Abstract
Objective
Childhood onset growth hormone deficiency (COGHD) is associated with low bone mineral density (BMD). Adults with persistent COGHD may be at risk for insufficient bone accrual or bone loss during adulthood. The purpose of this study was to identify BMD predictors and characterize the effects of GH replacement on BMD in COGHD adults with persistent GHD.
Design
Retrospective analysis of the KIMS database.
Methods
Variables predicting standardized BMD (sBMD) were identified. The effect of GH replacement (3 years) on BMD was examined.
Results
314 COGHD adults (148 women, 166 men; 62 non-naïve, 178 semi-naïve, and 74 true naïve, depending on length and timing of previous GH replacement), who had BMD measured in lumbar spine (LS) and femoral neck (FN) at study entry.
In semi-naïve subjects, a longer gap in GH replacement between childhood and adulthood was predictive of lower sBMD in the FN (r= −0.18, P=0.038). Thyrotropin deficiency predicted lower sBMD in the LS (r= −0.16,P=0.052). In true naïve patients, a longer gap between onset of pituitary disease and study entry (r= −0.35,P=0.012) and female gender (r= −0.27,P=0.043) independently predicted lower sBMD in the FN. There were no differences in BMD increases between non-naïve, semi-naïve and true naïve subjects on GH replacement.
Conclusions
In semi-naïve subjects a longer interval off GH replacement was associated with lower sBMD in the FN. Among true naïve patients, a longer gap between the onset of pituitary disease and GH replacement, and female gender predicted lower sBMD in the FN.
Keywords: Bone mineral density, childhood onset growth hormone deficiency, hypopituitarism, pituitary, thyrotropin
Introduction
Growth hormone deficiency (GHD) of either childhood or adult onset is associated with low bone mineral density (BMD), which may be more severe in childhood onset GHD (COGHD) in comparison with adult onset GHD 1. Up to approximately 40 % of patients with COGHD may have persistent GHD when retested in adulthood 2–4. As growth hormone (GH) may have an important role in optimizing bone accrual in individuals who have completed their statural growth 5, patients with COGHD persisting in adulthood may be at risk for insufficient bone accrual or accelerated bone loss as a result of discontinuation of GH replacement after completion of linear growth.
Growth hormone acts on bone both directly and indirectly, via endocrine and paracrine synthesis of insulin-like growth factor 1 (IGF-1) 6–10. In addition to GH and IGF-1, several other hormones have significant effects on bone mineralization, including thyroid hormone, glucocorticoids and sex steroids 11–14. Patients with COGHD may have multiple pituitary hormone deficiencies in addition to GHD, the presence of which, along with hormone replacement therapies, may influence BMD in this population.
Studies of patients with COGHD and persistent GHD after the completion of linear growth have yielded mixed results with regards to BMD outcomes in response to GH replacement, with some 15–17, but not all 18, studies suggesting that the resumption of GH replacement in young adulthood may lead to additional increases in BMD.
To further clarify whether patients with COGHD persisting in adulthood are at risk for developing lower BMD in adulthood as a result of interruptions in GH replacement and characterize the role of additional pituitary hormone deficiencies on BMD in this population in a clinical practice setting, data from the large KIMS database were extracted and analyzed. This database comprises over 16,000 adult GHD patients, including approximately 3,000 COGHD individuals. To further investigate the effects of GH replacement in this population, data regarding BMD changes on GH replacement in adulthood were examined in these patients, who were stratified based on the presence of previous GH replacement and the length of treatment gap (interval) between GH replacement in childhood and adulthood.
Subjects and Methods
Subjects
The KIMS (Pfizer International Metabolic Study) database was queried for adult subjects of both genders, meeting all of the following inclusion criteria: persistent GHD of childhood onset (based on stringent diagnostic criteria 19, Supplementary Table 1) as previously described 20, and recent BMD data available at KIMS entry (BMD measured by dual energy X ray absorptiometry (DXA) on a Hologic, Lunar/GE or Norland densitometer within 6 months before to one month after beginning GH replacement in adulthood).
A subpopulation was then identified, additionally meeting the following inclusion criteria: continuous (at least 90 % of the time) GH replacement for three years since KIMS entry, and BMD data available at 3 years since study entry, measured by DXA on the same densitometer used to acquire baseline BMD data.
All study subjects had provided written informed consent at the time of enrollment into KIMS. The study was conducted according to the principles of the Declaration of Helsinki 21. The majority of subjects were enrolled in European study centers.
Methods
The KIMS database was queried to extract demographic and clinical data on all study subjects, based on information submitted by each participating clinical center. Data extracted included age at diagnosis of pituitary disease, age at diagnosis of GHD, age at KIMS entry, duration of GHD (estimated as the difference between age at KIMS entry minus age at diagnosis of GHD), duration of GH replacement in childhood, gap (interval) between GH replacement in childhood and adulthood (i.e. KIMS entry), gender, body weight, height SDS and body mass index (BMI), prevalent fracture, cause of GHD, peak GH response on stimulation testing, type and number of additional pituitary hormone deficiencies, glucocorticoid replacement dose (converted to hydrocortisone equivalent dose, using a relative potency for cortisone acetate: hydrocortisone: prednisone or prednisolone: dexamethasone of 25 mg: 20 mg: 5 mg: 0.6 mg), levothyroxine replacement dose (when available)22. Study subjects were classified as being non-naïve (treated continuously or being off GH replacement shorter than 6 months before enrolment into KIMS), semi-naïve (treated in childhood, but being off GH replacement longer than 12 months before KIMS entry), and true-naïve (patients never treated in childhood, who were beginning GH replacement at KIMS entry).
Laboratory data included IGF-1 standard deviation scores (SDS), calculated based on serum IGF-1 levels measured centrally in the laboratories listed below.
Serum IGF-1 levels were assayed at Kabi Pharmacia, Stockholm, Sweden between 1994 and October 1997. Thereafter, IGF-1 assays were performed at Sahlgrenska University Hospital, Gothenburg, Sweden. Between 1994 and November 2002 a radioimmunoassay was used to assay IGF-1 in serum specimens after acid/ethanol precipitation of IGF binding proteins (Nichols Institute, San Juan Capistrano, CA, USA) 16. Subsequently (until September 2006), a chemiluminescence immunoassay was employed (Nichols Advantage system). Thereafter, the Immulite 2500 (DPC Siemens, Deerfield, IL, USA) method has been used to measure serum IGF-1 levels.
The following formulae were used to calculate IGF-1 SDS: SDS = (ln(IGF-1)-(5.95 – 0.0197 x age))/0.282 (between 1994 – 1997); SDS = (ln(IGF-1)-(15.92 – 0.0146 x age))/0.272 (between 1997 – 2002); and (from 2002 onwards) based on data from the study of Brabant et al 23.
