Abstract
The effects of differences in smoke concentration and exposure duration in Sprague Dawley rats to determine variation in type and severity of the testis apoptosis were evaluated. The daily dosages were 10, 20 and 30 non-filter cigarettes for a period of 2, 4, 6, 8 and 12 weeks. Mainstream smoke exposure suppressed body weight gain in all regimens. A dose-related increase in plasma nicotine concentration was observed in smoke-exposed groups for 4, 6, 8 and 12 week regimens. Histopathological examination of the exposed groups showed disturbances in the stages of spermatogenesis, tubules atrophying and these appeared to be dose-related. Cytoplasmic caspase-3 immunostaining was detected both in Sertoli cells and germ cells in smoke-exposure groups. An increase in TUNEL-positive cells of testicular cells was observed after 6 weeks of cigarette exposure. The results indicate that cigarette exposure concentration and duration have interaction effect to induce apoptosis in the rat testes.
Keywords: Testis, Apoptosis, Biomarkers, Dose–response, Reproductive tract, Inhalation toxicology
1. Introduction
At present, more than 1.1 billion people worldwide are exposed to cigarette smoke (CS). Every year an estimate of 5 million people die from tobacco-related diseases (Ezzati and Lopez, 2003). CS is also well known to be reproductively toxic to males. CS and its harmful components have been documented to be associated with sperm damage such as DNA strand break (Fraga et al., 1996, La Maestra et al., 2015, Marchetti et al., 2011), DNA adducts (Potts et al., 1999), chromosomal abnormality (Yauk et al., 2007) and plasma membrane integrity (Ramlau-Hansen et al., 2007). Billig et al. (1996) think that apoptosis is supposed to be an important physiologic mechanism that limits the number of germ cells in the seminiferous epithelium of testes.
Apoptosis is an active process by which cells are programed to die under a wide range of physiologic and developmental stimuli. Although apoptosis is an important physiologic mechanism that helps to maintain a balance between cell proliferation and cell death, a range of pathologic processes may stimulate it. An increase in apoptosis has been shown to occur in testis following experimental cryptorchidism (Shikone et al., 1994), hypophysectomy (Tapanainen et al., 1993) and exposure to toxic agents such as cocaine (Li et al., 1999) and chemotherapeutic agent such as mitomycin-C (Nakagawa et al., 1997).
In the area of inhalation toxicology, Haber’s rule (Gaylor, 2000) is commonly used to estimate the relationship between the exposure dosimetry and the biological effect based on the theory that an equivalent integrated dose of concentration (C) × time (t) induces a comparable toxic effect (k). However, few studies explored the interactions of exposure concentration and duration on the apoptosis of testis. Therefore, in this study, some crucial parameters were studied to determine the influence of these parameters on the apoptosis of testis when a constant c × t dose of cigarette smoke was exposed, and to determine the relationship between cigarette smoke exposure and the degree of apoptosis in testicular cells.
2. Materials and methods
2.1. Animals
Eighty adult male Sprague Dawley rats (male, 5 weeks old) were held for 1 week in quarantine status prior to initial smoke exposure. Animals were housed 5 per cage in polypropylene boxes during the course of the experiment. The animals were maintained in a temperature (21 °C) and humidity (50%) controlled room in a 12/12 h light/dark cycle and provided with unrestricted access to water and food. This protocol was performed in a contract laboratory accredited by the American Association for Accreditation of Laboratory animals (AAALAC) and approved by the Animal Ethics Committee of First Affiliated Hospital of Xinjiang Medical University (IACUC-20131105011).
2.2. Experimental procedures
Eighty rats were divided into sixteen groups and each group has five rats. The No. 1 and No. 15 groups of rats were respectively exposed to the smoke of 10, 20 and 30 nonfilter cigarettes for a period of 2, 4, 6, 8 and 12 months. The rats in No.16 group were exposed only to filtered and humidified air, which are called sham rats.
2.3. Tobacco smoke exposures
Rats were placed in a smoking machine (model TE-10; Teague Enterprises, Davis, CA, USA) which smoked the cigarettes under the Federal Trade Commission conditions (35 mL/puff, 8 puff/min, 2 s duration). The smoke was collected through a chimney and then delivered to the whole body chambers. Mainstream cigarette smoke was determined to contribute 11% of total suspended particulate matter (TSP), which was also directed into the sidestream cigarette smoke that contributed to 89% of TSP (Yu et al., 2002). Mainstream and sidestream cigarette smoke were both introduced to a conditioning chamber and left for 2–4 min to dilute and age the smoke. The smoke content in the inhaled air was determined by continuously monitoring the concentration of TSP and carbon monoxide in the chamber for 5 h/day, 5 days/week.
After tobacco smoke exposure, each rat was given 0.4 mL/100 g of sodium pentobarbital. Blood of rats was collected into ethylenediaminetetraacetic (EDTA) tubes and then centrifuged for 30 min. Aliquots of the plasma specimens were stored at – 80 °C and were ready to use in the following nicotine and cotinine concentration determination. All rats were observed daily and the general states were recorded. Body weights (BW) of rats were measured weekly since the initial day of smoke exposure.
2.4. Concentrations of nicotine and cotinine in plasma
The concentrations of nicotine and cotinine in plasma were determined using the method reported by Massadeh et al. (2009). A 2.5 mL aliquot of dichloromethane–hexane (1:1 v/v) was used for one-step extraction. Plasma samples were alkalinized with 100 μL of 2.5 M NaOH and then a 0.5 mL aliquot of plasma was added into a screw-capped tube with 100 μL of internal standard (2 ppm diphenylamine in 50% methanol). The organic layer was transferred to a new tube which contained 10 μL of glacial acetic acid, and then vortex mixed at 3500 rpm for 3 min. The organic phase was evaporated under a stream of nitrogen at 35 °C until dryness, and then was reconstituted to 100 μL with hexane. A 2 μL aliquot was injected manually into the GC–MS. The operational parameters were: injector temperature of 300 °C and transfer line temperature of 300 °C. Oven temperature was programed from 120 to 220 °C (20 °C/min), and then held for 2 min. The selected ions were: m/z 162 for nicotine and 176 for cotinine.
