Introduction
Phaeohyphomycosis is a heterogeneous group of opportunistic infections that are becoming increasingly more prevalent as pathogens in immunocompromised individuals. We present a rare case of cutaneous phaeohyphomycosis caused by Biatriospora mackinnonii in a renal transplant patient. This case also highlights the importance of molecular techniques in the diagnosis of this rare disease.
Case report
A 65-year-old Mexican-American woman was evaluated in the dermatology department for a right forearm cutaneous lesion. Her medical history was significant for diabetes and hypertension leading to end-stage renal disease. The patient underwent cadaveric renal transplant 5 months earlier and was currently taking tacrolimus and mycophenolic acid. Approximately 3 months before her presentation, she noted the rapid onset of a painless papule on her right distal forearm that subsequently progressed insidiously into a large nodule. It was subsequently drained as a presumed hematoma, and she completed a short course of cephalexin. She then traveled to Colima, Mexico. During the trip she denied outdoor exposures but did admit to attempting to drain the lesion at home using insulin needles. Upon returning to the United States, the lesion was drained again as a presumed hematoma, and she completed a course of clindamycin without improvement. A radiograph of her forearm and wrist at that time showed soft tissue swelling without foreign body or underlying abnormality. Physical examination found a 5- × 5-cm symmetric dome-shaped exophytic nodule located on her right forearm with a diffuse overlying hemorrhagic eschar that was easily removed revealing underlying pink friable tissue (Fig 1). A rim of erythema surrounded this lesion. No lymphadenopathy was appreciated.
Fig 1.
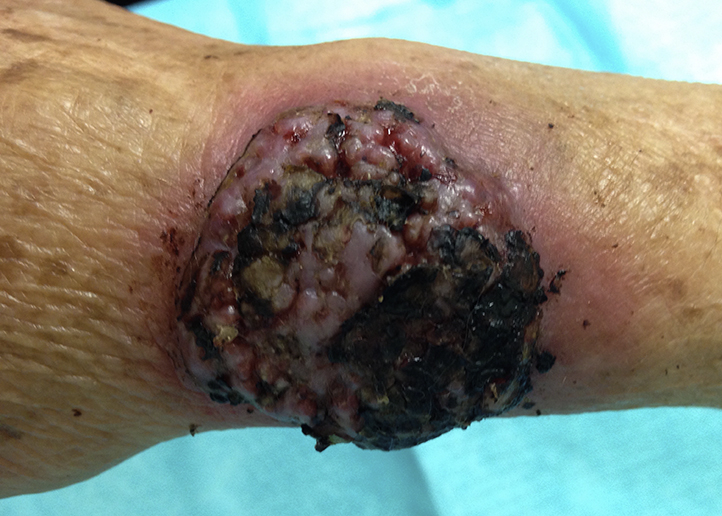
Cutaneous phaeohyphomycosis caused by B mackinnonii. Exophytic nodule with overlying eschar on dorsal forearm/wrist.
Punch biopsy skin specimens were obtained for both tissue culture and histology. Hematoxylin and eosin–stained slides showed dermal involvement by fungal hyphal elements, marked irregular pseudoepitheliomatous hyperplasia of the epidermis, and moderate-to-severe acute and chronic dermal inflammation with microabscesses (Fig 2). There was an absence of granules, and no sclerotic bodies were noted. Periodic acid-Schiff and Gomori methenamine silver stains found the fungal elements to be predominantly in the mid to reticular dermis, and Fontana-Masson staining confirmed this finding (Fig 3).
Fig 2.
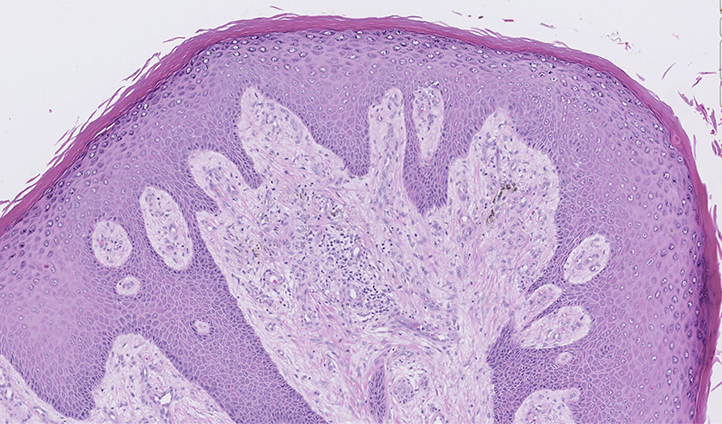
Hematoxylin-eosin staining shows exuberant pseudoepitheliomatous hyperplasia of the epidermis with dense acute and chronic dermal inflammation.