Bone densitometric data extracted included BMD and Z scores from two skeletal sites (including posterior anterior lumbar spine (LS) and femoral neck (FN)). At any skeletal site, measured BMD values vary between different densitometers, as diverse bone edge detection algorithms and calibration standards are employed by densitometer manufacturers. Predictive equations have been developed in order to facilitate direct comparisons between BMD data obtained on different densitometers 24, 25. These equations yield standardized BMD (sBMD) values, which can be used in population studies. Herein, sBMD data were calculated based on BMD values and the predictive equations for LS and FN, as previously described 20, 24, 25.
Statistical analysis
The Student’s t-test was used to analyze normally distributed data and the Wilcoxon rank sum test or the sign rank test was used in the analysis of non-parametric variables. Univariate as well as stepwise, multivariate regression analysis was used to identify variables independently predicting sBMD (in LS or FN). Independent variables introduced in the multivariate regression analysis model were subject age, age at onset of pituitary disease, age at diagnosis of GHD, gender, height SDS (calculated based on published reference data) 26, interval between onset of pituitary disease and study entry, estimated GHD duration, estimated duration of pediatric GH replacement, cause of GHD (idiopathic versus all others), gap (interval) length between GH replacement in childhood and KIMS entry, peak GH level on stimulation testing, and the presence or absence of deficiencies of gonadotropins, corticotropin, thyrotropin or antidiuretic hormone. Nominal variables were assigned numerical codes for the purpose of analysis.
The Statistical Analysis System (SAS Institute, Inc., Cary, NC, USA) was used in all statistical procedures. Data were expressed as median (10th percentile, 90th percentile) or mean ± SE, and statistical significance was accepted at a P<0.05 level.
Results
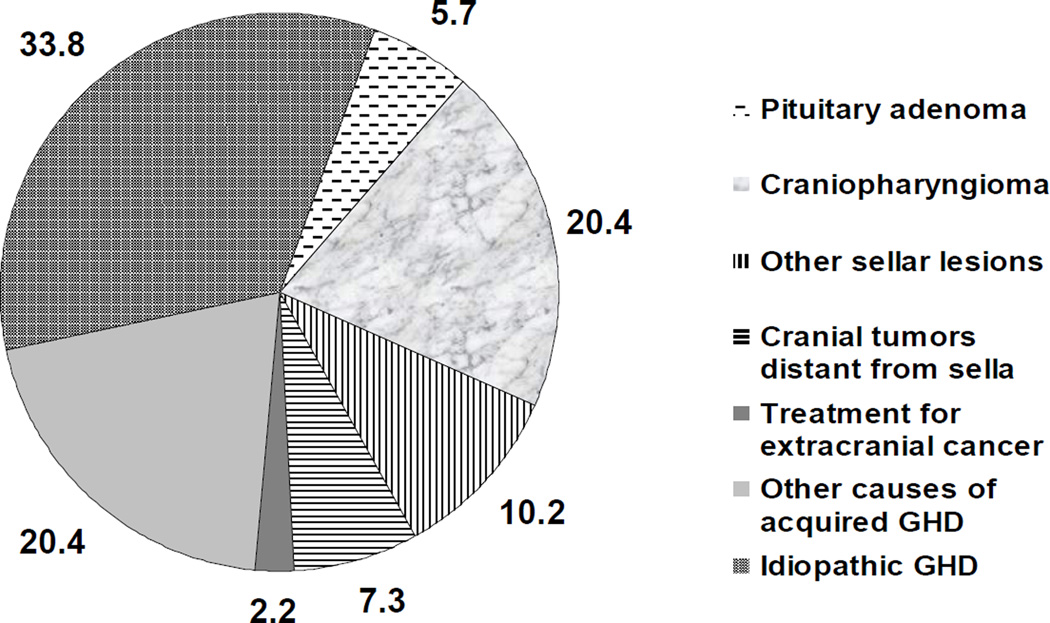
There were 314 adult subjects, all diagnosed with COGHD based on stringent criteria (Supplementary Table 1) as previously described 20. There were 148 women (47.1 %) and 166 men (52.9 %). The majority of study subjects were Caucasian from western European countries, and were enrolled in 89 participating centers. Data on the causes of GHD in this population are shown in Figure 1. The most common single cause of GHD was idiopathic (33.8 %), followed by craniopharyngioma (20.4 %). Pituitary adenoma was relatively uncommon (5.7 %), as anticipated in a population of patients with COGHD, and was present in 14.9 % of true naïve, 2.2 % of semi-naïve, and 4.8 % of non-naïve patients (P = NS).
Figure 1.
Causes of growth hormone deficiency in the study population (percent total), based on a previously defined classification list33.
Additional characteristics of the study population, stratified as being non-naïve (62 patients, 19.7 %), semi-naïve (178 subjects, 56.7 %), or true naïve (74 patients, 23.6 %), are shown in Table 1.
Table 1.
Characteristics of the study population, stratified by the gap length between growth hormone replacement in childhood and adulthood. Study subjects were classified as being non-naïve (treated continuously or being off GH replacement shorter than 6 months before enrolment into KIMS), semi-naïve (treated in childhood, but being off GH replacement longer than 12 months before KIMS entry), and true-naïve (patients never treated in childhood, who were beginning GH replacement at KIMS entry).