2.5. Immunohistochemistry analyses
Immunohistochemical analyses of anti-caspase-3 primary antibodies were performed to detect apoptosis. The formalin-fixed testes removed from the SD rats were dehydrated and embedded in paraffin according to standard protocols (Yao et al., 2010). These 4 μm paraffin sections were cut on a microtome, taken onto glass slides, and treated with poly-l-lysine. Sections were dewaxed in xylene and treated with a graded ethanol series after incubating at 37 °C overnight and 60 °C for an hour. Meanwhile, endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 15 min. After washing with deionized water and then with phosphate-buffered saline (three times, 5 min each), the sections were placed in microwave irradiation for antigen retrieval using 0.01 M citrate buffer (pH 6.0) for 12 min (700 W), and were then washed with deionized water and PBS buffer (three times, 5 min each). Samples were incubated at 4 °C with the primary antibodies (caspase-3; catalog number ab2302, abcam, Cambridge, UK) diluted (1:100 concentration) with antibody diluents (catalog number TA-125-UD Thermo Fisher Scientific, Fremont, CA, USA), and followed by washing with PBS (3 × 5 min). Samples were then incubated with secondary antibody, biotinylated goat antipolyvalent (catalog number TP-125-BN, Thermo Fisher Scientific, Fremont, CA, USA) for 20 min and washed with PBS. Finally, the sections were soaked in DAB (3,3′ diaminobenzidine) (Thermo Fisher Scientific, catalog number TA-125-HD, Fremont, CA, USA) for 10 min and stained with Harris Hematoxylin. The slides were covered with xylol-based mounting, and observed under Leica DM 4000 B light microscope (Wetzlar, Germany).
The results were analyzed by Image-Pro Plus software (version 6.0, Media Cybernetics, Silver Spring, MD) using the method introduced by Xavier (Xavier et al., 2005). Briefly, five digital images with 3254 × 2448 pixel resolution and ×400 magnification of each slice were captured on a Leica DFC320 digital camera (Leica Microsystems Digital Imaging, Cambridge, UK), which is driven by Leica DFC Twain software V.6.3.0 (Leica Microsystems Digital Imaging). Immunohistochemical parameters assessed include (a) mean stained area, (b) mean intensity of stain, and (c) mean integrated optical density (mean IOD). The optical density was calibrated and the area of interest was set as: H (hue), 0–33; S (saturation), 0–255; I (intensity), 0–255. The image was converted to gray scale image, and the values were counted.
2.6. TUNEL analysis
In situ terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) immunofluorescent staining was used for detection of DNA damage (Pradhan et al., 2013). The formalin fixed, paraffin-embedded tissues were dewaxed in xylene and rehydrated through a graded series of ethanol and double-distilled water followed by a 5 min PBS wash. Sections were placed in a plastic jar containing 200 mL 0.1 M citrate buffer, pH 6.0 and incubated for 5 min under 350 W microwave irradiation. After rinsing the slides twice with PBS, 50 μL TUNEL reaction mixture (Roche Diagnostics, Mannheim, Germany) per sample was added and slides were incubated for 60 min at 37 °C in a humidified atmosphere in the dark. The slides were then washed several times with PBS buffer and analyzed under a laser scanning confocal microscope with an excitation range of 450–500 nm (e.g., 465 nm) and detection range of 515–565 nm (green). Converter-POD was added to sections and 30 min at 37 °C followed. Labeled apoptotic cells were identified by treating the slides with DAB substrate for 10 min. Counterstaining of nuclei was performed with Immunomaster’s Hematoxylin by incubating the sections at room temperature for 7 min. For negative control, TUNEL reaction mixture was replaced with label solution (without terminal transferase).
2.7. Statistical analysis
All data were reported as mean ± standard deviation (SD), and statistical analysis was performed using SPSS vs.16.0 (SPSS, Inc., Chicago, USA). Body weight and organ weight were analyzed by ANOVA followed by Dunnett’s test. The factorial design was used to determine the influence of exposure concentration and the duration of cigarette smoke on nicotine and cotinine levels in plasma, mean stained area, mean intensity of stain, and mean integrated optical density (IOD) of caspase-3 staining in rats. A significance level of p ⩽ 0.05 was used for all comparisons.
3. Results
3.1. Weights and general status
The smoke exposure atmosphere (TMP and CO) was well controlled in this study. There was no unscheduled removal due to early death or moribund condition. Immediately following the smoke exposure, all smoke-exposed animals displayed decreased locomotor activity, ataxic gait, irregular respiration, nasal noise, and salivation. These phenomena disappeared by the next morning in early weeks of the exposure, however, after 4 weeks these signs became worse and worse. Body weights (Fig. 1) of all groups increased steadily throughout the exposure period. The smoke-exposed animals gained body weight slower than sham group, and this trend appeared to be dose-related. The 10-nonfilter cigarette groups generally had a higher body weight than the 30-nonfilter cigarette groups. The weight gains of smoke-exposed animals were significantly less than the sham group when the exposure duration is more than 2 weeks according to the results of statistical analysis. Body weight and organ weight at terminal sacrifice are shown in Table 1. The relative testis weight (testis weight/body weight) of most smoke-exposed groups was significantly higher than the sham group, and this trend appeared to be dose-related. The 30-nonfilter cigarette groups generally had a higher relative testis weight than the 10-nonfilter cigarette groups. Those changes were comparable among the equivalent exposure groups.