Fig 3.
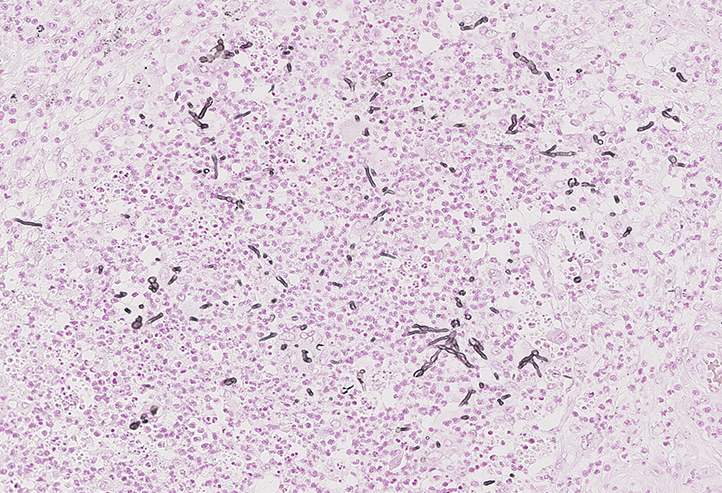
Fontana-Masson staining shows septate hyphae within the dermis.
Tissue culture recovered a velvety dark grey mold with a dark reverse after 10 days of incubation (Fig 4). Microscopic examination found septate hyphae but no other identifying structures (ie, conidia). DNA sequencing of the internal transcribed spacer and D1/D2 regions of the ribosome was performed and identified the organism as B mackinnonii (formerly Pyrenochaeta mackinnonii). Cultures for acid-fast bacilli were negative, but bacterial culture grew many pan-susceptible Klebsiella pneumoniae. Histoplasma antigen and blastomyces antibody were not detected, and a complete blood count with differential was within normal limits. Chest radiograph was nonrevealing. Magnetic resonance imaging of the right forearm showed a large T1/T2 hyperintense mass that appeared confined to the skin. The patient was started on itraconazole with a known interaction with tacrolimus requiring close monitoring and a decrease in tacrolimus dosing. After 2 weeks of pretreatment with itraconazole, 200 mg twice daily, the lesion was excised, and a split-thickness skin graft was used to close the surgical site. She was continued on itraconazole to complete a 6-month course. After 8 months of close postoperative follow-up, there is no evidence of recurrence.
Fig 4.
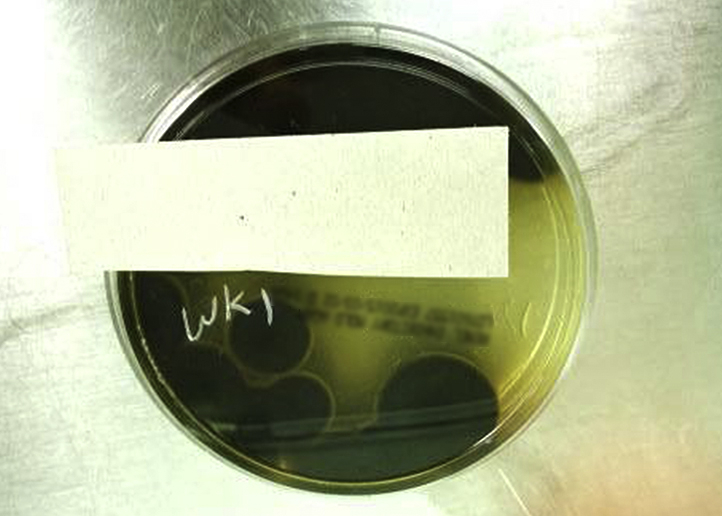
Growth of B mackinnonii on Remel Inhibitory Mold Agar incubated at 28°C for 10 days.
