Abstract
Brain derived neurotropic factor (BDNF) is emerging as an important player in airway inflammation, remodeling, and hyperreactivity. Separately, there is increasing evidence that sex hormones contribute to pathophysiology in the lung. BDNF and sex steroid signaling are thought to be intricately linked in the brain. There is currently little information on BDNF and sex steroid interactions in the airway, but is relevant to understanding growth factor signaling in the context of asthma in men vs. women. In this study, we assessed the effect of sex steroids on BDNF expression and secretion in human airway smooth muscle (ASM). Human ASM was treated with estrogen (E2) or testosterone (T, 10nM each) and intracellular BDNF and secreted BDNF measured. E2 and T significantly reduced secretion of BDNF; effects prevented by estrogen and androgen receptor inhibitor, ICI 182,780 (1uM) and flutamide (10uM), respectively. Interestingly, no significant changes were observed in intracellular BDNF mRNA or protein expression. High affinity BDNF receptor, TrkB, was not altered by E2 or T. E2 (but not T) significantly increased intracellular cyclic AMP levels. Notably, Epac1 and Epac2 expression were significantly reduced by E2 and T. Furthermore, SNARE complex protein SNAP25 was decreased. Overall, these novel data suggest that physiologically relevant concentrations of E2 or T inhibit BDNF secretion in human ASM, suggesting a potential interaction of sex steroids with BDNF in the airway that is different from brain. The relevance of sex steroid-BDNF interactions may lie in their overall contribution to airway diseases such as asthma.
Keywords: Neurotrophin, Estrogen, Testosterone, Epac, SNAP25
Introduction
Exaggerated airway narrowing in diseases such as asthma and chronic bronchitis (Chapman and Irvin, 2015; Jha et al., 2015) result from airway hyperreactivity as well “remodeling” that involved increased hypertrophy and hyperplasia of airway smooth muscle (ASM) mass(Elliot et al., 2015; Fayon et al., 2015; Lin et al., 2015; Prakash, 2013). In addition to pro-inflammatory mediators such as cytokines, structural and functional changes to the airway involve the effects of growth factors (Aravamudan et al., 2012b; Doherty and Broide, 2007; Prakash, 2013; Thompson et al., 2015). Accordingly, understanding mechanisms that regulate growth factor production in the airway is a relevant but underexplored area.
Nerve growth factor, brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3) and neurotrophin 4 (NT4) are all members of the neurotrophin family of growth factors (Prakash and Martin, 2014). There is now increased interest in the role of neurotrophins in non-neuronal tissues including the lung. In asthma, allergy, and even lung cancer there is an observed alteration in BDNF and receptor (Hoyle, 2003; Prakash et al., 2010; Watanabe et al., 2015). A role for BDNF in enhancing airway inflammation effects in ASM has been reported (Aravamudan et al., 2012a; Braun et al., 2004; Sathish et al., 2013a; Scuri et al., 2010; Thompson et al., 2015; Vohra et al., 2013). In the lung, BDNF can be produced by multiple cell types including epithelium, sensory neurons, immune cells and most notably by ASM (Meuchel et al., 2011; Prakash et al., 2010; Prakash and Martin, 2014; Scuri et al., 2010; Thompson et al., 2015). Specifically, we and others have also shown that BDNF is actively released by ASM and can have autocrine effects of increasing ASM proliferation and [Ca2+]i mechanisms (Abcejo et al., 2012; Aravamudan et al., 2012a; Prakash et al., 2010; Scuri et al., 2010; Vohra et al., 2013). What is less understood are the mechanisms regulating BDNF secretion.
Consistent with the greater understanding of BDNF regulation in the neuronal system, many studies have explored on the protective effects of estrogen in increasing BDNF production and thereby potentiating neuronal growth in the central nervous system (Aguirre and Baudry, 2009; Chakrabarti et al., 2014; Sato et al., 2007; Spence and Voskuhl, 2012). Testosterone has been shown to have a complementary role in regulating BDNF in the CNS to enhance neuronal health (Li et al., 2012; Spence and Voskuhl, 2012; Yang et al., 2004). In neurons, the secretory mechanisms for BDNF have also been explored and are thought to involve formation of synaptic vesicles, SNARE complexes, and cAMP/Ca2+-dependent release (Angleson et al., 1999; Greenberg et al., 2009; Schoch et al., 2001; Shimojo et al., 2015). However, the mechanisms underlying BDNF secretion induced by steroid hormones in non-neuronal cells are not clear.
The relevance of sex steroids, or their regulation of BDNF in the lung lies in the increasing acknowledgment that sex differences in asthma exist, and that sex steroids (particularly estrogen) can influence airway structure and function (Melgert et al., 2007; Townsend et al., 2012a). However, it remains unclear whether sex steroids are detrimental or beneficial in the airway. For example, we have demonstrated an acute bronchodilatory role for estrogen via effects on epithelial cells as well as ASM (Sathish et al., 2015a; Townsend et al., 2011; Townsend et al., 2013). However, progesterone has been shown to increase airway hyperreactivity in sensitized male mice (Hellings et al., 2003) whereas testosterone exhibits bronchodilator effects similar to estrogen (Bordallo et al., 2008; Kouloumenta et al., 2006). Yet, clinical data show increased asthma in women (which may also reflect complex effects on the immune system) (Melgert et al., 2007; Townsend et al., 2012a). Regardless of whether sex steroids are protective or detrimental, the mechanisms by which they produce effects in the airway are still being explored.
Given that BDNF enhances ASM [Ca2+]i and other aspects of asthma pathophysiology, and that some sex steroids (particularly estrogens) are bronchodilatory, we hypothesized that sex steroids decrease BDNF in the airway (i.e. different from what occurs in the CNS). Negative regulation of BDNF via sex steroids could provide a novel, potentially targetable pathway for blunting the effect of growth factors in airway diseases.