Figure 1.
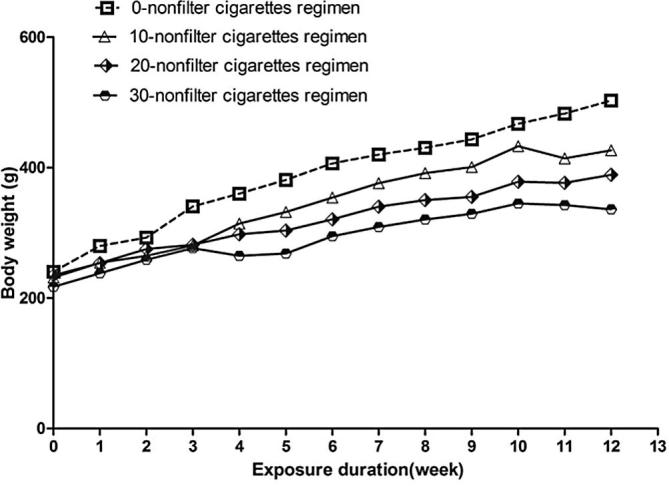
Body weight. Rats were sham exposed or were exposed to different doses of cigarettes over 12 weeks. Error bars have been omitted for clarity (mean, n = 5 per group).
Table 1.
Body and organ weights.
| Exposure duration | 2 w |
4 w |
6 w |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure concentration (cigarettes/day) | 0 | 10 | 20 | 30 | 0 | 10 | 20 | 30 | 0 | 10 | 20 | 30 |
| Final body weight (g) | 292.8 ± 17.2 | 276.0 ± 14.7 | 252.4 ± 25.9⁎ | 241.8 ± 12.7⁎⁎ | 360.2 ± 32.5 | 322.4 ± 20.2 | 274.0 ± 31.8⁎⁎ | 255.2 ± 28.4⁎⁎ | 406.4 ± 32.1 | 369.6 ± 28.7 | 299.4 ± 39.9⁎⁎ | 283.4 ± 44.3⁎⁎ |
| Testis (g) | 3.5 ± 0.3 | 2.9 ± 0.2 | 2.8 ± 0.4 | 2.6 ± 0.7 | 3.5 ± 0.3 | 3.5 ± 0.4 | 3.1 ± 0.4 | 3.0 ± 0.7 | 3.5 ± 0.3 | 3.4 ± 0.4 | 3.2 ± 0.4 | 3.0 ± 0.7 |
| Epididymis (g) | 1.3 ± 0.2 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 1.3 ± 0.2 | 1.2 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.2 |
| Prostate (g) | 0.9 ± 0.3 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.9 ± 0.3 | 0.5 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.9 ± 0.3 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Seminal vesicle (g) | 1.2 ± 0.5 | 0.7 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.3 | 1.2 ± 0.5 | 0.9 ± 0.3 | 0.7 ± 0.2 | 0.4 ± 0.1 | 1.2 ± 0.5 | 0.9 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.2 |
| Testis/BW | 0.7 ± 0.1 | 1.1 ± 0.0⁎⁎ | 1.1 ± 0.1⁎⁎ | 1.±0.3⁎⁎ | 0.7 ± 0.1 | 1.0 ± 0.1⁎⁎ | 1.1 ± 0.1⁎⁎ | 1.2 ± 0.3⁎⁎ | 0.7 ± 0.1 | 0.9 ± 0.1⁎ | 1.1 ± 0.1⁎⁎ | 1.1 ± 0.1⁎⁎ |
| Epididymis/BW | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0⁎⁎ | 0.3 ± 0.0⁎⁎ | 0.3 ± 0.1⁎⁎ | 0.3 ± 0.0 | 0.3 ± 0.0⁎⁎ | 0.3 ± 0.0⁎⁎ | 0.3 ± 0.0⁎ |
| Prostate/BW | 0.2 ± 0.1 | 0.1 ± 0.0⁎⁎ | 0.1 ± 0.0⁎⁎ | 0.1 ± 0.0⁎⁎ | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.0⁎ | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Seminal vesicle/BW | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1⁎ | 0.4 ± 0.1⁎⁎ | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 |
| Exposure duration | 8 w |
12 w |
||||||
|---|---|---|---|---|---|---|---|---|
| Exposure concentration (cigarettes/day) | 0 | 10 | 20 | 30 | 0 | 10 | 20 | 30 |
| Final body weight (g) | 430.6 ± 36.9 | 412.4 ± 36.9 | 369.0 ± 34.4⁎⁎ | 294.0 ± 19.6⁎⁎ | 503.0 ± 34.8 | 426.6 ± 16.4⁎⁎ | 389.2 ± 29.1⁎⁎ | 336.3 ± 17.2⁎⁎ |
| Testis (g) | 3.5 ± 0.3 | 3.0 ± 0.9 | 3.1 ± 0.2 | 3.0 ± 0.2 | 3.5 ± 0.3 | 3.3 ± 0.2 | 3.3 ± 0.3 | 3.0 ± 0.4 |
| Epididymis (g) | 1.3 ± 0.2 | 1.1 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.2 |
| Prostate (g) | 0.9 ± 0.3 | 0.6 ± 0.5 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.9 ± 0.3 | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.5 ± 0.2 |
| Seminal vesicle (g) | 1.2 ± 0.5 | 1.3 ± 0.3 | 1.2 ± 0.2 | 0.7 ± 0.3 | 1.2 ± 0.5 | 1.4 ± 0.4 | 1.1 ± 0.4 | 0.8 ± 0.2 |
| Testis/BW | 0.7 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.1 | 1.0 ± 0.1⁎⁎ | 0.7 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.1⁎ |
| Epididymis/BW | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.0⁎⁎ | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0⁎ | 0.3 ± 0.0⁎⁎ |
| Prostate/BW | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0⁎⁎ | 0.1 ± 0.0⁎⁎ | 0.20 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| Seminal vesicle/BW | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
The values are mean ± SE.