| Variable | Non-naïve (N = 62) |
Semi-naïve (N = 178) |
True naïve (N = 74) |
Non- naïve versus semi- naïve P value |
Semi-naïve versus true naïve P value |
Non-naïve versus true naïve P value |
|---|---|---|---|---|---|---|
| Age at onset of pituitary disease (yr) |
9.6 (2.9, 16.8) | 9.1 (3.6, 14.8) | 14.4 (7.5, 17.3) | NS | < 0.001 | < 0.001 |
| Age at diagnosis of GHD (yr) |
14.0 (4.8, 23.0) | 14.5 (5.9, 32.3) | 21.2 (15.6, 45.7) | NS | < 0.001 | < 0.001 |
| Age at study entry (yr) | 22.5 (18.4, 39.6) | 27.5 (19.8, 39.8) | 30.6 (18.2, 51.6) | 0.022 | 0.095 | < 0.010 |
| Estimated duration of GH deficiency (yr) |
11.7 (1.4, 25.8) | 12.2 (0.4, 24.2) | 1.5 (0, 16.8) | NS | < 0.001 | < 0.001 |
| Total yr GH replaced before study entry |
8.0 (1.1, 14.0) | 6.0 (2.0, 12.0) | 0 (0, 0) | 0.096 | NA | NA |
| IGF-1 SDS at study entry |
−1.4 (−4.9, 1.8) | −4.1 (−6.3, −2.3) | −4.1 (−7.0, −2.3) | < 0.001 | NS | < 0.001 * |
| Number of additional pituitary hormone deficiencies |
3 (0, 4) | 3 (0, 4) | 3 (0, 4) | NS | NS | NS |
| Height (cm) | 163 (150, 177) | 162 (149, 178) | 159 (139, 174) | NS * | 0.038 * | 0.030 * |
| Height SDS at study entry |
−0.86 (−3.18, 0.52) | −1.30 (−3.47, 0.28) | −1.63 (−4.40, 0.40) | NS* | 0.070* | 0.023* |
| BMI (kg/m2) | 24.1 (19.1, 33.5) | 25.1 (19.3, 35.4) | 27.4 (20.9, 34.9) | NS | < 0.010 | 0.011 |
| Lumbar spine sBMD (mg/cm2) |
989.5 (795.9, 1183) |
1005 (817.4, 1247) |
989.5 (809.4, 1190) |
NS | NS | NS |
| Lumbar spine Z score | −1.8 (−3.0, 1.6) | −1.2 (−2.3, 1.1) | −1.8 (−2.9, 0.4) | NS | 0.087 | NS * |
| Proportion of subjects with lumbar spine Z score < - 2.0 |
29.4 % | 17.6 % | 37.5 % | NS | NS | NS |
| Femoral neck sBMD (mg/cm2) |
885.6 (690.6, 1084) |
866.9 (682.1, 1160) |
840.9 (689.3, 1128) |
NS | NS | 0.099 |
| Femoral neck Z score | −0.4 (−1.8, 1.3) | −0.9 (−2.8, 0.5) | −0.6 (−2.1, 1.3) | 0.074 * | NS * | NS * |
| Proportion of subjects with femoral neck Z score < - 2.0 |
7.1 % | 20.4 % | 13.6 % | NS | NS | NS |
| Proportion of subjects with prevalent fracture |
16.4 % | 19.5 % | 29.2 % | NS | NS | NS |
Data shown as median (10th and 90th percentiles). Unless otherwise noted, P values were obtained using the Wilcoxon rank sum test.
P values were obtained using the t-test.
Abbreviations: sBMD: standardized bone mineral density; GHD: growth hormone deficiency; IGF-1: insulin-like growth factor 1; NS: not significant; SDS: standard deviation score.
True naïve subjects were older at the time of onset of pituitary disease or at the time of diagnosis of GHD in comparison with those in the other two groups (non-naïve and semi-naïve). In addition, true naïve subjects were slightly older than semi-naïve patients at the time of study entry, the latter also being slightly older than non-naïve subjects at study entry. True naïve subjects were shorter in height and heavier (based on BMI data) than patients belonging to the other two groups. Non-naïve subjects had higher IGF-1 SDS at time of study entry in comparison with those belonging to the other two groups, likely as a result of continuous GH replacement.
Glucocorticoid replacement therapy was reported in approximately 59 % of subjects in all three groups (P = NS), of whom 81 % were treated with hydrocortisone, 14 % with cortisone acetate, and the remaining subjects with prednisolone or dexamethasone. Glucocorticoid dosing, expressed as hydrocortisone equivalent dose, was similar in all three groups [non-naïve: 20 mg/day (10 mg/day, 30 mg/day), semi-naïve: 20 mg/day (10 mg/day, 30 mg/day); true naïve: 20 mg/day (10 mg/day, 30 mg/day), P = NS].
At study entry, there were no significant differences in sBMD values between the three subject groups. However, non-naïve patients showed a trend towards higher sBMD in the femoral neck in comparison with true naïve patients. In addition, semi-naïve patients showed a trend towards higher BMD Z scores in the lumbar spine than true naïve subjects, and non-naïve patients showed a trend towards higher BMD Z scores in the femoral neck than semi-naïve subjects.
