Peripheral T-cell lymphomas (PTCL), representing 5-10% of all non-Hodgkin lymphomas, are a heterogeneous group of disorders associated with an overall poor prognosis (1). Autologous stem cell transplantation (SCT) shows some efficacy in relapsed or refractory PTCL, with 3- to 5-year survival rates ranging from 33% to 69% in various retrospective studies (2). Several prospective studies have also evaluated up-front autologous SCT in first remission for PTCL, and some experts endorse this approach as the current standard of care (3). Given the relatively rare and heterogeneous nature of these malignancies, and the lack of large prospective trials, better means of assessing response to chemotherapy and identifying candidates for transplantation are needed.
Positron emission tomography using [18F]fluorodeoxyglucose (FDG-PET) is a useful modality in both the initial staging of PTCL and in re-staging after relapse or treatment (4). In other aggressive lymphomas such as diffuse large B-cell lymphoma and Hodgkin lymphoma, PET performed after salvage chemotherapy has been shown to have important prognostic implications for risk of relapse after subsequent SCT (5,6,7). Although obtaining a pre-transplantation FDG-PET is routine clinical practice, the ability of FDG-PET to predict long-term post-transplantation outcomes in PTCL has not been established.
In this retrospective study of 48 patients with PTCL, we compared the 3-year PFS and OS of patients with positive and negative pre-transplantation FDG-PET studies. Patients were included if they carried a histologically confirmed diagnosis of PTCL, underwent autologous or allogeneic stem cell transplantation at our institution between August 1, 2003 and August 1, 2012, were at least 18 years of age at the time of transplantation, and underwent an FDG-PET study after the initiation of chemotherapy and no more than 110 days prior to transplantation. A variety of PET systems and image acquisition protocols were used during the study period. Accredited nuclear medicine physicians interpreted FDG-PET studies at the time of acquisition. These individuals were not blinded to patients’ clinical information or to prior FDG-PET results. For retrospective analysis, both digital images and initial image reports were reviewed when available (for 36/48 and 48/48 patients, respectively). FDG-PET studies were interpreted as positive or negative according to the International Harmonization Project in Lymphoma guidelines (8). Data contributing to the International Prognostic Index (IPI) (9) and Prognostic Index for PTCL-U (PIT) (10) at the time of relapse pre-transplantation (or at the time of presentation for those transplanted in first remission) were determined when possible from the medical record (for 43/48 patients). Variables for the PET-positive and -negative groups were compared using the chi-square statistic or Fisher's Exact test for categorical and the Kruskal-Wallis test for continuous variables. Primary endpoints were OS and PFS. Overall survival was defined as time from date of transplantation to death due to any cause or censored at the last follow-up. Progression-free survival was defined as from date of transplantation to relapse or death from any cause. Univariate and multivariate analysis through Cox proportional-hazards models were used to evaluate selected variables for OS and PFS. SAS version 9.3 (Cary, NC) was used to perform all statistical analysis.
Baseline demographic data and prognostic variables are displayed in Table I. 52% of patients presented with a new diagnosis of PTCL; the remainder received pretransplantation chemotherapy for relapsed disease. The median age at transplantation was 56 (range 25-73). 77% of patients presented with advanced (stage III-IV) disease prior to pre-transplantation chemotherapy, and 46% had documented bone marrow involvement. A variety of chemotherapy regimens were employed prior to transplantation, the most common being CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone)-like regimens (n = 9), platinum-based regimens (n = 19), and a frontline regimen incorporating CHOP, methotrexate, and GVD (gemcitabine, vinorelbine, doxorubicin) (n = 11). Thirty-seven of the patients underwent autologous transplantation and 11 underwent allogeneic transplantation. For all but one patient, type of transplant was determined prior to the pre-transplant FDG-PET.
Table I.