Materials and methods
Human ASM Cells and Tissue
The techniques for isolating human ASM cells and tissue have been previously described (Abcejo et al., 2012; Sathish et al., 2015a; Vohra et al., 2013). Briefly, 3rd–6th generation human bronchi were obtained from lung specimens incidental to patient thoracic surgery at Mayo Clinic for focal, non-infectious causes (typically lobectomies for focal cancers). Normal lung areas were identified with the help of the pathologist (approved by Mayo Clinic Institutional Review Board). Samples were immersed in ice cold Hanks’ Balanced Salt Solution (HBSS, Invitrogen, Carlsbad, CA) and epithelium-denuded ASM tissue or enzymatically-dissociated cells from such tissues were used for experiments. For cells, cultures (<3rd passage) were maintained under standard conditions of 37°C (5% CO2, 95% air), while tissues were used fresh. Prior to experimentation, cells were serum starved for 48h. The presence of smooth muscle markers was used to ensure lack of phenotypic change.
Sex Steroid Treatements
ASM cells or tissue were treated with estrogen or testosterone (E2, 10nM final concentration, Sigma-Aldrich, St. Louis, MO, USA) or testosterone (T, 10nM final concentration, Sigma) in serum deficient media for 48h. Conditioned media from cell lysates and tissue homogenates were collected for BDNF expression and secretion. To determine the mechanisms by which estrogen and testosterone may affect BDNF, ASM cells were first incubated for 30 min to plasma membrane receptor inhibitors for estrogen (ICI 182,780 (ICI); 1μM, Tocris, New Orleans, LA) or testosterone (flutamide (FLU); 10μM, Tocris), prior to E2 or T administration.
In pilot studies, we explored a range of progesterone concentrations on BDNF expression and secretion and found no effect. Accordingly, progesterone-based experiments were not pursued further.
RNA isolation and q-PCR
Human ASM cells were harvested after 24h of E2 or T treatment and total RNA extracted using RNeasy micro kit (Qiagen, Valencia, CA). Complementary DNA (cDNA) was synthesized using Transcription kit (Roche, Indianapolis, IN), and was amplified using an LC480 LightCycler (ABI; Carlsbad, CA). Standard qPCR techniques (optimized for the Roche LC480 Light Cycler) were used. BDNF forward primer is 5′CATTCACGCTCTCCAG3′, reverse primer is 5′AACCACGATGTGACTCC3′. GAPDH forward primer is 5′AAGGTGAAGGTCGGAGTCAACGGATT3′, reverse primer is 5′CCATGGAATTTGCCATGGGAGGAATC3′. Data are presented as fold change in comparison to an endogenous control (GAPDH) and relative to untreated control.
BDNF and cyclic AMP (cAMP) ELISA
Conditioned medium from ASM cells and tissue treated with E2 or T were concentrated by centrifugal filtration using an Amicon Ultra-15 (3K) filter (Millipore, Billerica, USA) at 3,724 g for 1h. Concentrated supernatants and cell lysates were measured for BDNF and cAMP, respectively by Quantikine ELISA kits (R&D, Minneapolis, MN) according to the manufacturer’s instructions and using a FlexStation 3 microplate reader (Molecular Device, Sunnyvale, CA) set to 450 nm (wavelength correction set to 540 nm). Absorbance readings from test samples were compared to the manufacturer-provided standards. cAMP and BDNF concentrations were normalized to protein concentration of lysate or homogenate, and adjusted for centrifugation concentration.
Western blot analysis
Standard western blot techniques were used (Criterion Gel System, Bio-Rad, Hercules, CA, 4–15% or 15% gradient gels). Equal volume of conditioned medium or cell lysate as appropriate was tested and normalized to homogenate protein concentration (for medium) or GAPDH (for lysate). Primary antibodies used include: rabbit polyclonal antibody for BDNF (sc20981; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal antibody for TrkB (ab33655; Abcam, Cambridge, MA, USA), rabbit monoclonal antibody for Epac1 (ab109415; Abcam), rabbit polyclonal antibody for Epac2 (ab124189; Abcam), rabbit polyclonal antibody for SNAP25 (ab5666; Abcam), rabbit monoclonal antibody for SNAP47 (ab172609; Abcam), rabbit monoclonal antibody for GAPDH (14C10;Cell Signaling Technology, MA, USA), and monoclonal antibody for B-actin (ab5316; Abcam). Fluorescent secondary antibodies were used from Li-Cor Biosciences (Lincoln, Nebraska, USA). Concentration of primary and secondary antibody was used per manufacture’s recommendation. Protein expression was determined as relative fluorescence compared to housekeeping protein.
Statistical analysis
Differences between groups were analyzed using Student’s t-test or one way ANOVA with Dunnet’s post hoc analysis. Densitometric data are presented as bar graphs depicting Mean ± SEM, using SigmaPlot software version 12.3 (Systat Software Inc, London, UK). Statistical significance was set at p < 0.05. Samples from a minimum of four patients were used for all experiments, with three repeats per sample.
Results
Estrogen and Testosterone Inhibit BDNF Secretion (But Not Expression)
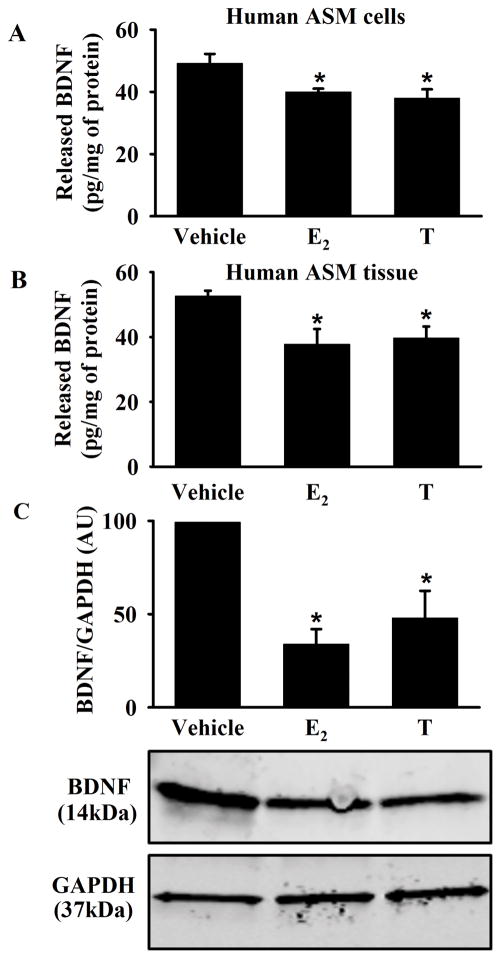
Steroidal treatment significantly reduced BDNF secretion in human ASM cells (Figure 1A, p<0.05). To ensure these effects were not unique to the cultured cell environment, fresh human ASM tissue was similarly treated with E2 or T. ELISA and western blot analysis confirmed significant decreases in BDNF secretion as a result of E2 or T treatment (Figure 1B, and C, p<0.05). No significance difference was noted between E2 vs. T effects.