In semi-naïve patients, there was a median (10th percentile, 90th percentile) gap (interval) of 8.2 yr (2.1 yr, 20.8 yr) between pediatric GH replacement and onset of GH replacement in adulthood. In this group, there was an association between duration of pediatric GH replacement and age at termination of pediatric GH replacement (r = 0.25, P = 0.001).
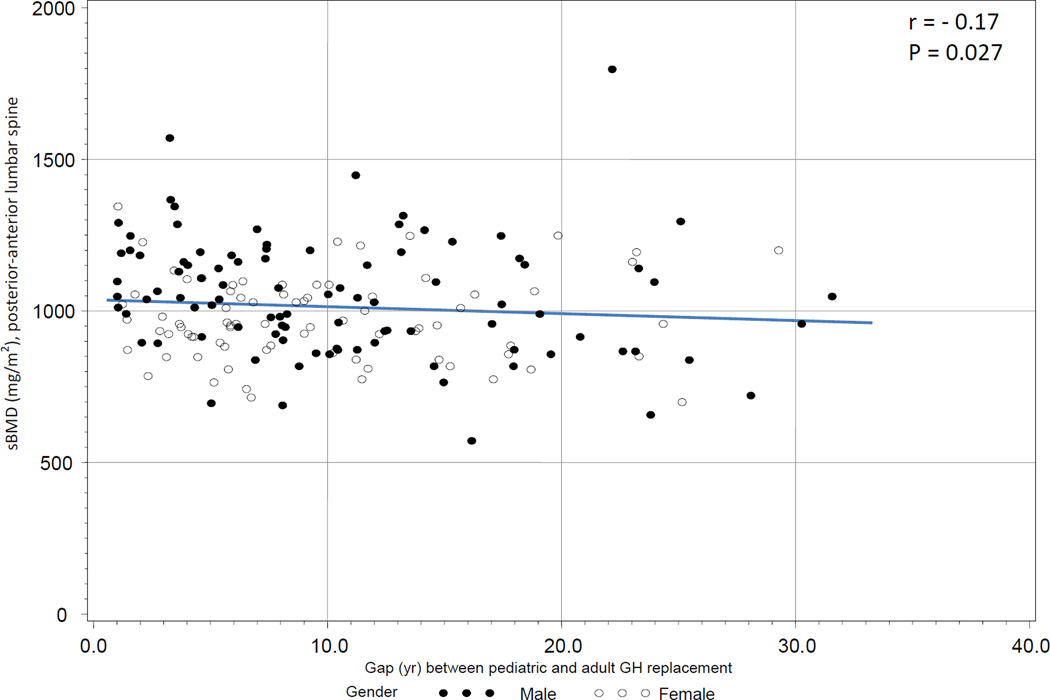
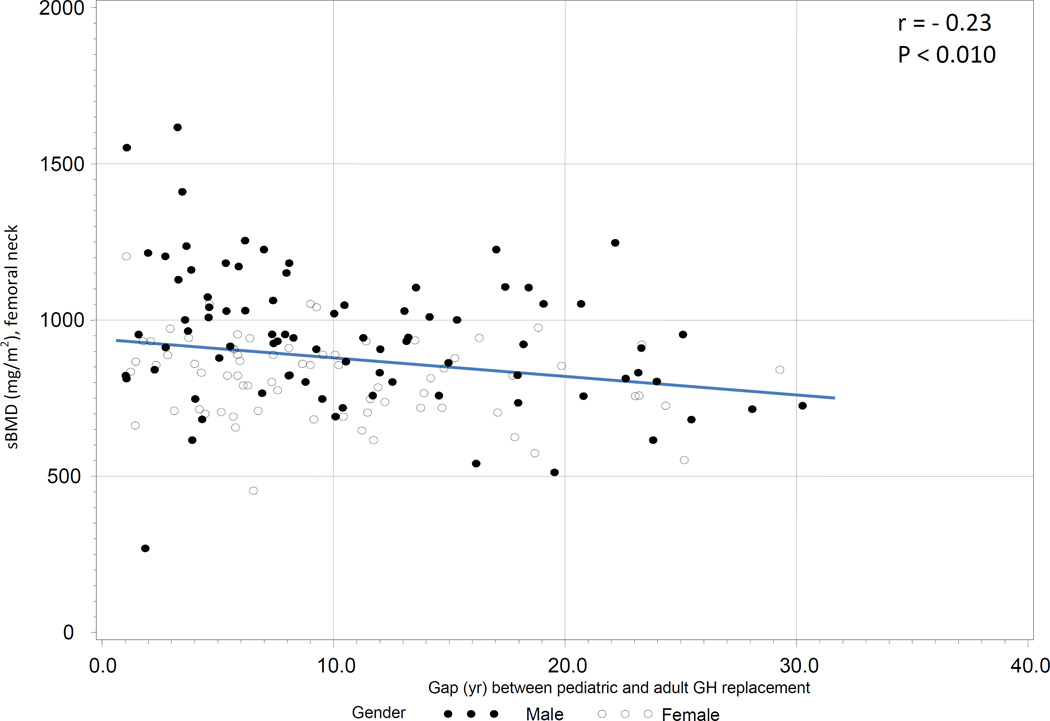
To examine the association between sBMD and the gap in GH replacement in this group, both univariate and multivariate regression analyses were conducted. On univariate analysis, there was a negative association between sBMD in the LS and the gap in GH replacement (r = −0.17, P = 0.027, Figure 2a) as well as a negative association between sBMD in the FN and the gap in GH replacement (r = −0.23, P < 0.010, Figure 2b).
Figure 2a.
a. Association between standardized BMD (posterior anterior lumbar spine) and gap length between GH replacement in childhood and adulthood in semi-naïve subjects (treated in childhood, but being off GH replacement longer than 12 months before KIMS entry).
b. Association between standardized BMD (femoral neck) and gap length between GH replacement in childhood and adulthood in semi-naïve subjects (treated in childhood, but being off GH replacement longer than 12 months before KIMS entry).
On multivariate regression analysis, the negative association between sBMD and gap in GH replacement remained significant in the FN (r = −0.18, P = 0.038), but not in the LS. We also examined the association between sBMD in the FN and gap in GH replacement in the subgroup of subjects aged < 25 yr old at study entry (n = 79). The gap in GH replacement between pediatric and adult GH replacement in this population was 4.5 yr (1.4 yr, 8.3 yr). There was no association between sBMD and gap in GH replacement in this subject subgroup.
Thyrotropin deficiency (defined as the presence of low free thyroxine levels with inappropriately normal serum thyrotropin) was an independent predictor of lower sBMD in the LS (r = −0.16, P = 0.036). However, in the final regression model, which additionally included peak GH level on stimulation testing as a possible predictor, the statistical significance of this observation became borderline (r = −0.16, P = 0.052). Of note, there was no association between sBMD and levothyroxine dose (in mcg/kg) in these patients (data not shown).
To identify predictors of sBMD in the other two groups (non-naïve and true naïve subjects), additional regression analyses were conducted. In non-naïve subjects, older age at study entry (r = 0.41, P = 0.027) and female gender (r = −0.40, P = 0.018) independently predicted sBMD in the FN (higher sBMD in older patients and lower sBMD in women) on multivariate regression analysis. No predictors of sBMD in the LS were identified in this group.
In true naïve subjects, greater height SDS at study entry (r = 0.43, P < 0.001) and higher peak GH level on stimulation testing (r = 0.35, P = 0.003) independently predicted higher sBMD in the LS on multivariate regression analysis. In addition, a longer gap between the onset of pituitary disease and study entry (r = −0.35, P = 0.012) and female gender (r = −0.27, P = 0.043) independently predicted lower sBMD in the FN of true naïve patients on multivariate regression analysis.
We also examined predictors of sBMD in the FN in the subgroup of true naïve subjects aged < 25 yr old at study entry (n = 29), using multivariate regression analysis. A longer gap between the diagnosis of GHD and onset of GH replacement in this subject subgroup (r = −0.41, P = 0.047) independently predicted lower sBMD in the FN. In addition, greater height SDS at study entry independently predicted higher sBMD in the FN (r = 0.44, P = 0.048).
Patient characteristics of the study subpopulation followed on GH replacement for 3 years after study entry, stratified as being non-naïve (26 patients, 26.8 %), semi-naïve (52 subjects, 53.6 %), or true naïve (19 patients, 19.6 %), are shown in Table 2a.
Table 2.