Patient characteristics.
| All Patients (n = 48) | PET-Negative (n = 31) | PET-Positive (n = 17) | P Value | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | Value (Range) | n (%) | Value (Range) | n (%) | Value (Range) | n (%) | |
| Gender | 0.56 | ||||||
| Male | 28 (58) | 17 (55) | 11 (65) | ||||
| Female | 20 (42) | 14 (45) | 6 (35) | ||||
| Age | 56 (25-73) | 53 (29-67) | 58 (25-73) | 0.75 | |||
| Diagnosis | 0.78 | ||||||
| ALCL, ALK + | 4 (8) | 3 (10) | 1 (6) | ||||
| ALCL, ALK − | 7 (15) | 4 (13) | 3 (18) | ||||
| AILT | 19 (40) | 11 (35) | 8 (47) | ||||
| PTCL-NOS | 18 (38) | 13 (42) | 5 (29) | ||||
| Transplanted in first remission | 25 (52) | 16 (52) | 9 (53) | 1.0 | |||
| Prognostic Variables | |||||||
| Age > 60 | 15 (31) | 11 (35) | 6 (35) | 1.0 | |||
| Stage III-IV | 37 (77) | 23 (74) | 14 (82) | 0.72 | |||
| LDH > 250 U/L | 23 (53) | 18 (67) | 5 (31) | 0.03 | |||
| Performance Status 2-4 | 9 (19) | 6 (19) | 3 (18) | 1.0 | |||
| ENS > 1 | 23 (48) | 14 (45) | 9 (53) | 0.76 | |||
| BM involvement | 22 (46) | 16 (52) | 6 (35) | 0.37 | |||
| IPI Score | 0.20 | ||||||
| 0-1 | 12 (28) | 7 (26) | 5 (31) | ||||
| 2 | 13 (30) | 6 (22) | 7 (44) | ||||
| 3-5 | 18 (42) | 14 (52) | 4 (25) | ||||
| PIT Score | 0.10 | ||||||
| 0 | 12 (28) | 5 (19) | 7 (44) | ||||
| 1 | 9 (21) | 5 (19) | 4 (25) | ||||
| 2-4 | 22 (51) | 17 (63) | 5 (31) | ||||
| Transplant Type | 0.04 | ||||||
| Autologous | 37 (77) | 27 (87) | 10 (59) | ||||
| Allogeneic | 11 (23) | 4 (13) | 7 (41) | ||||
| Interval from last chemotherapy to PET (days) | 20 (3-101) | 21 (3-100)* | 18.5 (9–37)** | 0.56 | |||
| Interval from PET to SCT (days) | 46 (8-101) | 49 (8-89) | 37 (12-101) | 0.39 | |||
Known for 24/31 patients.
Known for 10/17 patients.
PET indicates positron emission tomography; ACL, ALK+, anaplastic large-cell lymphoma, anaplastic lymphoma kinase-positive; AILT, angioimmunoblastic T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; LDH, lactate dehydrogenase; ENS, extranodal sites; BM, bone marrow; IPI, International Prognostic Index; PIT, Prognostic Index for Peripheral T-Cell Lymphoma; and SCT, stem cell transplantation.
Thirty-one of the 48 patients (65%) achieved a negative FDG-PET prior to transplantation, including 64% of patients transplanted in first remission and 65% of patients treated for relapse. Patients with positive and negative FDG-PET were well-matched with respect to prognostic variables at baseline; IPI and PIT score did not differ between the groups. Patients with a positive FDG-PET were significantly more likely to undergo allogeneic transplantations than those with a negative FDG-PET, however (p = 0.04) (Table I). FDG-PET was performed at the completion of pre-transplantation chemotherapy for 20 of the 48 patients (42%); for the remaining patients, at least one additional cycle of chemotherapy was delivered after FDG-PET and prior to transplantation. Rates of PET-negativity for patients who underwent FDG-PET midway through chemotherapy and at the completion of chemotherapy were 57% and 75%, respectively.