Figure 1. Effect of estrogen (E2) and Testosterone (T) on secretion of brain-derived neurotrophic factor (BDNF) in human airway smooth muscle (ASM).
A) BDNF secretion by human ASM cells was significantly decreased following E2 or T treatment. B) Similarly, 48h exposure of E2 or T significantly reduced BDNF secretion from fresh human ASM tissue. C) BDNF release was significantly decreased in tissue treated conditioned media. Released BNDF was normalized to GAPDH from matching tissue homogenate. Data are presented as mean ± SE, n = minimum of 4 patients, p < 0.05. *indicates significant effect of E2 or T from vehicle.
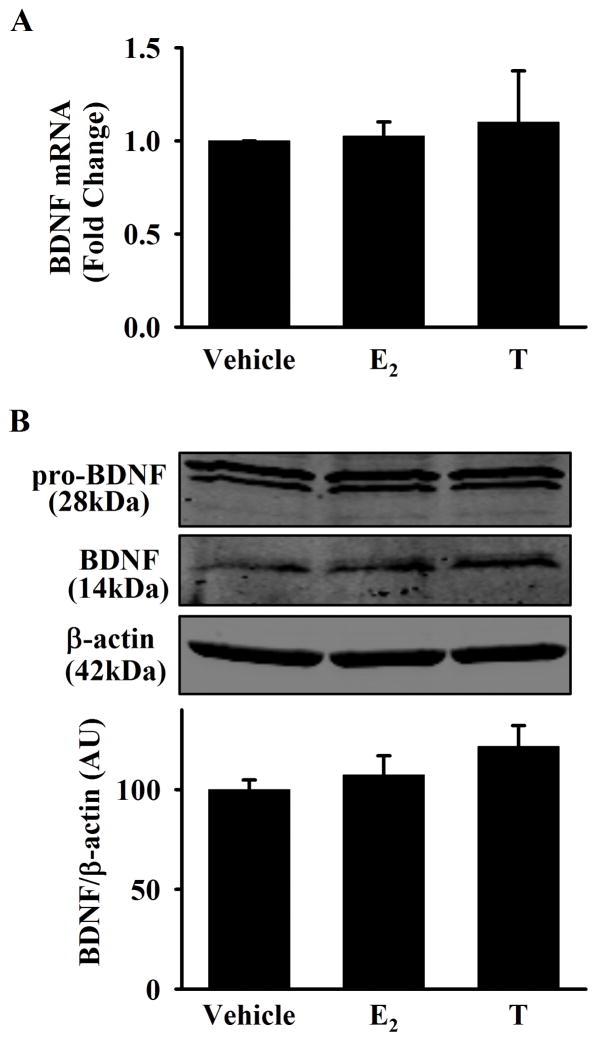
To explore the impact of E2 and T treatment on BDNF expression, mRNA and intracellular protein levels were measured. In human ASM cells, there was no significant change in BDNF gene expression after 24h exposure to E2 or T (Figure 2A). Protein expression of both pro-BDNF, and mature BDNF in cell lysates also did not significantly change (Figure 2B).
Figure 2. Effect of E2 and T on BDNF expression in human ASM.
A) Exposure of E2 or T (24 h) did not alter BDNF mRNA expression in human ASM cells (measured by qPCR). B) No significant difference in BDNF expression was observed via western blot analysis of cell lysates following exposure to E2 or T (48 h). Bar graph is total pro-BDNF and BDNF expression normalized to β-actin. Data are presented as mean ± SE, no = minimum of 4 patients, p < 0.05. *indicates significant effect of E2 or T from vehicle.
Estrogen and Testosterone Do Not Alter TrkB expression
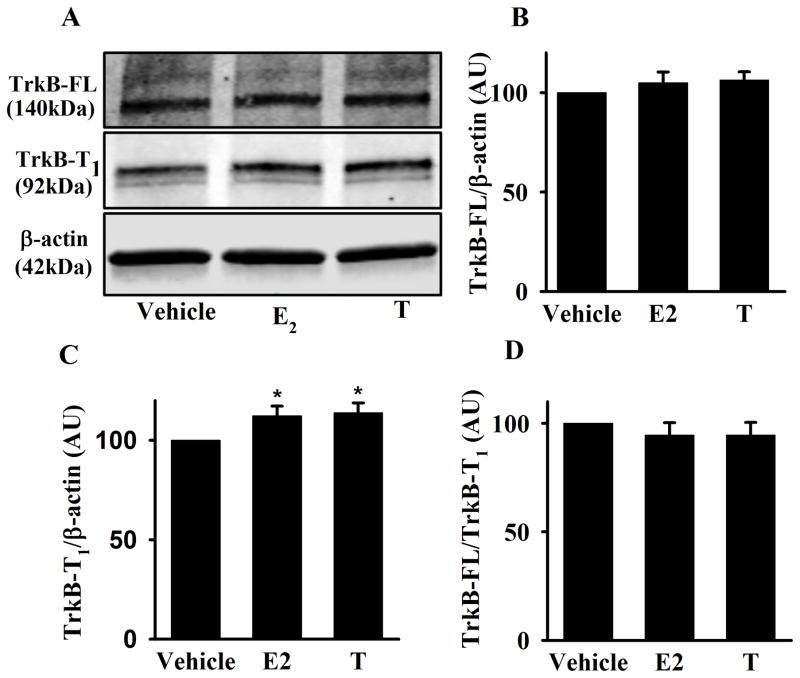
Human ASM cells were exposed to E2 or T for 48h. There was no significant change in the full length TrkB receptor expression (Figure 3A, and B). Although there was a significant increase in the truncated TrkB receptor isoform, TrkB-T1 (Figure 3A, and C, p<0.05), the ratio of TrkB-FL to TrkB-T1 was not significantly changed with E2 or T treatment (Figure 3D).
