but recently has been paired with another newly emerged targeted antiviral, sofosbuvir. Sofosbuvir is a nucleotide analog inhibitor of hepatitis C viral polymerase.1 In a recent randomized trial that compared patients on combined simeprevir/sofosbuvir with or without ribavirin, a rash was seen in 13.6% of the patients on the combined therapy without ribavirin.3 To date, there are few clear descriptions of this rash in the medical literature. We present a case of a photo-induced drug eruption in a patient on simeprevir and sofosbuvir for hepatitis C.
Case report
A 65 year-old white woman, 1 year status post–liver transplant and currently undergoing treatment for hepatitis C, was referred to the dermatology clinic by her primary care physician for evaluation of painful, clear, fluid-filled blisters on the lower lip, right cheek, and dorsal hands after not responding to 2 courses of acyclovir. Two weeks before onset of this eruption, she was started on combined simeprevir/sofosbuvir regimen for hepatitis C infection. Her other medications included tacrolimus and everolimus for transplant immunosuppression, levothyroxine, insulin, alendronate, amlodipine, omeprazole, simvastatin, and calcium with vitamin D, none of which had been started within the past 6 months. She had no history of medication allergies and denied any similar rashes in the past. The patient denied prolonged sun exposure before onset of the eruption.
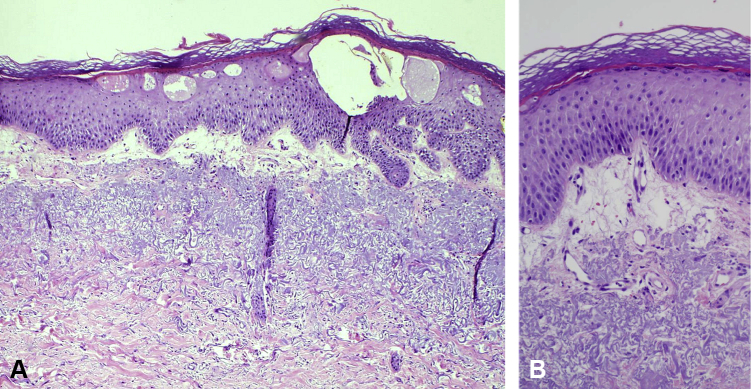
When examined in the dermatology clinic, she presented with a large erosion of the lower vermilion with honey-colored and hemorrhagic crusting (Fig 1, A). There were also ill-defined erythematous plaques with overlying tense bullae on the dorsolateral hands, right more so than left (Fig 1, B). Wound culture of the lip found normal skin flora. Herpes simplex virus polymerase chain reaction testing of a bulla on the right hand was negative. A punch biopsy of the right dorsal hand found spongiotic dermatitis with marked papillary dermal edema and a perivascular infiltrate of composed of lymphocytes, few neutrophils, and rare eosinophils (Fig 2, A and B). There was no evidence of herpetic infection on pathology. The clinical and histologic features were consistent with a drug eruption presumed secondary to treatment for her hepatitis C. At the time of diagnosis, the patient was almost halfway through her 12-week course of simeprevir/sofosbuvir and elected to complete the antiviral treatment. Therefore, she was prescribed triamcinolone 0.1% cream for her hands and desonide 0.05% cream for her lips. The rash significantly improved with topical steroids within 2 weeks and resolved on completion of the antiviral medications.
Fig 1.
Photo-distributed eruption in a patient on simeprevir. The patient presented with a large erosion of the lower vermilion with honey-colored and hemorrhagic crusting (A) and ill-defined erythematous plaques with overlying tense bullae on the dorsolateral hands (B).
Fig 2.
Histopathology shows spongiotic dermatitis with marked papillary dermal edema and a perivascular infiltrate of composed of lymphocytes, few neutrophils, and rare eosinophils. (A and B, Hematoxylin-eosin stain; original magnifications: A, ×100 and B, ×200.)