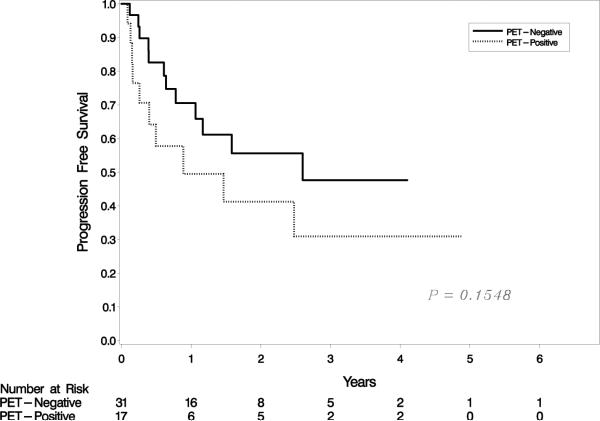
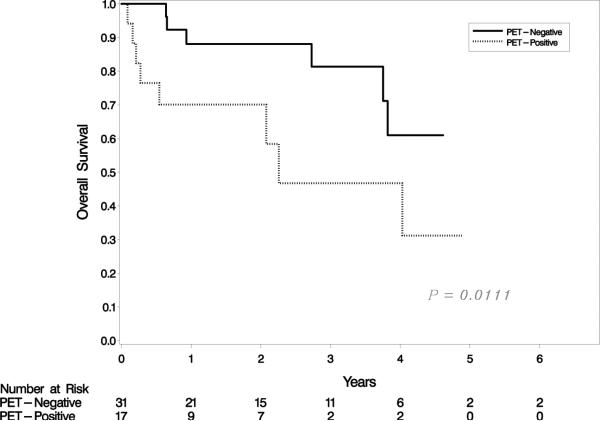
At median follow-up of 1.6 years (range, 0.1-6.8 years), 3-year PFS and OS for the entire cohort of patients were 41% (95% CI 24%- 58%) and 65% (95% CI 45%-79%), respectively (Figure 1a). A negative pre-transplantation FDG-PET was not associated with improved 3-year PFS. PFS for PET-negative and PET-positive patients was 48% (95% CI 24%-68%) and 31% (95% CI 9% – 56%), respectively (p = 0.15) (Figure 1b). In contrast, a significant association was observed between OS and pre-transplantation FDG-PET result. For PET-negative patients, 3-year OS was 81%p (95% CI 56%-93%), as compared to 31% (95% CI 6%-62%) for PET-positive patients (p = 0.01) (Figure 1c). When patients who underwent autologous and allogeneic transplantations were analyzed separately, neither PFS nor OS was associated with pre-transplantation FDG-PET result in either group (data not shown). Similarly, neither PIT nor IPI score was predictive of PFS or OS in our cohort (data not shown).
Figure 1.
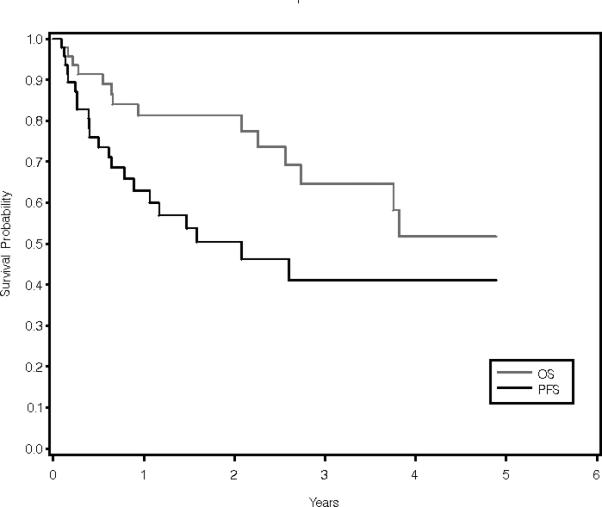
PFS and OS for the entire cohort (a); PFS by FDG-PET result (b); and OS by FDG-PET result (c).
In this cohort of PTCL patients, a negative FDG-PET prior to transplantation was not associated with improved 3-year PFS. Our findings are in contrast to classical Hodgkin lymphoma (7,11,12) and B-cell non-Hodgkin lymphoma (5,6) where a negative pre-transplantation FDG-PET has been consistently prognostic of PFS and/or OS. It is important to note, however, that as a retrospective study, this analysis was limited by the heterogeneity of the pre-transplantation chemotherapy received, and by the inclusion of both autologous and allogeneic transplantations with several different conditioning regimens. A variety of FDG-PET systems and acquisition protocols were employed as well, and the timing of FDG-PET was not standardized, although these variations represent actual clinical practice. Our cohort included patients with several histological subtypes of PTCL, each of which may be characterized by a different natural history (1) and response to therapy (13). Despite these limitations, our data represent the first analysis of the prognostic value of pre-transplantation FDG-PET in PTCL to our knowledge. The lack of demonstrable prognostic utility we observed questions the routine use of pre-transplantation FDG-PET in this patient population, and highlights the need for larger, prospective studies to address this issue.
Though PET-positive and PET-negative patients were well-matched overall with respect to demographic and prognostic factors, patients with a positive FDG-PET were significantly more likely to receive allogeneic transplantation than those with a negative FDG-PET (Table I). For 98% of patients, FDG-PET result did not influence the decision to pursue allogeneic vs. autologous transplantation because the transplantation decision was made before the FDG-PET was performed. Instead, the higher frequency of positive FDGPET prior to allogeneic transplantation may be a reflection of more aggressive disease in patients directed toward allogeneic transplantation. If PET-positive patients were affected by more aggressive disease, however, this was not indicated by the IPI and PIT prognostic models, which showed equivalent risk profiles in the PET-positive and PET-negative groups (Table I).