Figure 3. Effect of E2 and T on TrkB expression in human ASM.
A) Western blot analysis of TrkB-FL and TrkB-T1 in human ASM cells exposed to E2 or T for 48h. B) There was no significant change in TrkB-FL expression between experimental groups, C) Conversely, both E2 and T treatment significantly increased TrkB-T1 expression. D) However, the ratio of TrkB-FL to TrkB-T1 was found not to be significantly different. Data are presented as mean ± SE, n = minimum of 4 patients, p < 0.05. *indicates significant effect of E2 or T from vehicle.
Role of Estrogen and Testosterone Receptors
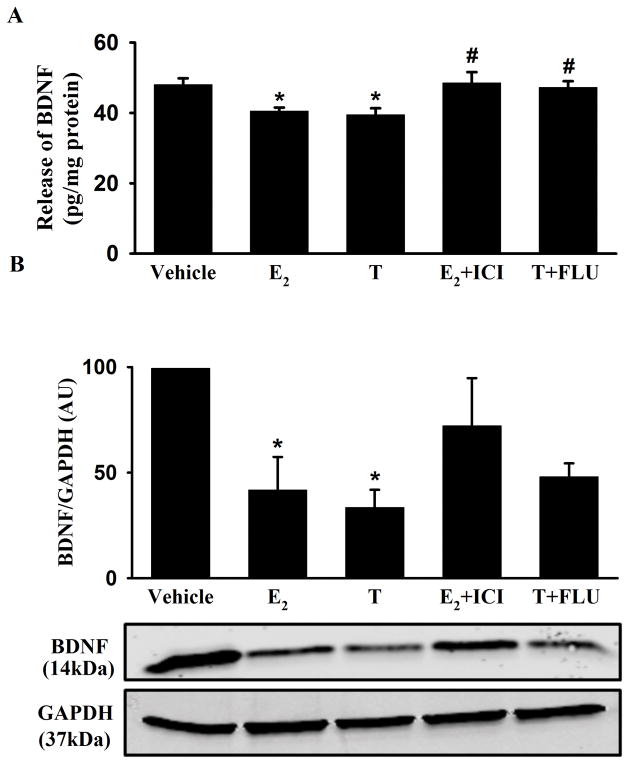
Human ASM cells or tissue were pretreated with the estrogen receptor (ER) antagonist ICI (1μM) or androgen receptor (AR) antagonist FLU (10μM) followed by 48h E2 or T treatment in the continued presence of the antagonist. In human ASM, the blunting of BDNF secretion by E2 or T was significantly reversed (almost to vehicle control) by inhibition of ER or AR (Figure 4A, p<0.05). Measurement of BDNF in conditioned media from tissue exposed to ICI or FLU prior to E2 and T treatment also demonstrated a trend towards return towards vehicle control but was not statistically significant (Figure 4B).
Figure 4. Effect of estrogen or androgen receptor inhibition on BDNF secretion in human ASM.
Human ASM cells were treated for 30 min with 1 μM estrogen receptor (ER) antagonist ICI 182,780 (ICI) or androgen receptor (AR) antagonist 10 μM Flutamide (FLU) prior to 48h exposure of E2 or T, respectively. A) BDNF secretion was restored to vehicle control in the presence of ICI and FLU. B) Western blot analysis of human ASM tissue conditioned media showed the same trend, but was not statistically significant. GAPDH from matching tissue homogenate was used to normalize the conditioned media. The experimental data are presented as mean ± SE, n = minimum of 4 patients, p < 0.05. *indicates significant effect of E2 and T from vehicle. #indicates significant effect of inhibitors from E2 or T.
Effect of Estrogen and Testosterone on cAMP pathway
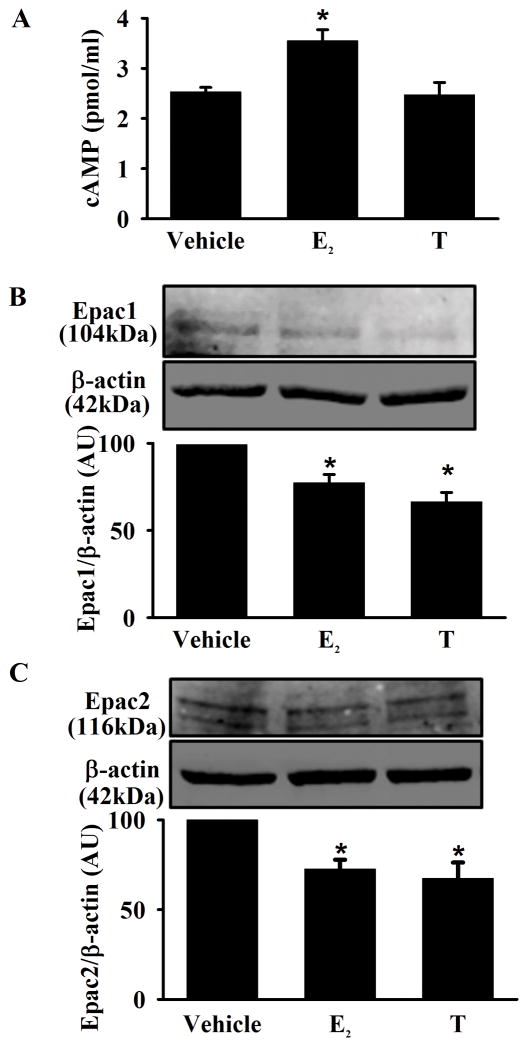
cAMP levels and cAMP-regulated guanine nucleotide exchange factor (Epac) expression were measured via ELISA and western blot analysis. Exposure to E2 significantly increased intracellular cAMP levels in ASM compared to vehicle, whereas T exposure had no effect (Figure 5A, p<0.05). Interestingly, expression of the regulatory proteins Epac1 and Epac2 were significantly reduced in human ASM cells exposed to either E2 or T (Figure 5B and C, p<0.05).