Discussion
The term phaeohyphomycosis was first introduced by Ajello et al1 in 1974 to describe infections caused by dark-walled, also known as dematiaceous or melanized, fungi that are clinically, pathologically, and mycologically distinct from classic chromoblastomycosis. In 1983, phaeohyphomycosis was further subclassified by McGinnis2 into 4 clinical presentations: superficial, cutaneous and corneal, subcutaneous, and systemic. Recent advances in molecular techniques led to the ability to rapidly and accurately speciate nonsporulating melanized molds. This finding is very important, as different species may have tropism for different organs and varying susceptibilities to antifungals.3 Gene sequencing of the internal transcribed spacer region remains one of the most widely accepted molecular methods because of its ability to discriminate between inter- and intraspecies variations, a high polymerase chain reaction amplification success rate, and enriched datasets deposited in many gene banks.3 Using gene sequencing we identified B mackinnonii as the causative species in this case. In a recent phylogenetic study, Pyrenochaeta mackinnonii was accommodated into the new genus Nigrograna as Nigrograna mackinnonii.4 Subsequently, the classification was revised again and the genus Nigrograna was reduced to the synonym of Biatriospora—thus the most current nomenclature for this species is B mackinnonii. To our knowledge, this species has only been implicated in one case of phaeohyphomycosis to date.3 There have been two cases in the literature describing this species as the causative agent of eumycotic mycetoma.4 Until recently, many reported cases of phaeohyphomycosis did not include the causative species or did so based on descriptions of the macroscopic and microscopic morphology. Thus, the true prevalence of this and other species implicated in phaeohyphomycosis may be understated because of lack of and unreliability of nonmolecular strain identification methods. The histologic criterion for diagnosis of phaeohyphomycosis is based solely on the finding of dematiaceous mycelia.5, 6
There are no standardized treatment protocols for the treatment of phaeohyphomycosis but voriconazole, posaconazole, and itraconazole show the most consistent in vitro activity against this group of fungi.7 Oral itraconazole is considered the empiric drug of choice given the most clinical experience with this drug in the literature.8 For cutaneous nodules in particular, surgery alone has been effective in many cases, but oral antifungals are often used as co-adjunctive therapies especially in immunosuppressed patients to prevent dissemination.7 Biatriospora is a genus that is widely distributed in soil, on wood, and other plant debris and as plant pathogens.9 Phaeohyphomycosis is often preceded by a history of trauma, but the role of injury in this case is unclear. Although cutaneous phaeohyphomycosis is a rare entity, there should be a high index of suspicion in renal transplant recipients presenting with pigmented skin lesions to allow for timely diagnosis and management.
Acknowledgments
The authors thank Narayana Meduri for photography assistance in the microbiology laboratory.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Ajello L., Georg L.K., Steigbigel R.T., Wang C.J. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia. 1974;66(3):490–498. [PubMed] [Google Scholar]
- 2.McGinnis M.R. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8(1):1–16. doi: 10.1016/s0190-9622(83)70001-0. [DOI] [PubMed] [Google Scholar]
- 3.Santos D.W., Padovan A.C., Melo A.S. Molecular identification of melanised non-sporulating moulds: a useful tool for studying the epidemiology of phaeohyphomycosis. Mycopathologia. 2013;175(5-6):445–454. doi: 10.1007/s11046-012-9608-x. [DOI] [PubMed] [Google Scholar]
- 4.de Gruyter J., Woudenberg J.H., Aveskamp M.M. Redisposition of phoma-like anamorphs in Pleosporales. Stud Mycol. 2013;75(1):1–36. doi: 10.3114/sim0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajello L. The gamut of human infections caused by dematiaceous fungi. Med Mycol. 1981;22(1):5. [Google Scholar]
- 6.Pec J., Palencarova E., Plank L. Phaeohyphomycosis due to Alternaria spp. and Phaeosclera dematioides: a histopathological study. Mycoses. 1996;39(5-6):217–221. doi: 10.1111/j.1439-0507.1996.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhary A., Meis J.F., Guarro J. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin Microbiol Infect. 2014;20(Suppl 3):47–75. doi: 10.1111/1469-0691.12515. [DOI] [PubMed] [Google Scholar]
- 8.Desnos-Ollivier M., Bretagne S., Dromer F., Lortholary O., Dannaoui E. Molecular identification of black-grain mycetoma agents. J Clin Microbiol. 2006;44(10):3517–3523. doi: 10.1128/JCM.00862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badali H., Chander J., Gulati N. Subcutaneous phaeohyphomycotic cyst caused by Pyrenochaeta romeroi. Med Mycol. 2010;48(5):763–768. doi: 10.3109/13693780903440383. [DOI] [PubMed] [Google Scholar]


