Abstract
Zinc oxide (ZnO) has broad applications in various areas. Nanoparticle synthesis using plants is an alternative to conventional physical and chemical methods. It is known that the biological synthesis of nanoparticles is gaining importance due to its simplicity, eco-friendliness and extensive antimicrobial activity. Also, in this study we report the synthesis of ZnO nanoparticles using Trifolium pratense flower extract. The prepared ZnO nanoparticles have been characterized by UV–Vis absorption spectroscopy, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), and scanning electron microscopy (SEM) with Energy dispersive X-ray analysis (EDX). Besides, this study determines the antimicrobial efficacy of the synthesized ZnO nanoparticles against clinical and standard strains of S. aureus and P. aeruginosa and standard strain of E. coli.
Keywords: ZnO nanoparticles, Plant, Antibacterial activity
1. Introduction
The synthesis of nanoparticles has been considered as the preference field in the nanotechnology sector due to the properties of materials based on size (Prakasham et al., 2014). Nowadays, the green synthesis of metal nanoparticles is an interesting issue of nanoscience. Also, there is growing attention to the biosynthesis of metal nanoparticles using organisms. Among these organisms, plants seem to be the best candidates and they are suitable for the large-scale biosynthesis of nanoparticles. Nanoparticles produced by plants are more stable and more varied in shape and size in comparison with those produced by other organisms (Ramesh et al., 2014). Metal oxides with nanostructure have attracted considerable interest in many areas of technology (Sangeetha et al., 2011). Among metal oxide nanoparticles, zinc oxide (ZnO) has received much attention in the recent past. ZnO nanostructures are the forefront of research due to their unique properties and wide applications (Rouhi et al., 2013). There are different methods used for the synthesis of zinc oxide nanoparticles: direct precipitation, homogeneous precipitation, solvothermal method, sonochemical method, reverse micelles, sol gel method, hydrothermal, thermal decomposition, and microwave irradiation (Kolekar et al., 2013). The biological method of the synthesis of ZnO nanoparticles is gaining importance due to its simplicity, eco-friendliness and extensive antimicrobial activity (Gunalan et al., 2012). According to Mahanty et al., 2013, the use of eco-friendly biosynthesized nanoparticles as an alternative to the chemically synthesized ones would help control chemical toxicity in the environment.
Based on the literature, the synthesis of ZnO nanoparticles using plants has been carried out using milky latex of Calotropis procera, Aloe vera extract (Salam et al., 2014), Ocimumbasilicum L. var. Purpurascens, Parthenium hysterophorus L. (Rajiv et al., 2013), Citrus aurantifolia extract (Samat and Nor, 2013), Plectranthus amboinicus (Vijayakumar et al., 2015). Moreover, the synthesis of ZnO nanoparticles using orange juice was reported by Jha et al., 2011. Yuvakkumar et al., 2015 used Nephelium lappaceum L. (rambutan) peel extract in the biosynthesis of zinc oxide nanocrystals. The advantage of using ZnO nanoparticles is that they strongly inhibit the action of pathogenic microbes when used in small concentration (Applerot et al., 2009). On the basis of the literature, it is known that ZnO demonstrates significant growth inhibition of a broad spectrum of bacteria. Lakshmi et al., 2012 studied the antibacterial activity of ZnO nanoparticles, namely, clinical isolates: Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, and Staphylococcus aureus. Divyapriya et al., 2014 synthesized ZnO nanoparticles using the ethanol extract of Murraya Koenigii. The results indicated that ZnO nanoparticles showed effective antibacterial activity against Gram negative and Gram positive bacteria. Gunalan et al., 2012 showed that the green synthesized ZnO has a stronger inhibitory effect than chemically synthesized nanoparticles. This study used the following bacterial strains: S. aureus, Serratia marcescens, Proteus mirabilis, Citrobacter freundii, and fungal strains: Aspergillus flavus, Aspergillus nidulans, Trichoderma harzianum, and Rhizopus stolonifer.