Figure 5. Effect of E2 and T on cAMP levels and Epac expression in human ASM.
A) Exposure of E2 significantly increased intracellular cAMP compared to vehicle. There was no significant effect with T exposure. B and C) Expression of the regulatory proteins Epac1 and Epac2 was significantly reduced in the presence of E2 or T. Data are presented as mean ± SE, n = minimum of 5 patients, p < 0.05. *indicates significant effect of E2 and T from vehicle.
Effect of Estrogen and Testosterone on SNARE Proteins
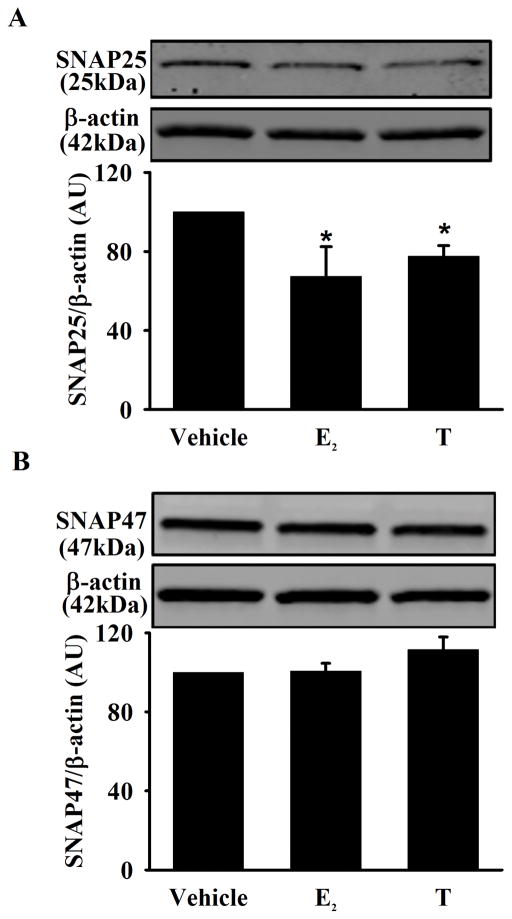
Based on data in neurons (Shimojo et al., 2015), we explored the role of secretory vesicle regulatory proteins in E2 and T effects on BDNF, focusing on the secretory vesicular proteins SNAP25 and SNAP47. SNAP25 expression was significantly decreased in cells exposed to E2 or T (Figure 6A, p<0.05). In contrast, there was no effect of either sex steroid on SNAP47 expression (Figure 6B).
Figure 6. Effect of E2 and T on secretory vesicular proteins (SNAP25 and SNAP 47) in human ASM.
A) Exposure of E2 or T significantly decreased SNAP25 expression compared to vehicle. B) There was no significant difference in SNAP47 expression between experimental groups. Data are presented as mean ± SE, n = minimum of 5 patients, p < 0.05. *indicates significant effect of E2 and T from vehicle.
Discussion
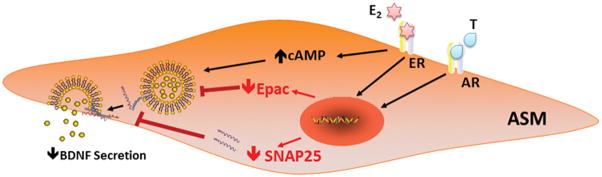
Studies in the brain have linked BDNF and sex steroids in the context of sex differences in incidence/severity of stress, depression, schizophrenia, and responses to pain (Greenberg et al., 2014; Nishinaka et al., 2015; Zhang et al., 2014). Furthermore, BDNF and estrogen signaling are thought to be intricately linked in the brain (Numakawa et al., 2014). However, there is currently no information on whether and how BDNF and sex hormones interact in the context of non-neuronal tissues including the lung, where both BDNF and sex steroids are emerging topics in terms of airway function and dysfunction. As a first step in deciphering interactions between sex steroids and BDNF, the present study demonstrates that in human ASM, physiologically relevant concentrations of estrogen or testosterone act through ERs and ARs respectively to decrease basal BDNF secretion, potentially via the cAMP-Epac pathway and inhibition of vesicular release. In this regard, the current study provides novel insights into the mechanisms by which sex steroids affect BDNF in the airway (Figure 7), and importantly highlights differences in such interactions between the CNS and the lung.
Figure 7. Potential mechanisms by which E2 and T affect BDNF secretion in human ASM.
Inhibition of BDNF secretion (but not BDNF expression) in human ASM by sex steroids may be mediated via cAMP-Epac pathway and/or reduced SNAP25 vesicular protein expression.
BDNF is a member of the neurotrophin growth factor family and is extensively distributed in the non-neuronal, peripheral system (Prakash et al., 2010). Recent studies, including our own, clearly indicate the importance of BDNF in human airway in the context of asthma and other airway diseases (Aravamudan et al., 2012a; Britt et al., 2015; Dagnell et al., 2007; Pan et al., 2010; Prakash and Martin, 2014; Prakash et al., 2009; Sathish et al., 2013a; Thompson et al., 2015; Vohra et al., 2013; Watanabe et al., 2015). Initially, in the endoplasmic reticulum BDNF is synthesized as a precursor protein (Lessmann and Brigadski, 2009; McDonald and Chao, 1995; Robinson et al., 1995) that is cleaved to produce pro-BDNF which is further sorted into either secretory vesicle. Such vesicular pro-BDNF can be converted intracellularly into mature BDNF (by endoproteases or convertases (Mowla et al., 2001)) or more commonly gets released into the extracellular space as pro-BDNF where it is cleaved by factors such as plasmin and matrix metalloproteinases into mature BDNF (Pang et al., 2004; Yamamori et al., 2013). Cellular action of BDNF involves high-affinity tropomyosin related kinase receptor TrkB, where the full-length TrkB-FL isoform is required for activity, and the truncated isoform TrkB-T1 is of unclear relevance, but may play a dominant negative role by sequestration of BDNF (Aven et al., 2014; Prakash and Martin, 2014). We have previously shown that both TrkB receptor isoforms are expressed in human ASM (Prakash and Martin, 2014; Prakash et al., 2009). Thus, multiple levels exist for the regulation of BDNF expression, secretion and autocrine/paracrine action. Accordingly, understanding the underlying mechanisms is key to determining how BDNF may influence the airway structure and function.