| a. Characteristics of the study subpopulation followed longitudinally (on GH replacement in adulthood after study entry). Study subjects were classified as being non-naïve (treated continuously or being off GH replacement shorter than 6 months before enrolment into KIMS), semi-naïve (treated in childhood, but being off GH replacement longer than 12 months before KIMS entry), and true-naïve (patients never treated in childhood, who were beginning GH replacement at KIMS entry). | ||||||
|---|---|---|---|---|---|---|
| Variable | Non-naïve (N = 26) |
Semi-naïve (N = 52) |
True naïve (N = 19) |
Non- naïve versus semi- naïve P value |
Semi-naïve versus true naïve P value |
Non-naïve versus true naïve P value |
| Age at onset of pituitary disease (yr) |
10.3 (2.7, 17.1) | 8.8 (3.7, 13.6) | 11.9 (5.9, 17.5) | NS | < 0.010 * | NS |
| Age at diagnosis of GHD (yr) |
14.9 (4.1, 24.3) | 13.4 (5.2, 30.7) | 20.4 (15.3, 56.4) | NS | < 0.001 | < 0.001 |
| Age at study entry (yr) | 24.9 (19.4, 44.7) | 26.2 (19.4, 36.5) | 30.6 (17.4, 57.6) | NS | NS | NS |
| Estimated duration of GH deficiency (yr) before study entry |
13.0 (1.2, 28.0) | 11.9 (0.4, 22.0) | 1.0 (0.0, 27.6) | NS | < 0.001 | < 0.010 |
| Total yr GH replaced before study entry |
7.8 (1.0, 14.0) | 5.5 (2.0, 13.0) | 0 (0, 0) | NS | NA | NA |
| IGF-1 SDS at study entry | −0.6 (−4.0, 2.0) | −3.9 (−5.5, −1.7) | −3.5 (−4.3, −1.7) | < 0.001 | NS | < 0.010 * |
| Number of additional pituitary hormone deficiencies |
3 (0, 4) | 3 (0, 4) | 2 (0, 4) | NS | NS | NS |
| Height (cm) at study entry | 165 (156, 177) | 167 (148, 180) | 161 (134, 173) | NS | NS | NS |
| Height SDS at study entry | −0.78 (−3.14, 0.67) | −1.07 (−3.30, 0.40) | −1.62 (−5.09, 0.82) | NS | NS * | NS |
| BMI (kg/m2) at study entry | 24.7 (18.9, 36.8) | 25.2 (19.7, 35.4) | 26.5 (20.0, 33.8) | NS | NS | NS |
| GH dose (mg/day) at 3 years |
0.5 (0.3, 0.9) | 0.6 (0.3, 1.0) | 0.5 (0.1, 1.0) | NS | NS | NS |
| IGF-1 SDS at 3 years | 0.3 (−3.1, 1.9) | −0.4 (−3.0, 1.3) | −0.3 (−1.9, 1.9) | NS | NS | NS |
| b. Effects of 3 years of GH replacement in adulthood on BMD, stratified by the gap length between growth hormone replacement in childhood and adulthood. Study subjects were classified as being non-naïve (treated continuously or being off GH replacement shorter than 6 months before enrolment into KIMS), semi-naïve (treated in childhood, but being off GH replacement longer than 12 months before KIMS entry), and true-naïve (patients never treated in childhood, who were beginning GH replacement at KIMS entry). P values shown refer to within subgroup changes over baseline. | ||||||
|---|---|---|---|---|---|---|
| Variable | Non-naïve (N = 26) |
Non-naïve P value |
Semi-naïve (N = 52) |
Semi-naïve P value |
True naïve (N = 19) |
True naïve P value |
| Absolute change in lumbar spine BMD (g/cm2) |
0.015 (−0.050, 0.120) |
0.053 | 0.040 (−0.080, 0.160) |
< 0.010 | 0.060 (−0.060, 0.170) |
0.029 |
| Percent change in lumbar spine BMD (%) |
1.59 (−3.55, 12.37) |
0.035 | 3.91 (−6.59, 16.90) |
< 0.010 | 6.00 (−6.74, 17.89) |
0.032 |
| Absolute change in femoral neck BMD (g/cm2) |
0.020 (−0.020, 0.210) |
0.011 | 0.020 (−0.100, 0.140) |
0.013 | 0.065 (−0.140, 0.150) |
0.177 (NS) |
| Percent in femoral neck BMD (%) |
2.78 (−2.08, 22.45) |
< 0.010 | 2.89 (−10.1, 14.29) |
0.015 | 7.17 (−13.5, 19.0) |
0.173 (NS) |
Data shown as median (10th and 90th percentiles). Unless otherwise noted, P values were obtained using the Wilcoxon rank sum test.
P values were obtained using the t-test.
Abbreviations: BMD: bone mineral density; sBMD: standardized bone mineral density; GHD: growth hormone deficiency; IGF-1: insulin-like growth factor 1; SDS: standard deviation score.
Data shown as median (10th and 90th percentiles). P values were obtained using the Sign rank test.
Abbreviations: BMD: bone mineral density; sBMD: standardized bone mineral density; GHD: growth hormone deficiency; IGF-1: insulin-like growth factor 1; SDS: standard deviation score.
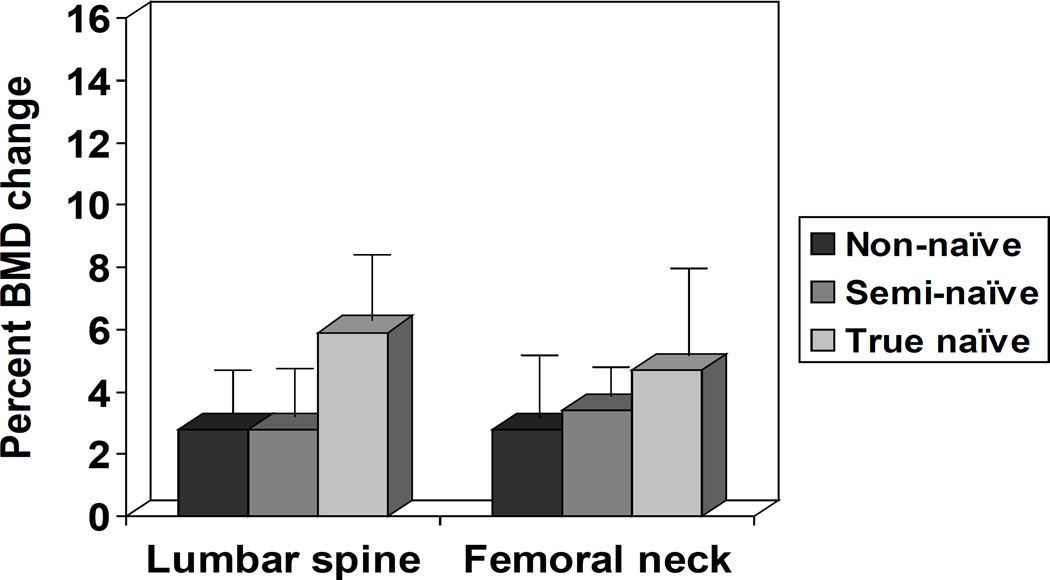
Data on the effects of GH replacement on BMD (absolute and percent changes) in the LS and FN are shown in Table 2b and Figure 3. There were significant increases in BMD in the LS of all three patient groups, including non-naïve, semi-naïve and true naïve patients. In addition, there were significant increases in BMD in the FN of non-naïve and semi-naïve subjects. However, in true naïve subjects there was a statistically non-significant increase in BMD in the FN. This finding did not change if true naïve subjects with IGF-1 SDS < −1.0 at 3 years were removed (data not shown). There were no predictors of BMD response to GH replacement identified on regression analysis (data not shown). There were no statistically significant differences between the changes (absolute or percent) in BMD (in the LS or FN) between the three groups, including non-naïve, semi-naïve and true naïve patients (P = NS).
Figure 3.
Effects of 3 years of GH replacement in adulthood on BMD (percent change over baseline), stratified by the gap length between growth hormone replacement in childhood and adulthood. Study subjects were classified as being non-naïve (treated continuously or being off GH replacement shorter than 6 months before enrolment into KIMS), semi-naïve (treated in childhood, but being off GH replacement longer than 12 months before KIMS entry), and true-naïve (patients never treated in childhood, who were beginning GH replacement at KIMS entry). There is no control (untreated) group. Data shown as mean ± SE.