The 3-year PFS (41%) and OS (65%) of the patients in this cohort are similar to values reported in previous studies of SCT as either upfront or salvage therapy in PTCL (2). Though a statistically significant association between a negative FDG-PET and improved 3-year OS was observed, this association was not seen for PFS. This discrepancy might be in part explained by the fact that six of the patients who achieved a negative FDG-PET prior to transplantation went on to receive a second or third transplantation, three of whom had not relapsed at the time of last follow-up. Thus, these individuals showed long-term post-transplantation OS, but not PFS.
This preliminary study suggests that in patients with PTCL, the risk of relapse remains high even after a negative pre-transplantation FDG-PET. To better define the role of this imaging modality, pre-transplantation FDG-PET should be incorporated into larger, prospective studies of SCT in PTCL, a possibility that is perhaps more feasible in light of the increasing role of up-front autologous SCT in treating these aggressive malignancies (2,3). Similarly, neither the IPI nor the PIT prognostic model was able to accurately risk-stratify patients in this cohort. Improved prognostic models are clearly needed to identify those patients most at risk of relapse, who might benefit from post-transplantation maintenance chemotherapy and/or molecularly targeted agents.
References
- 1.Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International Peripheral T-Cell and Natural Killer/T-Cell Lymphoma Study: Pathology Findings and Clinical Outcomes. J Clin Oncol. 2008;26(25):4124–30. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Housing C, Champlin RE. Stem-cell transplantation in T-cell non-Hodgkin's lymphomas. Annals of Oncology. 2011;22(7):1471–7. doi: 10.1093/annonc/mdr140. [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz AJ, Lunning M, Horwitz S. Should patients with aggressive peripheral T-cell lymphoma all be treated the same?: No... well yes, ... but maybe not for long. Cancer Journal. 2012;18(5):445–449. doi: 10.1097/PPO.0b013e31826aeeb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feeney J, Horwitz S, Gönen M, Schöder H. Characterization of T-cell lymphomas by FDG PET/CT. AJR Am J Roentgenol. 2010;195(2):333–40. doi: 10.2214/AJR.09.3665. [DOI] [PubMed] [Google Scholar]
- 5.Johnston P, Wiseman G, Micallef I. Positron emission tomography using F-18 fluorodeoxyglucose pre- and post-autologous stem cell transplant in non-Hodgkin's lymphoma. Bone Marrow Transplantation. 2008;41:919–925. doi: 10.1038/bmt.2008.82. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson M, Hoyt R, Roberts AW, et al. Improved survival for relapsed diffuse large B cell lymphoma is predicted by a negative pre-transplant FDG-PET scan following salvage chemotherapy. Br J Haematol. 2010;150(1):39–45. doi: 10.1111/j.1365-2141.2010.08162.x. [DOI] [PubMed] [Google Scholar]
- 7.Smeltzer JP, Cashen AF, Zhang Q, et al. Prognostic significance of FDG-PET in relapsed or refractory classical Hodgkin lymphoma treated with standard salvage chemotherapy and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1646–52. doi: 10.1016/j.bbmt.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of Positron Emission Tomography for Response Assessment of Lymphoma: Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25(5):571–8. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 9.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A Predictive Model for Aggressive Non-Hodgkin's Lymphoma. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 10.Gallamini A, Stelitano C, Calvi R, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103(7):2474–9. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 11.Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pre-transplant functional imaging predicts outcome following autologous stem cell transplant for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116:4934–4937. doi: 10.1182/blood-2010-05-282756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castagna L, Bramanti S, Balzarotti M, et al. Predictive value of early 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) during salvage chemotherapy in relapsing/refractory Hodgkin lymphoma (HL) treated with high-dose chemotherapy. Br J Haematol. 2009;145:369–372. doi: 10.1111/j.1365-2141.2009.07645.x. [DOI] [PubMed] [Google Scholar]
- 13.Escalón MP, Liu NS, Yang Y, et al. Prognostic factors and treatment of patients with Tcell non-Hodgkin lymphoma: the M. D. Anderson Cancer Center experience. Cancer. 2005;103(10):2091–8. doi: 10.1002/cncr.20999. [DOI] [PubMed] [Google Scholar]