Recent advancement in sex steroid biology indicates that sex steroids (estrogen and testosterone) play important roles in many pathophysiological processes of non-reproductive organs, including the lung (Townsend et al., 2012a). Sex steroid signaling is complex and involves multiple signaling pathways with both genomic and/or non-genomic mechanisms (Sathish et al., 2015b; Townsend et al., 2012a). Previously, we have shown rapid, non-genomic effects of physiologically-relevant concentrations of E2 on reduction of [Ca2+]i in human ASM (Townsend et al., 2010) with bronchodilatory effects via increasing cAMP production (Townsend et al., 2012b). In this regard, the findings of our current study support the idea that that estrogen increases cAMP in ASM. We now further show that both Epac1 and Epac2 expression are reduced in the presence of estrogen, suggesting an additional pathway for estrogens to blunt [Ca2+]i or other aspects of ASM signaling. Similar to estrogen, testosterone non-genomically relaxes pre-constricted rabbit tracheal smooth muscle (Kouloumenta et al., 2006) which may involve inhibition of voltage-dependent calcium channels (Bordallo et al., 2008; Montano et al., 2014). In contrast to non-genomic effects, very limited data is available in regards to genomic effects of sex steroids on human ASM. The current study suggests one possible genomic effect of either sex steroid may be modulation of BDNF.
Previously we have shown short term treatment of estrogen can induce an increase in cAMP levels in human ASM (Townsend et al., 2012b). cAMP signaling has also been shown to be involved in multiple cellular mechanisms including smooth muscle contraction, proliferation, trafficking and exocytosis processes (Billington et al., 2013; Sathish et al., 2015a; Thompson et al., 2015). Regulation of exocytosis through cAMP had been shown to be dependent on Epac1 and Epac2 expression (Schmidt et al., 2013; Shibasaki et al., 2007; Ulucan et al., 2007). In support, our recently published study on developing ASM cells also suggested a significant role of Epac2 in BDNF secretion (Thompson et al., 2015). Here we demonstrate that expression of Epac1 and Epac2 are decreased upon estrogen or testosterone treatment. Inhibition of critical mechanisms for vesicular release of BDNF via reduction of Epacs could be one pathway for estrogen and testosterone action in terms of BDNF. In line with our findings, previous studies’ using dorsal root ganglions also suggested the inhibitory effect of estrogen downstream of Epac in attenuation of PKC-epsilon translocation (Hucho et al., 2006). The regulatory role of testosterone on Epacs in vesicular release has not been explored.
Recent studies from our group propose that there is indeed endogenous production of BDNF from human ASM in both adult (Sathish et al., 2013b; Vohra et al., 2013) and developing ASM (Thompson et al., 2015) and that BDNF can be secreted by ASM. Our current study demonstrates an inhibitory effect of estrogen and testosterone on BDNF release in human ASM via Epacs. Furthermore, it appears that neither estrogens nor testosterone affect BDNF mRNA or protein expression. In addition, there is no significant effect of these sex steroids on high affinity receptor TrkB receptors. This finding is novel, and interestingly is contrary to what would be expected in the CNS where estrogens stimulate BDNF (Cersosimo and Benarroch, 2015; Chakrabarti et al., 2014; Pietranera et al., 2015; Spence and Voskuhl, 2012). The reasons for this difference are not clear, but may reflect cell-dependent heterogeneity of BDNF regulation.
Previous studies have suggested neuronal regulation of vesicular release through presynaptic modulation in expression of Syb2 and SNAP25 (Shimojo et al., 2015). Here, vesicular traffic/fusion with the plasma membrane, and consequently exocytosis of the vesicular cargo i.e. BDNF, is regulated by the formation of these key proteins contribution to the SNARE complex (Shimojo et al., 2015). SNAP47, another SNARE complex protein, plays a unique regulatory role in the SNARE complex exocytosis process. SNAP47 interacts with SNAP25 and promotes exocytosis of BDNF in a cell-autonomous manner in the CNS (Shimojo et al., 2015). The role of such vesicular proteins thought to be involved in BDNF secretion has not been explored in the lung. Estrogen and testosterone significantly reduced SNAP25 expression in human ASM, while SNAP47 expression was not affected. These results suggest that reduction in SNAP25 expression may contribute to decreasing vesicular release of BDNF in human ASM. What would be interesting to determine is whether release of other factors by ASM, including cytokines, are also regulated via these mechanisms.
In conclusion, our results demonstrate that estrogen and testosterone inhibit BDNF secretion via two possible inhibitory mechanisms, cAMP-Epac and/or SNAP25 in human ASM. Given the increasing recognition of both sex steroids and BDNF in airway disease, and their modulation by inflammation (e.g. in the context of disease), their interaction may be important in the regulation of airway structure and function.