Discussion
Childhood onset GHD persists in adulthood in a subset of patients, who may be at risk for developing lower BMD as a result of failure of bone mineral accrual or accelerated bone loss during delayed or interrupted GH replacement in adulthood. Such interruptions may be common among pediatric patients after the completion of statural growth, who may not continue to follow-up with their treating physician or transition their care to an adult endocrinologist 27.
The hypothesis of the present study was that interruptions in GH replacement in adulthood may be detrimental to BMD. In addition, it was hypothesized that deficiencies of other pituitary hormones, besides GH, may influence BMD, as has been shown in a population of adult onset GHD subjects 20. To facilitate direct comparisons between BMD values obtained on densitometers from different manufacturers, densitometric data were converted to sBMD to allow cross-sectional analysis of BMD data 24, 25.
The study population was defined to include COGHD subjects with persistent GHD in adulthood, based on stringent diagnostic criteria, who were stratified based on the presence and length of treatment gap (interval) length between pediatric and adult GH replacement. This stratification resulted in 3 groups, including non-naïve, semi-naïve and true naïve. In semi-naïve patients, a longer gap in GH replacement was associated with lower sBMD in the FN. In true naïve patients, a longer interval between the onset of pituitary disease and GH replacement in adulthood as well as female gender were independently associated with lower sBMD in the FN. In the LS of semi-naïve patients, thyrotropin deficiency (central hypothyroidism) was independently associated with lower sBMD.
In the subgroup of true naïve patients aged < 25 year old at study entry, a longer gap between the diagnosis of GHD and onset of GH replacement independently predicted lower sBMD in the FN. Greater height SDS at study entry independently predicted higher sBMD in the FN. In another subgroup analysis, there was no association between the gap in GH replacement and sBMD in semi-naïve patients aged < 25 yr old at study entry. However, not only was this subgroup size considerably smaller, but also the gap in GH replacement was considerably shorter than in the entire cohort. Thus, this additional analysis does not exclude the possibility that some of our patients failed to accrue bone mass during the gap period.
These findings are consistent with a relevant role of GH during bone mineral accrual in young adulthood 28, and suggest that interruptions in GH replacement during adulthood may be detrimental to bone health. It is possible that GH replacement may help prevent bone loss and preserve bone mineralization during adulthood. On subgroup analysis, we found no association between the gap in GH replacement and sBMD in semi-naïve patients < 25 yr old at study entry. However, not only was the subgroup size considerably smaller, but also the gap in GH replacement was considerably shorter. Thus, this additional analysis does not exclude the possibility that some of our patients failed to accrue bone mass during the gap period.
It is also conceivable that patients with longer gap in GH replacement between childhood and adulthood were undertreated as children, thus accounting for their lower bone density. Of note, data on pediatric GH dose replacement were not available in our study. However, to account for this possibility, we have included height SDS and duration of pediatric GH replacement in our analysis and noted that our findings remained robust to this adjustment.
As such, these observations are of clinical significance. Based on the present data, an estimate of a safe gap in GH replacement (with regards to BMD) cannot be made. However, the current findings underscore the importance of minimizing the interval in GH replacement during adulthood, which might help optimize skeletal outcomes. Taken together with previously published data suggesting that a gap in GH replacement may also be associated with less favorable systemic lipid profiles 29, our observations are consistent with the notion that interruptions in GH replacement during adulthood may be detrimental to overall health.
The mechanism mediating the association between thyrotropin deficiency and lower sBMD is not clear, and could involve a consequence of excessive or insufficient thyroid hormone replacement. It is also possible that GHD severity could be a confounder in the relationship between thyrotropin deficiency and sBMD, as the degree of thyrotropin deficiency has been associated with GHD severity 30. It may also be noted that exogenous thyroid hormone excess has been associated with lower BMD 12. As free thyroxine levels were not available in most patients, additional data are needed to clarify this association. Nevertheless, the present observations suggest that thyroid hormone replacement be carefully titrated to minimize its possible adverse effects on bone health 14, 31.
An additional objective of the present study was to examine the effects of GH replacement in COGHD subjects with persistent GHD in adulthood. This goal was considered to be of interest, as the findings of previous studies have not been consistent (Table 3) 15–18. The present study observations indicated that there was a significant increase in BMD in the LS of non-naïve, semi-naïve, and true naïve subjects after 3 years of GH replacement (without significant differences between the 3 groups), and are consistent with a relevant role of GH replacement in bone accrual in this population 27.
Table 3.
Effects of GH replacement on lumbar spine BMD (or Z score) in randomized controlled clinical trials, including childhood onset GH deficient young adults with GHD persisting in adulthood, as well as the present uncontrolled study.
| First author | Publication year |
Study population size (n) |
Study duration (months) |
Change (expressed as mean/median) over baseline in treated group |
Change (expressed as mean/median) over baseline in control group |
P value |
|---|---|---|---|---|---|---|
| Shalet SM et al 15 | 2003 | 149 | 24 | 6.1 % (BMD) | 3.1 % (BMD) | 0.027 |
| Underwood LE et al 16 | 2003 | 64 | 24 | 5.2 % (BMD) | 1.3 % (BMD) | 0.001 |
| Mauras N et al 18 | 2005 | 58 | 24 | −0.29 (Z score) | −1.08 (Z score) | 0.086 |
| Conway GS et al 17 | 2009 | 160 | 24 | 6.0 % (BMD) | 2.0 % (BMD) | < 0.001 |
| Present study | 97 | 36 | 1.59 % (non-naïve); 3.91 % (semi-naïve); 6.0 % (true-naïve)* |
Not applicable | < 0.05* |
P values for percent increase in BMD (over baseline) as follows: non-naïve (P = 0.035); semi-naïve (P < 0.010) and true naïve subjects (P = 0.032).
Abbreviations: BMD: bone mineral density; GHD: growth hormone deficiency
Broadly similar were the effects of 3 years of GH replacement on BMD in the FN, with the exception that the increase in FN BMD was not statistically significant in true naïve subjects. It may be noted that the subgroup of true naïve subjects followed prospectively was relatively small, which may have limited our ability to detect a statistically significant change in BMD or identify predictors of response to GH replacement. In addition, data on compliance with GH replacement throughout the study period were not available, nor were data on calcium and vitamin D intake, all of which could influence the effects of GH replacement on BMD.
Strengths of the present study include the stringent definition of GHD and the overall large study population. However, any study based on analysis of previously collected data has limitations, arising from the inclusion of subjects with available data, based on local clinical practice. As a result of applying stringent inclusion criteria, 10.5 % of COGHD patients in the KIMS database were eligible to participate and were included in this analysis. In addition, the current data pertain to a population of predominantly Caucasian patients and may not be applicable to patients of other racial groups. True naïve adult subjects with COGHD are rather atypical in practice, which justifies analysis of these data as a separate group in the authors’ opinion.