P ⩽ 0.05.
P ⩽ 0.01 for the corresponding sham.
3.2. Concentrations of nicotine and cotinine in plasma
The levels of nicotine and cotinine in plasma were measured as biomarkers of cigarette smoke exposure. A dose-related increase in plasma nicotine concentration (Fig.2A) was observed in smoke-exposed groups for 4, 6, 8 and 12 week regimens. The 8 week regimen groups had approximately 2–3 times higher levels than other regimen groups at equivalent daily doses. The concentration of cotinine (Fig.2B) did not show the similar results as nicotine. However, factorial design revealed a synergistic effect of exposure concentration and duration on plasma cotinine levels (F = 3.158, p < 0.01).
Figure 2.
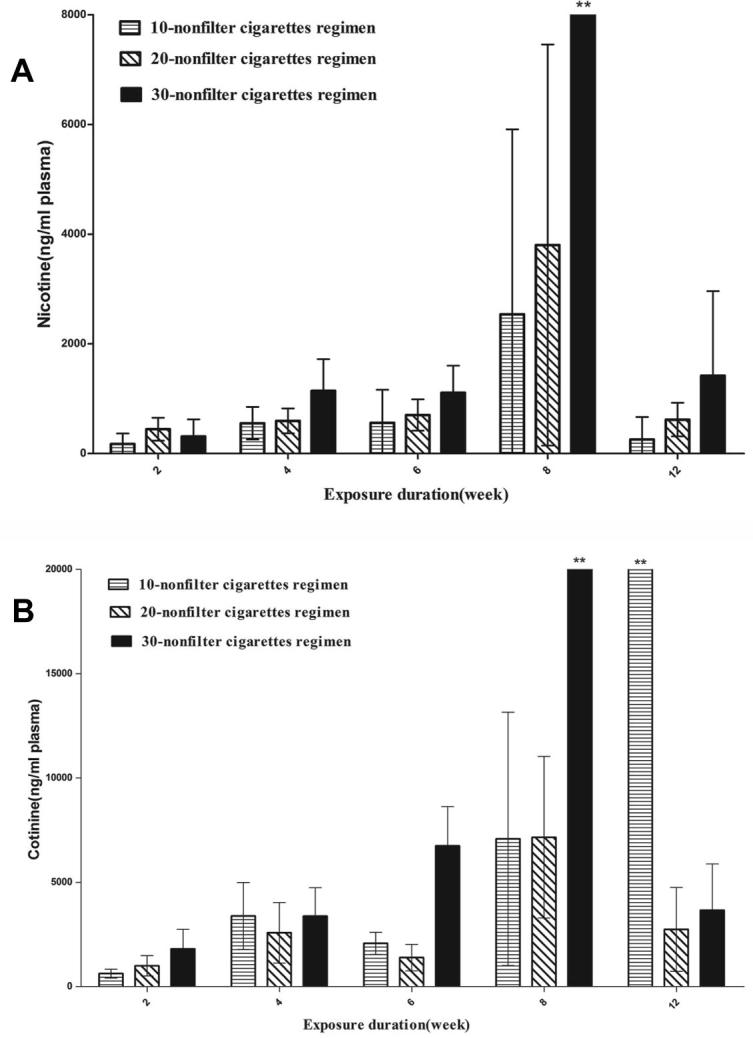
Exposure markers. Blood was collected at the end of 2, 4, 6, 8 and 12 weeks after cigarette smoke exposure, and nicotine (A) and cotinine (B) concentrations were measured in plasma. The values are mean ± SD. **P ⩽ 0.01 for the respective sham.