Acknowledgments
Funding Support: Supported by NIH R01 grants HL088029 (Prakash), HL56470 (Prakash), HL012349 (Sathish)
References
- Abcejo AJ, Sathish V, Smelter DF, Aravamudan B, Thompson MA, Hartman WR, Pabelick CM, Prakash YS. Brain-derived neurotrophic factor enhances calcium regulatory mechanisms in human airway smooth muscle. PLoS One. 2012;7(8):e44343. doi: 10.1371/journal.pone.0044343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre CC, Baudry M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci. 2009;29(3):447–454. doi: 10.1111/j.1460-9568.2008.06591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angleson JK, Cochilla AJ, Kilic G, Nussinovitch I, Betz WJ. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nat Neurosci. 1999;2(5):440–446. doi: 10.1038/8107. [DOI] [PubMed] [Google Scholar]
- Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med. 2012a;16(4):812–823. doi: 10.1111/j.1582-4934.2011.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravamudan B, Thompson M, Pabelick C, Prakash YS. Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J Cell Mol Med. 2012b;16(4):812–823. doi: 10.1111/j.1582-4934.2011.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aven L, Paez-Cortez J, Achey R, Krishnan R, Ram-Mohan S, Cruikshank WW, Fine A, Ai X. An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28(2):897–907. doi: 10.1096/fj.13-238212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington CK, Ojo OO, Penn RB, Ito S. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther. 2013;26(1):112–120. doi: 10.1016/j.pupt.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordallo J, de Boto MJ, Meana C, Velasco L, Bordallo C, Suarez L, Cantabrana B, Sanchez M. Modulatory role of endogenous androgens on airway smooth muscle tone in isolated guinea-pig and bovine trachea; involvement of beta2-adrenoceptors, the polyamine system and external calcium. Eur J Pharmacol. 2008;601(1–3):154–162. doi: 10.1016/j.ejphar.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Neuhaus-Steinmetz U, Quarcoo D, Glaab T, McGregor GP, Fischer A, Renz H. Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br J Pharmacol. 2004;141(3):431–440. doi: 10.1038/sj.bjp.0705638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt RD, Jr, Thompson MA, Kuipers I, Stewart A, Vogel ER, Thu J, Martin RJ, Pabelick CM, Prakash YS. Soluble guanylate cyclase modulators blunt hyperoxia effects on calcium responses of developing human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2015;309(6):L537–542. doi: 10.1152/ajplung.00232.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo MG, Benarroch EE. Estrogen actions in the nervous system: Complexity and clinical implications. Neurology. 2015;85(3):263–273. doi: 10.1212/WNL.0000000000001776. [DOI] [PubMed] [Google Scholar]
- Chakrabarti M, Haque A, Banik NL, Nagarkatti P, Nagarkatti M, Ray SK. Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res Bull. 2014;109:22–31. doi: 10.1016/j.brainresbull.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DG, Irvin CG. Mechanisms of airway hyper-responsiveness in asthma: the past, present and yet to come. Clin Exp Allergy. 2015;45(4):706–719. doi: 10.1111/cea.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnell C, Kemi C, Klominek J, Eriksson P, Skold CM, Eklund A, Grunewald J, Olgart Hoglund C. Effects of neurotrophins on human bronchial smooth muscle cell migration and matrix metalloproteinase-9 secretion. Translational research : the journal of laboratory and clinical medicine. 2007;150(5):303–310. doi: 10.1016/j.trsl.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007;19(6):676–680. doi: 10.1016/j.coi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Elliot JG, Jones RL, Abramson MJ, Green FH, Mauad T, McKay KO, Bai TR, James AL. Distribution of airway smooth muscle remodelling in asthma: relation to airway inflammation. Respirology. 2015;20(1):66–72. doi: 10.1111/resp.12384. [DOI] [PubMed] [Google Scholar]
- Fayon M, Andrieux A, Bara I, Rebola M, Labbe A, Marthan R, Berger P. An age-wise comparison of human airway smooth muscle proliferative capacity. PLoS One. 2015;10(3):e0122446. doi: 10.1371/journal.pone.0122446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29(41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellings PW, Vandekerckhove P, Claeys R, Billen J, Kasran A, Ceuppens JL. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy. 2003;33(10):1457–1463. doi: 10.1046/j.1365-2222.2003.01743.x. [DOI] [PubMed] [Google Scholar]
- Hoyle GW. Neurotrophins and lung disease. Cytokine Growth Factor Rev. 2003;14(6):551–558. doi: 10.1016/s1359-6101(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur J Neurosci. 2006;24(2):527–534. doi: 10.1111/j.1460-9568.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- Jha A, Sharma P, Anaparti V, Ryu MH, Halayko AJ. A role for transient receptor potential ankyrin 1 cation channel (TRPA1) in airway hyper-responsiveness? Canadian journal of physiology and pharmacology. 2015;93(3):171–176. doi: 10.1139/cjpp-2014-0417. [DOI] [PubMed] [Google Scholar]
- Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. Non-genomic effect of testosterone on airway smooth muscle. Br J Pharmacol. 2006;149(8):1083–1091. doi: 10.1038/sj.bjp.0706936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neuroscience research. 2009;65(1):11–22. doi: 10.1016/j.neures.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Li M, Masugi-Tokita M, Takanami K, Yamada S, Kawata M. Testosterone has sublayer-specific effects on dendritic spine maturation mediated by BDNF and PSD-95 in pyramidal neurons in the hippocampus CA1 area. Brain Res. 2012;1484:76–84. doi: 10.1016/j.brainres.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Lin AH, Shang Y, Mitzner W, Sham JS, Tang WY. Aberrant DNA Methylation of Phosphodiesterase 4D: Effect on Airway Smooth Muscle Cell Phenotypes. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2015-0079OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald NQ, Chao MV. Structural determinants of neurotrophin action. J Biol Chem. 1995;270(34):19669–19672. doi: 10.1074/jbc.270.34.19669. [DOI] [PubMed] [Google Scholar]
- Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep. 2007;7(2):143–150. doi: 10.1007/s11882-007-0012-4. [DOI] [PubMed] [Google Scholar]