Another study limitation involves the lack of densitometer cross-calibration between participating centers. It may be noted that central quality control and cross-calibration procedures are generally employed in clinical trials. However, the present study represents a pharmaco-epidemiological survey of data obtained in clinical practice. To mitigate the limitations arising from the lack of cross-calibration between densitometers, we have employed the standardized BMD methodology, as already described.
In pediatric GHD populations, (areal) BMD (as measured by DXA) is influenced by patients’ height 27, 28. Our study population consisted of adult, rather than pediatric, COGHD patients. Nevertheless, to minimize any potential confounding effect of patients’ height, this variable was introduced as a potential predictor in all multivariate analyses.
It may be noted that sBMD values in true naïve patients were not significantly decreased in comparison with those in semi-naïve or non-naïve patients at study entry. However, true naïve patients were significantly older at the time of onset of pituitary disease and GHD diagnosis, and had a shorter GHD duration in comparison with the other two groups at study entry. As a consequence, it is possible that true naïve patients may have been relatively protected from the deleterious effects of untreated GHD on bone density.
The present study findings explained a relatively small proportion of the total variability in sBMD, underscoring the potential role of additional genetic, nutritional and lifestyle factors which may influence sBMD. Nevertheless, the aims of the present study were limited to the investigation of the role of the gap (interval) in GH replacement as well as endocrine variables as potential predictors of bone mineralization.
A substantial proportion of our study subjects had Z scores < −2.0 in the LS. It has been suggested that isolated GHD of childhood or adult onset is associated with normal BMD 32. However, the majority of the subjects in the present study had multiple pituitary hormone deficiencies, limiting the applicability of the conclusions of the earlier article to our study 32. Of note, there were no available data on the time of fracture in our study population. Therefore, fracture incidence could not be estimated based on our data.
In conclusion, a longer gap in GH replacement in adults with COGHD persisting in adulthood (or a longer interval between the onset of pituitary disease and GH replacement in true naïve patients) was negatively associated with bone mineralization (in FN) in this population. Thyrotropin deficiency was also associated with lower bone mineralization (in LS) in semi-naïve subjects, albeit with borderline statistical significance. Growth hormone replacement for 3 years led to significant gains in BMD in most patient groups and skeletal sites. Overall, these findings, based on data from clinical practice, affirm the role of GH in optimizing bone accrual in COGHD patients in transition to adulthood and/or maintaining bone mineral density during adulthood.
Supplementary Material
Acknowledgments
All authors of the present study would like to express their gratitude to the clinicians who provided the primary data on their patients.
KIMS is sponsored by Pfizer Inc. Drs. King, Koltowska – Häggstrom, Wajnrajch, and Peter Jönsson are full-time employees of Pfizer Inc. Drs. Tritos, Hamrahian, Greenspan, Cook and Biller were not compensated for their contributions to this manuscript.
Abbreviations
- sBMD
standardized bone mineral density
- BMI
body mass index
- DXA
dual energy X ray absorptiometry
- FN
femoral neck
- GH
growth hormone
- GHD
growth hormone deficiency
- GHRH
growth hormone releasing hormone
- IGF-1
insulin-like growth factor 1
- KIMS
Pfizer International Metabolic Study
- LS
posterior anterior lumbar spine
- SDS
standard deviation score
Footnotes
Disclosure statement: NT has been a recipient of research funding and lecture fees from Pfizer, Inc; spouse is an employee of Pfizer, Inc. AH has been a recipient of lecture fees from Novartis, Ipsen, and Pfizer, and has served as a consultant to Ipsen and Pfizer, Inc. SLG has been a recipient of research funding from Eli Lilly, Warner Chilcott, Tarsa; has consulted and served on an advisory board for Amgen and Merck. DK, MW, PJ and MKH are full-time employees of Pfizer Inc. BMKB has been a recipient of research funding from Pfizer, Eli Lilly, Novo-Nordisk and Serono; has served on an advisory board and received consulting fees from Pfizer and Novo-Nordisk.
References
- 1.Koranyi J, Svensson J, Gotherstrom G, Sunnerhagen KS, Bengtsson B, Johannsson G. Baseline characteristics and the effects of five years of GH replacement therapy in adults with GH deficiency of childhood or adulthood onset: a comparative, prospective study. J Clin Endocrinol Metab. 2001;86:4693–4699. doi: 10.1210/jcem.86.10.7896. [DOI] [PubMed] [Google Scholar]
- 2.Nicolson A, Toogood AA, Rahim A, Shalet SM. The prevalence of severe growth hormone deficiency in adults who received growth hormone replacement in childhood [see comment] Clin Endocrinol (Oxf) 1996;44:311–316. doi: 10.1046/j.1365-2265.1996.671492.x. [DOI] [PubMed] [Google Scholar]
- 3.Aimaretti G, Baffoni C, Bellone S, Di Vito L, Corneli G, Arvat E, Benso L, Camanni F, Ghigo E. Retesting young adults with childhood-onset growth hormone (GH) deficiency with GH-releasing-hormone-plus-arginine test. J Clin Endocrinol Metab. 2000;85:3693–3699. doi: 10.1210/jcem.85.10.6858. [DOI] [PubMed] [Google Scholar]
- 4.Longobardi S, Merola B, Pivonello R, Di Rella F, Di Somma C, Colao A, Ghigo E, Camanni F, Lombardi G. Reevaluation of growth hormone (GH) secretion in 69 adults diagnosed as GH-deficient patients during childhood. J Clin Endocrinol Metab. 1996;81:1244–1247. doi: 10.1210/jcem.81.3.8772606. [DOI] [PubMed] [Google Scholar]
- 5.Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84:4702–4712. doi: 10.1210/jcem.84.12.6182. [DOI] [PubMed] [Google Scholar]
- 6.Hutchison MR, Bassett MH, White PC. Insulin-like growth factor-I and fibroblast growth factor, but not growth hormone, affect growth plate chondrocyte proliferation. Endocrinology. 2007;148:3122–3130. doi: 10.1210/en.2006-1264. [DOI] [PubMed] [Google Scholar]