3.3. Histological changes
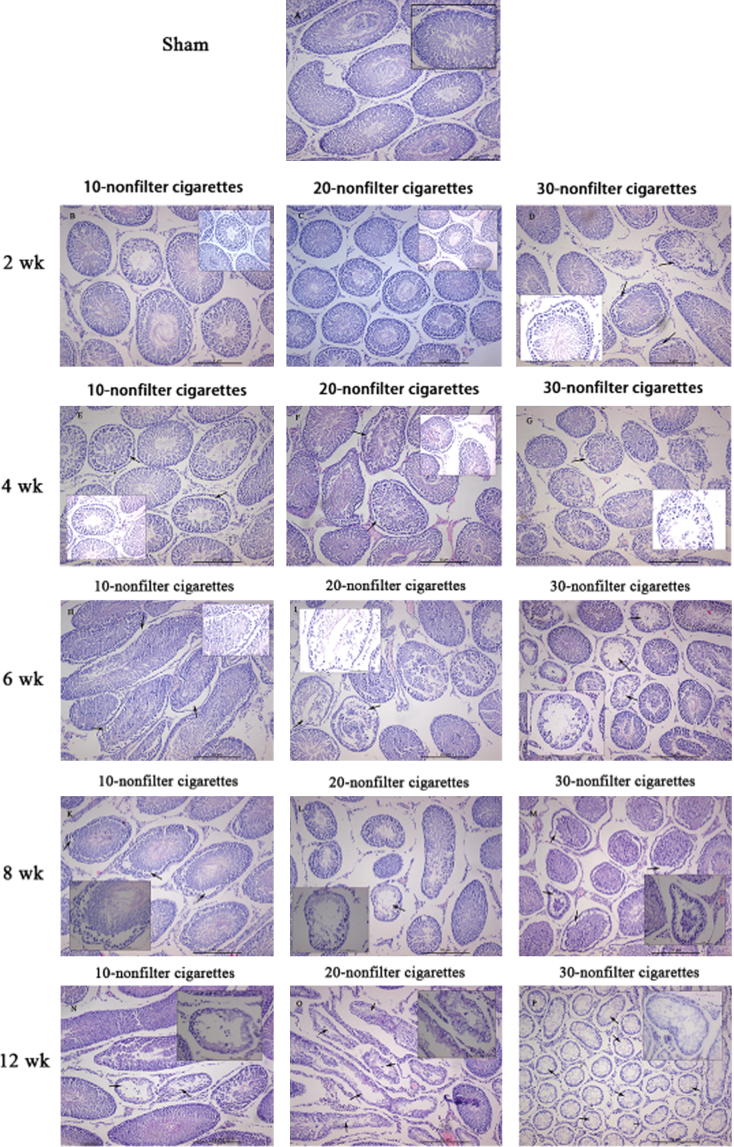
Typical histological images of animals are shown in Fig. 3. The testis of sham rats (Fig.3A) showed different stages in seminiferous elements comprising Sertoli cells, interstitial cells and germ cells, which were normal in appearance. The cigarette smoke exposed rats exhibited disturbance in diverse stages of spermatogenesis. The tunica propria and basement membrane were disrupted in a smoke dose-dependent manner in all exposure regimens. When the smoke exposure is longer than 6 weeks, apoptosis was observed in Sertoli cells, indicated by cell debris and vacuolization (Fig.3J). Decreased Leydig cells count, wider intercellular spacing and more fibroblasts in the interstitium were observed after 8 weeks of exposure (Fig.3K–M), which appeared to be dose-related. The reduction in the total count of spermatocytes, spermatogonia, spermatids, Sertoli cells and Leydig cells in the smoke-exposure groups were also observed.
Figure 3.
Morphology of cigarette smoke induced changes in the testicular tissues of rats. H&E stain for pathological assessment. (A) Testis from sham-exposed rat. (B)–(D) Section of testis from rat exposed to 10, 20, and 30 nonfilter cigarettes/day for 2 weeks. (E)–(G) Section of testis from rat exposed to 10, 20, and 30 nonfilter cigarettes/day for 4 weeks showing the disturbance in the various stages of spermatogenesis. (H)–(J) Section of testis from rat exposed to 10, 20, and 30 nonfilter cigarettes/day for 6 weeks and the arrow indicating the atrophic tubules. (K)–(M) Section of testis from rat exposed to 10, 20, and 30 nonfilter cigarettes/day for 8 weeks showing the wider spacing of intercellular. (N)–(P) Section of testis from rat exposed to 10, 20, and 30 nonfilter cigarettes/day for 12 weeks and the arrow indicating the vacuolization and cell debris of Sertoli cells. The decrease in the total count of spermatogonia, spermatocytes, spermatids, Leydig cells, and Sertoli cells was also noticed.
3.4. Caspase-3 immunohistology
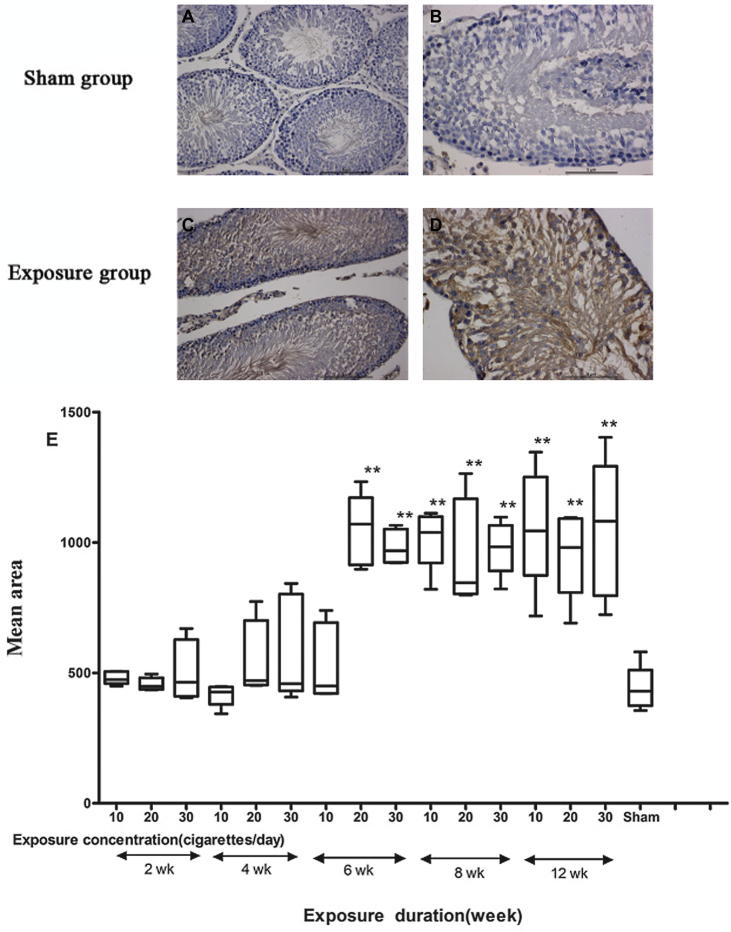
Cytoplasmic caspase-3 immunostaining was detected in the germ and Sertoli cells in smoke-exposed groups (Fig.4C and D) with immunohistochemical assays. These findings were confirmed by quantification of the mean area, which revealed a significant interaction of exposure concentration and duration (F = 3.830; p < 0.01). In comparison with the sham group, 6, 8, and 12 week regime groups showed larger expression areas (p < 0.05; Fig.4E). The interaction of exposure concentration and duration significantly affected the mean density and integrated optical density (IOD) (F = 4.113; p < 0.01; F = 7.979; p < 0.01) and most exposed-groups got a significant increase compared with sham group (p < 0.05; Fig.4F and G).