In this study, ZnO nanoparticles were synthesized using Trifolium pratense flower water extract. T. pratense L. (family: Leguminosae, section: Trifolium) contains anthocyanins, phenolic acids and small amounts of tannins, carotene, essential oils and vitamins C and E. T. pratense L. contains high levels of estrogenic isoflavones (genistein, daidzein, biochanin A and formononetin (Liu et al., 2001, Burdette et al., 2002). These isoflavones have cardiovascularprotective, cutaneoprotective and osteoprotective effects. Many of these effects of isoflavones have been considered to be related to their estrogenic or antioxidant activities (Occhiuto et al., 2009).
2. Materials and methods
2.1. UV–Vis spectra analysis
The sample was measured for its maximum absorbance using UV–Vis spectrophotometry. The optical property of ZnO nanoparticles was analyzed via ultraviolet and visible absorption spectroscopy (spectrophotometer Cary E 500) in the range of 280–800 nm.
2.2. Scanning electron microscopy (SEM)
The morphology of ZnO nanoparticles was examined by means of scanning electron microscopy (SU3500, Hitachi with spectral imaging system Thermo Scientific NSS (EDS), the tape of detector (BSE-3D), acceleration voltage (15.0 kV), working distance (11.6 mm), pressure (in the case of variable vacuum conditions) (40 Pa).
2.2.1. Fourier transform infra-red spectroscopy (FT-IR)
The binding properties of ZnO nanoparticles using T. pratense extract were investigated by FTIR analysis. The characterization involved Fourier transform infrared spectroscopy (FTIR) analysis of the dried powder of the synthesized ZnO nanoparticles by Perkin Elmer Spectrum 1000 spectrum in attenuated total reflection mode, and using the spectral range of 4000–400 cm−1 with the resolution of 4 cm−1.
2.2.2. Total reflection X-ray fluorescence analysis (TXRF)
The presence of ZnO nanoparticles in water extract of T. pratense was confirmed using the X-ray fluorescence spectrometer Bruker S2 TXRF Picofox, operated at 50 kV and 600 μA.
2.2.3. X-ray diffraction (XRD)
X-ray diffraction (XRD) studies of nanoparticles were carried out using BRUKER D8 ADVANCE brand ∗-2∗ configuration (generator-detector) X-ray tube copper S = 1.54 A and LYNXEYE PDS detector. The estimation of the size of particles was performed by Scherrer’s formula.
2.2.4. Antibacterial activity study
The antimicrobial activity of ZnO was evaluated against standard and clinical strain of Gram-positive and Gram-negative bacteria (standard strains: S. aureus ATCC 4163, E. coli ATCC 25922, P. aeruginosa ATCC 6749; clinical strains: S. aureus and P. aeruginosa) by the agar well diffusion method.
The wells of 8 mm diameter were punched into the Mueller–Hinton Agar (MHA, bioMerieux) having the test microorganism (at concentration about 5 × 105 CFU/ml). The wells were filled with 100 μl of ZnO at the concentration of 1028 μg/mL, 516 μg/mL and 256 μg/mL and 125 μg/mL. Gentamicin was used as the control. The plates were incubated for 18 h at 35° ± 1 °C. Antimicrobial activity was evaluated by measuring the inhibition zone against the test microorganisms.
2.2.5. Plant collection
Fresh and healthy flowers of T. pratense were collected from Wielkopolska region (Poland).
2.2.6. Preparation of plant extracts
The flowers of Trifolium pretense were washed twice with distilled water and air-dried for 5 days at room temperature. To 4.5 g powder of T. pratense flower, 200 ml of double distilled water was added. Then, the solution was stirred for 45 min in the temperature of 80 °C.
2.2.7. Synthesis of ZnO nanoparticles
The water extract was used for the synthesis of ZnO nanoparticles. Also, 30 ml extract was added to 30 ml 0.5 M ZnO. The solution was stirred for 4 h maintaining the temperature at 90 °C. After this time, the temperature was reduced to 30 °C. The solution was stirred for 24 h. The color of the solution was yellow. Then, the powdered annealing of remnants was carried out in a muffle furnace at 400 °C for 1 h. The white powder was obtained.