- 7.Ohlsson C, Nilsson A, Isaksson OG, Lindahl A. Effect of growth hormone and insulin-like growth factor-I on DNA synthesis and matrix production in rat epiphyseal chondrocytes in monolayer culture. J Endocrinol. 1992;133:291–300. doi: 10.1677/joe.0.1330291. [DOI] [PubMed] [Google Scholar]
- 8.Sims NA, Clement-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest. 2000;106:1095–1103. doi: 10.1172/JCI10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 11.Drinkwater BL, Nilson K, Chesnut CH, 3rd, Bremner WJ, Shainholtz S, Southworth MB. Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med. 1984;311:277–281. doi: 10.1056/NEJM198408023110501. [DOI] [PubMed] [Google Scholar]
- 12.Greenspan SL, Greenspan FS. The effect of thyroid hormone on skeletal integrity. Ann Intern Med. 1999;130:750–758. doi: 10.7326/0003-4819-130-9-199905040-00016. [DOI] [PubMed] [Google Scholar]
- 13.Tritos NA, Biller BM. Growth hormone and bone. Curr Opin Endocrinol Diabetes Obes. 2009;16:415–422. doi: 10.1097/MED.0b013e3283319e6d. [DOI] [PubMed] [Google Scholar]
- 14.Greenspan SL, Greenspan FS, Resnick NM, Block JE, Friedlander AL, Genant HK. Skeletal integrity in premenopausal and postmenopausal women receiving long-term L-thyroxine therapy. Am J Med. 1991;91:5–14. doi: 10.1016/0002-9343(91)90066-7. [DOI] [PubMed] [Google Scholar]
- 15.Shalet SM, Shavrikova E, Cromer M, Child CJ, Keller E, Zapletalova J, Moshang T, Blum WF, Chipman JJ, Quigley CA, Attanasio AF. Effect of growth hormone (GH) treatment on bone in postpubertal GH-deficient patients: a 2-year randomized, controlled, dose-ranging study. J Clin Endocrinol Metab. 2003;88:4124–4129. doi: 10.1210/jc.2003-030126. [DOI] [PubMed] [Google Scholar]
- 16.Underwood LE, Attie KM, Baptista J. Growth hormone (GH) dose-response in young adults with childhood-onset GH deficiency: a two-year, multicenter, multiple-dose, placebo-controlled study. J Clin Endocrinol Metab. 2003;88:5273–5280. doi: 10.1210/jc.2003-030204. [DOI] [PubMed] [Google Scholar]
- 17.Conway G, Szarras-Czapnik M, Racz K, Keller A, Chanson P, Tauber M, Zacharin M. Treatment for 24 months with recombinant human growth hormone has a beneficial effect on bone mineral density in young adults with childhood-onset growth hormone deficiency. Eur J Endocrinol. 2009;160:899–907. doi: 10.1530/EJE-08-0436. [DOI] [PubMed] [Google Scholar]
- 18.Mauras N, Pescovitz OH, Allada V, Messig M, Wajnrajch MP, Lippe B. Limited efficacy of growth hormone (GH) during transition of GH-deficient patients from adolescence to adulthood: a phase III multicenter, double-blind, randomized two-year trial. J Clin Endocrinol Metab. 2005;90:3946–3955. doi: 10.1210/jc.2005-0208. [DOI] [PubMed] [Google Scholar]
- 19.Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157:695–700. doi: 10.1530/EJE-07-0631. [DOI] [PubMed] [Google Scholar]
- 20.Tritos NA, Greenspan SL, King D, Hamrahian A, Cook DM, Jonsson PJ, Wajnrajch MP, Koltowska-Haggstrom M, Biller BM. Unreplaced sex steroid deficiency, corticotropin deficiency, and lower IGF-I are associated with lower bone mineral density in adults with growth hormone deficiency: a KIMS database analysis. J Clin Endocrinol Metab. 2011;96:1516–1523. doi: 10.1210/jc.2010-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riis P. Thirty years of bioethics: the Helsinki Declaration 1964–2003. New Rev Bioeth. 2003;1:15–25. doi: 10.1080/1740028032000131396. [DOI] [PubMed] [Google Scholar]
- 22.Filipsson H, Monson JP, Koltowska-Haggstrom M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. 2006;91:3954–3961. doi: 10.1210/jc.2006-0524. [DOI] [PubMed] [Google Scholar]
- 23.Brabant G, von zur Muhlen A, Wuster C, Ranke MB, Kratzsch J, Kiess W, Ketelslegers JM, Wilhelmsen L, Hulthen L, Saller B, Mattsson A, Wilde J, Schemer R, Kann P. Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Horm Res. 2003;60:53–60. doi: 10.1159/000071871. [DOI] [PubMed] [Google Scholar]
- 24.Genant HK, Grampp S, Gluer CC, Faulkner KG, Jergas M, Engelke K, Hagiwara S, Van Kuijk C. Universal standardization for dual x-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res. 1994;9:1503–1514. doi: 10.1002/jbmr.5650091002. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Fuerst T, Hui S, Genant HK. Standardization of bone mineral density at femoral neck, trochanter and Ward's triangle. Osteoporos Int. 2001;12:438–444. doi: 10.1007/s001980170087. [DOI] [PubMed] [Google Scholar]
- 26.Prader A, Largo RH, Molinari L, Issler C. Physical growth of Swiss children from birth to 20 years of age. First Zurich longitudinal study of growth and development. Helv Paediatr Acta Suppl. 1989;52:1–125. [PubMed] [Google Scholar]
- 27.Nguyen VT, Misra M. Transitioning of children with GH deficiency to adult dosing: changes in body composition. Pituitary. 2009;12:125–135. doi: 10.1007/s11102-008-0101-y. [DOI] [PubMed] [Google Scholar]
- 28.Baroncelli GI, Bertelloni S, Sodini F, Saggese G. Acquisition of bone mass in normal individuals and in patients with growth hormone deficiency. J Pediatr Endocrinol Metab. 2003;16(Suppl 2):327–335. [PubMed] [Google Scholar]
- 29.Koltowska-Haggstrom M, Geffner ME, Jonsson P, Monson JP, Abs R, Hana V, Hoybye C, Wollmann HA. Discontinuation of growth hormone (GH) treatment during the transition phase is an important factor determining the phenotype of young adults with non idiopathic childhood-onset GH deficiency. J Clin Endocrinol Metab. 2010;95:2646–2654. doi: 10.1210/jc.2009-2013. [DOI] [PubMed] [Google Scholar]
- 30.Jostel A, Ryder WD, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index. Clin Endocrinol (Oxf) 2009;71:529–534. doi: 10.1111/j.1365-2265.2009.03534.x. [DOI] [PubMed] [Google Scholar]
- 31.Duncan WE, Chang A, Solomon B, Wartofsky L. Influence of clinical characteristics and parameters associated with thyroid hormone therapy on the bone mineral density of women treated with thyroid hormone. Thyroid. 1994;4:183–190. doi: 10.1089/thy.1994.4.183. [DOI] [PubMed] [Google Scholar]
- 32.Hogler W, Shaw N. Childhood growth hormone deficiency, bone density, structures and fractures: scrutinizing the evidence. Clin Endocrinol (Oxf) 2010;72:281–289. doi: 10.1111/j.1365-2265.2009.03686.x. [DOI] [PubMed] [Google Scholar]
- 33.Abs R, Feldt-Rasmussen U. Growth hormone deficiency in adults: 10 years of KIMS. Oxford: Oxford PharmaGenesis™ Ltd; 2004. The KIMS 2004 Aetiology of growth hormone deficiency (KIMS classification list) pp. 346–348. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.