Figure 4.
Immunohistochemical analyses with caspase-3 for the detection of apoptosis. (A) and (B) Testicular tissues of sham group. (C) and (D) Testicular tissues of smoke-exposure groups. (E) Mean area, (F) mean density and (G) mean integrated optical density (IOD) of caspase-3 staining in each group. *P ⩽ 0.05, **P ⩽ 0.01 for the respective sham.
3.5. TUNEL assays
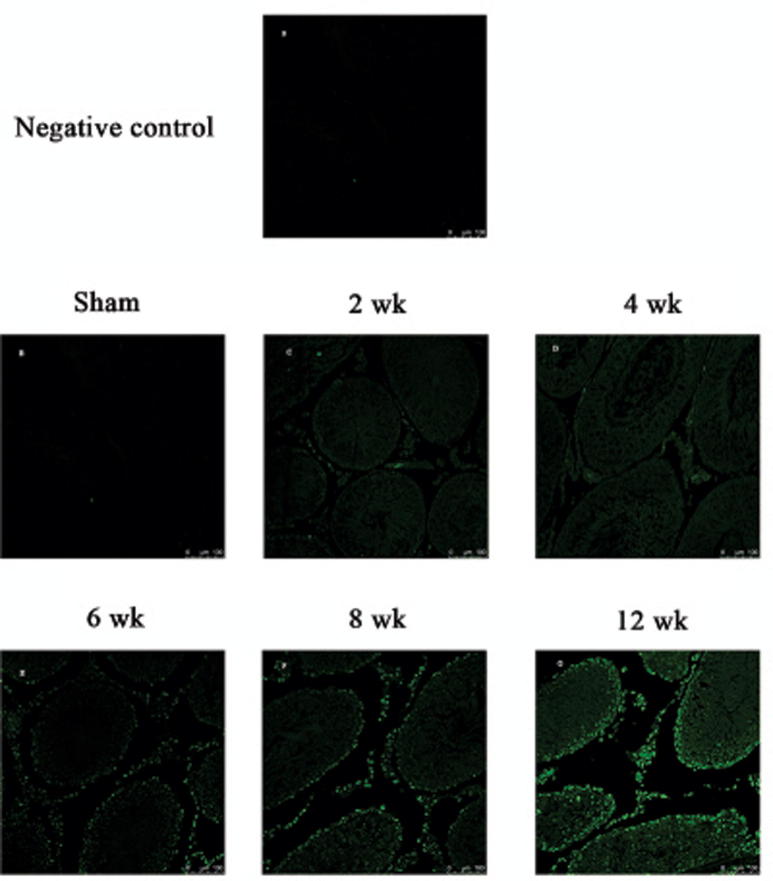
Few apoptotic cells in testicular sections of rats were detected with TUNEL assays after 2 and 4 week regimen smoke exposure (Figs. 5C, D and 6C, D). A dramatic increase in the number of apoptotic cells was observed in 6 week regimen and then again in 8 and 12 week smoke-exposed rats (Figs. 5F, G and 6F, G). Several positive cells grouped in clusters of two or more Leydig cells. These results suggested that a higher apoptosis rate of testis occurred after a 6-week cigarette smoke exposure on rats.
Figure 5.
TUNEL-stained rat testis cross-sections counterstained with hematoxylin depicting TUNEL-positive cells inside the seminiferous tubules. The arrows indicate TUNEL-positive cells. (A) negative control; (B) sham group; (C) 2 week regimens; (D) 4 week regimens; (E) 6 week regimens; (F) 8 week regimens; (G) 12 week regimens.
Figure 6.
TUNEL-stained rat testes cross-sections observed by laser scanning confocal microscope. (A) negative control; (B) sham group; (C) 2 week regimens; (D) 4 week regimens; (E) 6 week regimens; (F) 8 week regimens; (G) 12 week regimens.
4. Discussion
The present study was conducted to test Haber’s law for inhaled cigarette smoke using a variety of endpoints to assess different aspects of testis cell apoptosis. The morphological and biochemical alterations in testis were evaluated. The results indicated that there are significant differences among different smoke exposure regimens of the same total dose but different durations. For most of the analyzed parameters, higher intensity and longer exposure induced more significant effects. The data collected during the exposures confirmed that both the CO (gas/vapor) and TSP (particulate) phases of the inhalation atmospheres presented to the SD rats were well-controlled and provided appropriate data to compare the responses of the animals to smoke.
During the daily exposure period, smoke-exposed animals displayed decreased locomotor activity, ataxic gait, irregular respiration, nasal noise, and salivation. Their weight gains were reduced as the exposure concentration and duration increased, compared with the sham rats. This is consistent with previous observations from cigarette smoke inhalation studies (Bouley et al., 1975, Chowdhury, 1990) and probably a consequence of biological effects of nicotine and acrolein in cigarette smoke.
The relative organ weights (normalized to BW) of testes and epididymides at interim sacrifice in the 4, 8 and 12 week regimen groups were dose-related and significantly increased compared with sham rats, indicating that repeated smoke exposures might affect several reproductive organs in male rats.