2.3. Statistical analysis
The inhibition zone diameter data were analyzed using one way analysis of variance (ANOVA). The differences were considered significant at P value < 0.05.
3. Results and discussion
3.1. UV–Vis
The optical absorption spectra of ZnO dispersed in water were recorded using UV–Vis Spectrophotometer Cary E 500. UV spectra were measured at room temperature in a quartz cuvette with the path length of 1 cm. It is known that UV–Vis spectroscopy is the most widely used technique for the structural characterization of nanoparticles. Also, the absorbance of the reaction mixture was monitored after 24 h of reaction. Moreover, the absorbance measurements were performed after 48, 72, 96 and 120 h after the solution of ZnO nanoparticles was prepared. Fig. 1 shows the UV–Vis absorption spectrum of ZnO nanoparticles sample at different times. Typical exciton absorption at 283 nm was observed at room temperature. According to Gupta et al., 2014, the absorption edge systematically shifts to the lower wavelength or higher energy with the decreasing size of the nanoparticle.
Figure 1.
The UV–Vis absorption spectra for ZnO nanoparticles during 120 h.
3.2. Fourier transform infra-red spectroscopy (FT IR)
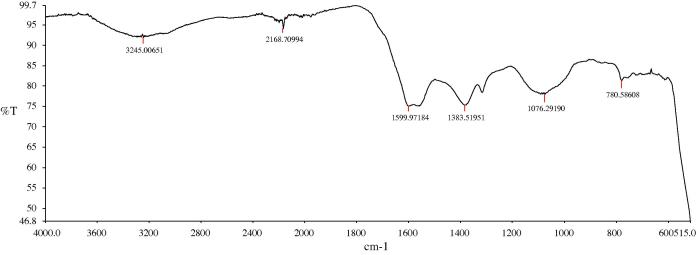
The results were further reinforced by FT-IR analysis, which showed the shifts and difference in areas of the peaks. FT-IR spectroscopy consists in measuring the absorption of IR radiations by a sample, and the results of such measurement are shown by means of a wavelength. The reading of the IR spectrum includes the interpretation of the interdependence between the absorption bands (vibrational bands) and the chemical compounds in the sample. By means of this technique, it is possible to identify the biomolecules in plant extracts which play the crucial role in the processes of reduction and stabilisation of the green synthesis of nanoparticles (Senthilkumar and Sivakumar, 2014).
Fig. 2 shows the IR spectrum of the sample dried at 400 °C for 1 h. According to Fig. 2, it is observed that the bands are at 3245 cm−1, 2168 cm−1, 1383 cm−1, 1599 cm−1, 1076 cm−1, 780 cm−1 and 515 cm−1. The FTIR spectrum of ZnO nanoparticles was recorded in the range of 500–4000 cm−1. The peak in the region between 400 and 600 cm−1 is allotted to Zn–O (Yuvakkumar et al., 2015). Also, the band located near 515 cm−1 is assigned to ZnO stretching vibration. The bands at 3245 cm−1 and 1599 cm−1 are characteristic for hydroxyl group (O–H). The peaks at 1383 cm−1 and 1076 cm−11 may be ascribed to –C–O and –C–O–C stretching modes. The band which appeared at 2168 cm−1 is due to C C stretching.
Figure 2.
FT-IR spectra of synthesized ZnO nanoparticles using Trifolium pratense flower water extract.
3.3. Total reflection X-ray fluorescence analysis (TXRF)
The elemental analysis of the sample used X-ray fluorescence (XRF). The sample was determined by the X-ray fluorescence spectrometer TXRF Bruker S2 Picofox, operated at 50 kV and 600 μA. The TXRF spectrum, shown in Fig. 3, presents the clear elemental composition profile of the synthesized ZnO nanoparticles. The intense signal at 8.63 keV strongly suggests that ZnO nanoparticles were the major elements of Trifolium pratense flower water extract.