Discussion
Photo-induced drug eruptions are responsible for only 8% of reported cutaneous adverse events from medications, and are classified into 2 categories, phototoxic or photoallergic, based on their pathogenesis. Photoallergic drug eruptions in contrast to phototoxic are immune-mediated and do not occur in all individuals exposed to the medication and solar radiation. Photoallergic reactions are usually eczematous clinically and have histology demonstrating spongiosis and vesiculation of the epidermis with dermal perivascular inflammation. Phototoxic eruptions occur much more commonly and often present as exaggerated sunburn with pathology exhibiting apoptotic keratinocytes and a dermal infiltrate of neutrophils and lymphocytes. It can be difficult to differentiate between phototoxic and photoallergic eruptions, which can be aided by photopatch testing, although this often does not change the management in these cases.4
The eczematous appearance, distribution, and histology of the eruption in our patient are consistent with a photo-induced drug eruption. Our patient did not recall significant sun exposure, but the ultraviolet light received through a windshield while driving would be sufficient to cause this eruption and explain the involvement of the dorsolateral hands. A recent publication by Simpson et al5 describes 2 patients presenting with a photo-distributed eruption after starting simeprevir/sofosbuvir for hepatitis C, which showed a lichenoid pattern on histology.5 We considered these cases to be photoallergic eruptions, but the lichenoid pattern on pathology, burning sensation experienced by both patients, and the hyperpigmentation seen in one of the cases are more consistent with a phototoxic mechanism.5, 6 Pathology in our case would favor a photoallergic reaction given the lack of apoptotic keratinocytes, but further photoprovocation testing is necessary to clearly delineate this mechanism.
In phase III clinical trials comparing simeprevir plus peginterferon and ribavirin with placebo plus peginterferon and ribavirin, there were similar rates of rash between the 2 groups. However, photosensitivity was seen in the simeprevir group at rates of 1.6% to 4% compared with less than 1% in the placebo group.7 In the phase III clinical trials for sofosbuvir, rates of rash were also similar in the treatment groups compared with placebo with no reports of photosensitivity.8, 9, 10 A recent trial of combined simeprevir/sofosbuvir with or without ribavirin demonstrated rash in 13.6% of the group without ribavirin and overall rate of photosensitivity of 5%.3 Simpson et al5 speculate that simeprevir is the causative agent of the eruptions in their report given the higher incidence of photosensitivity with simeprevir compared with placebo clinical trials, which has not been described with sofosbuvir.7 We agree that simeprevir is the likely causative agent in our patient given the photo-distributed nature.5
A recent trial found that combined simeprevir/sofosbuvir was more effective in achieving a sustained virologic response and was better tolerated than combined ribavirin, peginterferon, and sofosbuvir.1 The introduction of these 2 drugs has revolutionized the treatment of hepatitis C and are now the preferred therapy.7 Because of their success and predicted future use, a greater understanding of their dermatologic adverse events is necessary.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Pearlman B.L., Ehleben C., Perrys M. The Combination of Simeprevir and Sofosbuvir Is More Effective Than That of Peginterferon, Ribavirin, and Sofosbuvir for Patients With Hepatitis C-Related Child's Class A Cirrhosis. Gastroenterology. 2015;148:762–770.e2. doi: 10.1053/j.gastro.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Izquierdo L., Helle F., Francois C., Castelain S., Duverlie G., Brochot E. Simeprevir for the treatment of hepatitis C virus infection. Pharmacogenomics Pers Med. 2014;7:241–249. doi: 10.2147/PGPM.S52715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawitz E., Sulkowski M.S., Ghalib R. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 4.Drucker A.M., Rosen C.F. Drug-induced photosensitivity: culprit drugs, management and prevention. Drug Saf. 2011;34:821–837. doi: 10.2165/11592780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Simpson C.L., McCausland D., Chu E.Y. Photo-distributed lichenoid eruption secondary to direct anti-viral therapy for hepatitis C. J Cutan Pathol. 2015;42:769–773. doi: 10.1111/cup.12532. [DOI] [PubMed] [Google Scholar]
- 6.Epstein J.H. Phototoxicity and photoallergy. Semin Cutan Med Surg. 1999;18:274–284. doi: 10.1016/s1085-5629(99)80026-1. [DOI] [PubMed] [Google Scholar]
- 7.Childs-Kean L.M., Hand E.O. Simeprevir and sofosbuvir for treatment of chronic hepatitis C infection. Clin Ther. 2015;37:243–267. doi: 10.1016/j.clinthera.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Lawitz E., Mangia A., Wyles D. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson I.M., Gordon S.C., Kowdley K.V. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 10.Zeuzem S., Dusheiko G.M., Salupere R. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]