Microscopic examination of the testes revealed decreased total counts of Leydig cells, Sertoli cells, spermatogonia, spermatocytes and spermatids. Vacuolization and cell debris of the Sertoli cells were commonly observed and significantly increased in all smoke-exposed groups. A decrease in the count of Leydig cells, wider intercellular spacing and an increase in fibroblasts in the interstitium were observed after 8 weeks of exposure and this appeared to be dose-related. These results are comparable with previous studies that focused on spermatogenesis and Sertoli cells (La Maestra et al., 2015, Richthoff et al., 2008, Shi et al., 2001). For adult mammals, spermatogenesis is a dynamic process of cell differentiation characterized by mitotic and meiotic divisions, which transform the stem spermatogonia into final mature spermatozoa. Throughout the process of development and differentiation, a considerable number of germ cells die due to apoptosis to control the overproduction of male gametes (Rodriguez et al., 1997). It is well known that the paracrine signaling between Sertoli cells regulates the process of germ cell death. In the presence of exogenous stimulants, such as cadmium, nicotine, lead, and benzopiren some adverse processes occurred which affect testicular function, nonphysiologic apoptosis and DNA fractures and lead to impaired spermatogenesis and infertility. Clinical and epidemiological studies have shown that smoking can cause a decline in sperm density, sperm mobility, and semen volume (Al-Matubsi et al., 2011, Richthoff et al., 2008). Marchetti et al. suggested that tobacco smoke might be a male germ cell mutagen (Marchetti et al., 2011). These findings suggest that chronic cigarette smoke exposure leads to germ cell death in testes possibly increasing apoptosis, besides other potential mechanisms.
Recently, apoptosis has also been reported to have an important role in spermatogenesis in human testis (Tesarik et al., 2004), and increased apoptosis has been found to occur during maturation arrest (MA), hypo-spermatogenesis, and Sertoli cell only syndrome (Lin et al., 1997, Takagi et al., 2001). After a close examination of both caspase-3-positive and TUNEL-positive cells, a significant increase of apoptosis was evidenced by caspase-3 and TUNEL immunopositivity in testes of smoke-exposed rats. A synergistic effect of exposure concentration and duration was found. We also noticed that apoptotic cells were mainly located in the center of the tubules. In other word, if any spermatogonia are present in the clusters of apoptotic cells, they would be separated from the basement membrane of the seminiferous tubules. If this is the case, the apoptotic mechanism elicited in spermatogonia might be explained by a particular mode of cell death named anoikis (Moreno et al., 2006). This type of cell death has been found in adherent cells and is triggered by the separation from extracellular matrix. A similar mechanism might thus be involved in the apoptosis of germ cells of smoke-exposed SD rats.
5. Conclusion
CS imposed significant toxic effects on testis. This study demonstrated that the extent of testis apoptosis following cigarette smoke inhalation depends not only on exposure concentration but also on exposure duration. The evaluation of countermeasures against smoke-induced testis injury should be performed in multiple types of exposure scenarios.
Funding
This work was supported by the Nature Science Foundation of China [Grant No.: 81260433] and the Natural Science Foundation of Xinjiang Uygur Autonomous Region [Grant No.: 2014211C015].
Acknowledgments
We are grateful to Prof. Liu, Department of Occupational and Environmental Health, School of Public Health, Xinjiang Medical University, for her helpful discussion and suggestions.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Matubsi H.Y., Kanaan R.A., Hamdan F., Salim M., Oriquat G.A., Al H.O. Smoking practices in Jordanian people and their impact on semen quality and hormonal levels among adult men. Cent. Eur. J. Public Health. 2011;19:54–59. doi: 10.21101/cejph.a3629. [DOI] [PubMed] [Google Scholar]
- Billig H., Chun S.Y., Eisenhauer K., Hsueh A.J. Gonadal cell apoptosis: hormone-regulated cell demise. Hum. Reprod. Update. 1996;2:103–117. doi: 10.1093/humupd/2.2.103. [DOI] [PubMed] [Google Scholar]
- Bouley G., Dubreuil A., Godin J., Boudene C. Effects in the rat of a weak dose of acrolein inhaled continuously. Eur. J. Toxicol. Environ. Hyg. 1975;8:291–297. [PubMed] [Google Scholar]
- Chowdhury P. Endocrine and metabolic regulation of body mass by nicotine: role of growth hormone. Ann. Clin. Lab. Sci. 1990;20:415–419. [PubMed] [Google Scholar]
- Ezzati M., Lopez A.D. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362:847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- Fraga C.G., Motchnik P.A., Wyrobek A.J., Rempel D.M., Ames B.N. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat. Res. 1996;351:199–203. doi: 10.1016/0027-5107(95)00251-0. [DOI] [PubMed] [Google Scholar]
- Gaylor D.W. The use of Haber’s law in standard setting and risk assessment. Toxicology. 2000;149:17–19. doi: 10.1016/s0300-483x(00)00228-6. [DOI] [PubMed] [Google Scholar]