- Meuchel LW, Stewart A, Smelter DF, Abcejo AJ, Thompson MA, Zaidi SI, Martin RJ, Prakash YS. Neurokinin-neurotrophin interactions in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2011;301(1):L91–98. doi: 10.1152/ajplung.00320.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano LM, Espinoza J, Flores-Soto E, Chavez J, Perusquia M. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J Endocrinol. 2014;222(1):1–13. doi: 10.1530/JOE-14-0074. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276(16):12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Nishinaka T, Kinoshita M, Nakamoto K, Tokuyama S. Sex differences in depression-like behavior after nerve injury are associated with differential changes in brain-derived neurotrophic factor levels in mice subjected to early life stress. Neurosci Lett. 2015;592:32–36. doi: 10.1016/j.neulet.2015.02.053. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Richards M, Nakajima S, Adachi N, Furuta M, Odaka H, Kunugi H. The role of brain-derived neurotrophic factor in comorbid depression: possible linkage with steroid hormones, cytokines, and nutrition. Front Psychiatry. 2014;5:136. doi: 10.3389/fpsyt.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Rhode HK, Undem BJ, Myers AC. Neurotransmitters in airway parasympathetic neurons altered by neurotrophin-3 and repeated allergen challenge. Am J Respir Cell Mol Biol. 2010;43(4):452–457. doi: 10.1165/rcmb.2009-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306(5695):487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Pietranera L, Brocca ME, Roig P, Lima A, Garcia-Segura LM, De Nicola AF. Estrogens are neuroprotective factors for hypertensive encephalopathy. J Steroid Biochem Mol Biol. 2015;146:15–25. doi: 10.1016/j.jsbmb.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Prakash Y, Thompson MA, Meuchel L, Pabelick CM, Mantilla CB, Zaidi S, Martin RJ. Neurotrophins in lung health and disease. Expert Rev Respir Med. 2010;4(3):395–411. doi: 10.1586/ers.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol. 2013;305(12):L912–933. doi: 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Martin RJ. Brain-derived neurotrophic factor in the airways. Pharmacol Ther. 2014;143(1):74–86. doi: 10.1016/j.pharmthera.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Thompson MA, Pabelick CM. Brain-derived neurotrophic factor in TNF-alpha modulation of Ca2+ in human airway smooth muscle. Am J Respir Cell Mol Biol. 2009;41(5):603–611. doi: 10.1165/rcmb.2008-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RC, Radziejewski C, Stuart DI, Jones EY. Structure of the brain-derived neurotrophic factor/neurotrophin 3 heterodimer. Biochemistry. 1995;34(13):4139–4146. doi: 10.1021/bi00013a001. [DOI] [PubMed] [Google Scholar]
- Sathish V, Freeman MR, Long E, Thompson MA, Pabelick CM, Prakash YS. Cigarette Smoke and Estrogen Signaling in Human Airway Smooth Muscle. Cell Physiol Biochem. 2015a;36(3):1101–1115. doi: 10.1159/000430282. [DOI] [PubMed] [Google Scholar]
- Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther. 2015b;150:94–108. doi: 10.1016/j.pharmthera.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish V, Vanoosten SK, Miller BS, Aravamudan B, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Brain-derived neurotrophic factor in cigarette smoke-induced airway hyperreactivity. Am J Respir Cell Mol Biol. 2013a;48(4):431–438. doi: 10.1165/rcmb.2012-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish V, Vanoosten SK, Miller BS, Aravamudan B, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Brain-derived neurotrophic factor in cigarette smoke-induced airway hyperreactivity. Am J Respir Cell Mol Biol. 2013b;48(4):431–438. doi: 10.1165/rcmb.2012-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. beta-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–120. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Dekker FJ, Maarsingh H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev. 2013;65(2):670–709. doi: 10.1124/pr.110.003707. [DOI] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294(5544):1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Scuri M, Samsell L, Piedimonte G. The role of neurotrophins in inflammation and allergy. Inflamm Allergy Drug Targets. 2010;9(3):173–180. doi: 10.2174/187152810792231913. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci U S A. 2007;104(49):19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo M, Courchet J, Pieraut S, Torabi-Rander N, Sando R, 3rd, Polleux F, Maximov A. SNAREs Controlling Vesicular Release of BDNF and Development of Callosal Axons. Cell reports. 2015;11(7):1054–1066. doi: 10.1016/j.celrep.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33(1):105–115. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MA, Britt RD, Jr, Kuipers I, Stewart A, Thu J, Pandya HC, MacFarlane P, Pabelick CM, Martin RJ, Prakash YS. cAMP-mediated secretion of brain-derived neurotrophic factor in developing airway smooth muscle. Biochim Biophys Acta. 2015;1853(10 Pt A):2506–2514. doi: 10.1016/j.bbamcr.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Meuchel LW, Thompson MA, Pabelick CM, Prakash YS. Estrogen increases nitric-oxide production in human bronchial epithelium. J Pharmacol Exp Ther. 2011;339(3):815–824. doi: 10.1124/jpet.111.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Meuchel LW, Thompson MA, Pabelick CM, Prakash YS. Estrogen modulation of nitric oxide signaling in the airway. J Cell Physiol. 2013;228(4):688. doi: 10.1002/jcp.24227. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012a;33(1):1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol. 2012b;303(10):L923–928. doi: 10.1152/ajplung.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Thompson MA, Pabelick CM, Prakash YS. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2010;298(4):L521–530. doi: 10.1152/ajplung.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulucan C, Wang X, Baljinnyam E, Bai Y, Okumura S, Sato M, Minamisawa S, Hirotani S, Ishikawa Y. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293(3):H1662–1672. doi: 10.1152/ajpheart.00159.2007. [DOI] [PubMed] [Google Scholar]
- Vohra PK, Thompson MA, Sathish V, Kiel A, Jerde C, Pabelick CM, Singh BB, Prakash YS. TRPC3 regulates release of brain-derived neurotrophic factor from human airway smooth muscle. Biochim Biophys Acta. 2013;1833(12):2953–2960. doi: 10.1016/j.bbamcr.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Fajt ML, Trudeau JB, Voraphani N, Hu H, Zhou X, Holguin F, Wenzel SE. Brain Derived Neurotrophic Factor (BDNF) Expression in Asthma: Association with Severity and Type-2 Inflammatory Processes. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2015-0015OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori H, Hashimoto R, Ishima T, Kishi F, Yasuda Y, Ohi K, Fujimoto M, Umeda-Yano S, Ito A, Hashimoto K, Takeda M. Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment-resistant schizophrenia treated with clozapine. Neurosci Lett. 2013;556:37–41. doi: 10.1016/j.neulet.2013.09.059. [DOI] [PubMed] [Google Scholar]
- Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145(1):161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Chen DC, Tan YL, Tan SP, Wang ZR, Yang FD, Xiu MH, Hui L, Lv MH, Zunta-Soares GB, Soares JC. Gender difference in association of cognition with BDNF in chronic schizophrenia. Psychoneuroendocrinology. 2014;48:136–146. doi: 10.1016/j.psyneuen.2014.06.004. [DOI] [PubMed] [Google Scholar]