Figure 3.
TXRF spectrum of ZnO nanoparticles synthesized using Trifolium pratense flower water extract.
3.4. Scanning electron microscopy (SEM) and EDS analysis
The morphology of the nanostructures was studied using scanning electron microscopy (SEM). Fig. 4a and c present the SEM images of the obtained ZnO nanoparticles. The synthesized ZnO nanoparticles were agglomerated with a particle size ranging from below 100–190 nm. The numbers 1, 2, 3, 4, 5 indicate the points in which the measurement was made. To gain further insight into the features of ZnO nanoparticles, the analysis of the sample was performed using EDS techniques. The energy dispersive spectra of the samples obtained from the SEM-EDS analysis show that the sample prepared by the above route has pure ZnO phases (Kumar et al., 2013). The EDS studies of Fig. 4b and d present three peaks between 1 kV and 10 kV. Those maxima are directly related to zinc in the tested material. The results indicated that the reaction product was composed of high purity zinc nanoparticles. Additionally, the presence of highly pure ZnO is confirmed by X-ray diffraction XRD (Fig. 5).
Figure 4.
Scaning electron microscopy (a, c) and EDS profile (b, d) of ZnO nanoparticles synthesized using Trifolium pratense flower water extract.
Figure 5.
XRD patterns of synthesized ZnO nanoparticles synthesized using Trifolium pratense flower water extract.
3.5. XRD analysis
X-ray diffraction was taken to further confirm the zinc oxide phase of the nanoparticles. The XRD pattern of zinc oxide nanoparticles is shown in Fig. 5. The XRD peaks were identified as (1 0 0), (0 0 2), (1 0 1), (0 1 2), (1 1 0), (0 1 3), (2 0 0), (1 1 2), (0 0 4) and (2 0 2). The narrow and strong diffraction peaks indicate the well crystalline nature of zinc oxide. The size of ZnO nanoparticles was obtained by Debye–Scherrer’s formula given by the equation:
where:
D – the crystal size,
λ – the wavelength of the X-ray radiation (λ = 0.15406 nm) for CuKα,
K – usually taken as 0.89,
β – the line width at half-maximum height (Vijayalakshmi and Rajendran, 2012).
The Scherrer formula was used to calculate the particle sizes and was found to be in the range of 60–70 nm. XRD study confirmed the presence of even smaller particles than the SEM examination. The larger nanoparticles of ZnO (about 190 nm) in the sample result from the agglomeration of smaller nanoparticles, whose presence is confirmed by X-ray diffraction (XRD). The XRD method allowed for the identification of smaller sizes of nanoparticles. The agglomeration of smaller nanoparticles occurs due to the fact that we are dealing with biological material.
3.6. Antimicrobial activity
The antibacterial activity of ZnO nanoparticles was evaluated by measuring the zone of inhibition against the test organisms. The sizes of the zones of growth inhibition are presented in Table 1. The results indicated that ZnO nanoparticles synthesized from T. pratense flower extract showed effective antibacterial activity against all tested strains. Generally, the results showed that that the inhibitory effect of ZnO increased with the increase in concentration (P < 0.05). The results for the biologically synthesized ZnO were comparable to the results obtained for gentamicin (P > 0.05). ZnO nanoparticles synthesized using T. pratense flower extract were better against P. aeruginosa than gentamicin. In the case of the strains of S. aureus ATCC 4163, E. coli ATCC 25922, P. aeruginosa ATCC 6749, S. aureus, the sizes of the zones were similar. It is known that gentamicin is a compound received in chemical synthesis. Moreover, the antibacterial activity of gentamicin is famous. The presented ZnO nanoparticles are compounds obtained by using biological material. In addition, ZnO nanoparticles show similar efficacy.
Table 1.