- La Maestra S., De Flora S., Micale R.T. Effect of cigarette smoke on DNA damage, oxidative stress, and morphological alterations in mouse testis and spermatozoa. Int. J. Hyg. Environ. Health. 2015;218:117–122. doi: 10.1016/j.ijheh.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Li H., Jiang Y., Rajpurkar A., Dunbar J.C., Dhabuwala C.B. Cocaine induced apoptosis in rat testes. J. Urol. 1999;162:213–216. doi: 10.1097/00005392-199907000-00070. [DOI] [PubMed] [Google Scholar]
- Lin W.W., Lamb D.J., Wheeler T.M., Abrams J., Lipshultz L.I., Kim E.D. Apoptotic frequency is increased in spermatogenic maturation arrest and hypospermatogenic states. J. Urol. 1997;158:1791–1793. doi: 10.1016/s0022-5347(01)64130-2. [DOI] [PubMed] [Google Scholar]
- Marchetti F., Rowan-Carroll A., Williams A., Polyzos A., Berndt-Weis M.L., Yauk C.L. Sidestream tobacco smoke is a male germ cell mutagen. Proc. Natl. Acad. Sci. U.S.A. 2011;108:12811–12814. doi: 10.1073/pnas.1106896108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massadeh A.M., Gharaibeh A.A., Omari K.W. A single-step extraction method for the determination of nicotine and cotinine in Jordanian smokers’ blood and urine samples by RP-HPLC and GC–MS. J. Chromatogr. Sci. 2009;47:170–177. doi: 10.1093/chromsci/47.2.170. [DOI] [PubMed] [Google Scholar]
- Moreno R.D., Lizama C., Urzua N., Vergara S.P., Reyes J.G. Caspase activation throughout the first wave of spermatogenesis in the rat. Cell Tissue Res. 2006;325:533–540. doi: 10.1007/s00441-006-0186-4. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Nakamura N., Fujioka M., Mori C. Spermatogenic cell apoptosis induced by mitomycin C in the mouse testis. Toxicol. Appl. Pharmacol. 1997;147:204–213. doi: 10.1006/taap.1997.8246. [DOI] [PubMed] [Google Scholar]
- Potts R.J., Newbury C.J., Smith G., Notarianni L.J., Jefferies T.M. Sperm chromatin damage associated with male smoking. Mutat. Res. 1999;423:103–111. doi: 10.1016/s0027-5107(98)00242-5. [DOI] [PubMed] [Google Scholar]
- Pradhan P.M., Niraula S.R., Ghimire A., Singh S.B., Pokharel P.K. Tobacco use and associated factors among adolescent students in Dharan, Eastern Nepal: a cross-sectional questionnaire survey. BMJ Open. 2013;3:1–7. doi: 10.1136/bmjopen-2012-002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlau-Hansen C.H., Thulstrup A.M., Aggerholm A.S., Jensen M.S., Toft G., Bonde J.P. Is smoking a risk factor for decreased semen quality? A cross-sectional analysis. Hum. Reprod. 2007;22:188–196. doi: 10.1093/humrep/del364. [DOI] [PubMed] [Google Scholar]
- Richthoff J., Elzanaty S., Rylander L., Hagmar L., Giwercman A. Association between tobacco exposure and reproductive parameters in adolescent males. Int. J. Androl. 2008;31:31–39. doi: 10.1111/j.1365-2605.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez I., Ody C., Araki K., Garcia I., Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997;16:2262–2270. doi: 10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., Ko E., Barclay L., Hoang T., Rademaker A., Martin R. Cigarette smoking and aneuploidy in human sperm. Mol. Reprod. Dev. 2001;59:417–421. doi: 10.1002/mrd.1048. [DOI] [PubMed] [Google Scholar]
- Shikone T., Billig H., Hsueh A.J. Experimentally induced cryptorchidism increases apoptosis in rat testis. Biol. Reprod. 1994;51:865–872. doi: 10.1095/biolreprod51.5.865. [DOI] [PubMed] [Google Scholar]
- Takagi S., Itoh N., Kimura M., Sasao T., Tsukamoto T. Spermatogonial proliferation and apoptosis in hypospermatogenesis associated with nonobstructive azoospermia. Fertil. Steril. 2001;76:901–907. doi: 10.1016/s0015-0282(01)02732-7. [DOI] [PubMed] [Google Scholar]
- Tapanainen J.S., Tilly J.L., Vihko K.K., Hsueh A.J. Hormonal control of apoptotic cell death in the testis: gonadotropins and androgens as testicular cell survival factors. Mol. Endocrinol. 1993;7:643–650. doi: 10.1210/mend.7.5.8316250. [DOI] [PubMed] [Google Scholar]
- Tesarik J., Ubaldi F., Rienzi L., Martinez F., Iacobelli M., Mendoza C., Greco E. Caspase-dependent and -independent DNA fragmentation in Sertoli and germ cells from men with primary testicular failure: relationship with histological diagnosis. Hum. Reprod. 2004;19:254–261. doi: 10.1093/humrep/deh081. [DOI] [PubMed] [Google Scholar]
- Xavier L.L., Viola G.G., Ferraz A.C., Da C.C., Deonizio J.M., Netto C.A., Achaval M. A simple and fast densitometric method for the analysis of tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta and in the ventral tegmental area. Brain Res. Brain Res. Protoc. 2005;16:58–64. doi: 10.1016/j.brainresprot.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Yao C.J., Xu W.J., Gong X.L., Zhou Y., Yan Z.Q., Zhu Z.J., Wang Z.X., Li Q.L., Guo X.B., Wang L.Y., Ma D., Qiao Z.D. The role of Dby mRNA in early development of male mouse zygotes. Asian J. Androl. 2010;12:567–577. doi: 10.1038/aja.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauk C.L., Berndt M.L., Williams A., Rowan-Carroll A., Douglas G.R., Stampfli M.R. Mainstream tobacco smoke causes paternal germ-line DNA mutation. Cancer Res. 2007;67:5103–5106. doi: 10.1158/0008-5472.CAN-07-0279. [DOI] [PubMed] [Google Scholar]
- Yu M., Pinkerton K.E., Witschi H. Short-term exposure to aged and diluted sidestream cigarette smoke enhances ozone-induced lung injury in B6C3F1 mice. Toxicol. Sci. 2002;65:99–106. doi: 10.1093/toxsci/65.1.99. [DOI] [PubMed] [Google Scholar]