Antimicrobial activity of ZnO nanoparticles.
| Microorganisms | Zones of growth inhibition [mm] at concentration [μg/ml] |
|||||||
|---|---|---|---|---|---|---|---|---|
| ZnO |
Gentamicin |
|||||||
| 1280 | 516 | 256 | 125 | 1028 | 516 | 256 | 128 | |
| S. aureus ATCC 4163 | 31 | 30 | 26 | 22 | 31 | 30 | 28 | 27 |
| E. coli ATCC 25922 | 31 | 29 | 26 | 22 | 31 | 29 | 28 | 27 |
| P. aeruginosa ATCC 6749 | 28 | 24 | 26 | 21 | 23 | 20 | 29 | 26 |
| S. aureus | 31 | 24 | 27 | 28 | 31 | 25 | 29 | 28 |
| P. aeruginosa | 29 | 26 | 20 | 14 | 21 | 17 | 13 | 10 |
According to Divya et al., 2013, ZnO nanoparticles cause disruption of bacterial membranes probably by the production of reactive oxygen species, such as superoxide and hydroxyl radicals. Moreover, ZnO nanoparticles have positive zeta potential at their surface. This depends on the nature of the surface of different bacteria. Moreover, the antibacterial activity is reported to be dependent on the concentration of ZnO nanoparticles and the impact of the type of surfactant used. Also, ZnO nanoparticles could be attributed to the damage of the bacterial cell membrane and extrusion of the cytoplasmic contents thereby resulting in the death of the bacterium. On the basis of the research, it can be concluded that the inhibition of bacterial growth by ZnO nanoparticles could be attributed to the damage of the bacterial cell membrane and the extrusion of the cytoplasmic contents thereby resulting in the death of the bacterium (Divyapriya et al., 2014).
4. Conclusion
It is known that the green synthesis of ZnO nanoparticles is much safer and environmentally friendly as compared to chemical synthesis. In response to this assumption, this study demonstrates the green synthesis of ZnO nanoparticles using T. pratense flower water extract. The synthesized ZnO nanoparticles were characterized by UV–Vis absorption spectroscopy, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM) and Energy dispersive X-ray analysis (EDX). These methods confirmed the presence of the synthesized ZnO nanoparticles in the range of 60–70 nm. The larger nanoparticles of ZnO resulted from the agglomeration of smaller nanoparticles. Moreover, the synthesized ZnO nanoparticles exhibited high activity against S. aureus ATCC 4163, E. coli ATCC 25922, P. aeruginosa ATCC 6749, S. aureus and P. aeruginosa. Also, the green synthesis of ZnO nanoparticles using T. pratense flower water extract can be an alternative to chemical methods.
5. Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The research on the biosynthesis of ZnO nanoparticles using Trifolium pratense extract was carried out thanks to the laboratory of Adam Mickiewicz University Foundation in Poznań, established as a part of the project WND-POIG.05.01.00-00-058/2011 “Waste Cluster – raising the standards of waste management using new technologies”. The project is co-financed by the European Union from the European Regional Development Fund.
Footnotes
Peer review under responsibility of King Saud University.
References
- Applerot, Wiberg, E, Holleman A.F., 2009. Inorganic Chemistry. Elsevier 22: 24–34 Brayner, Basu, 2006. Elsevier 1225–1229.
- Burdette J.E., Liu J., Lantvit D., Lim E., Booth N., Bhat K.P., Hedayat S., Van Breemen R.B., Constantinou A.I., Pezzuto J.M., Farnsworth N.R., Bolton J.L. Trifolium pratense (red clover) exhibits estrogenic effects in vivo in ovariectomized Sprague-Dawley rats. J. Nutr. 2002;132(174):27–30. doi: 10.1093/jn/132.1.27. [DOI] [PubMed] [Google Scholar]
- Divya M.J., Sowmia C., Joona K., Dhanya K.P. Synthesis of zinc oxide nanoparticle from Hibiscus rosa-sinensis leaf extract and investigation of its antimicrobial activity. Res. J. Pharm. Biol. Chem. 2013;4(2):1137–1142. [Google Scholar]
- Divyapriya S., Sowmia C., Sasikala S. Synthesis of zinc oxide nanoparticles and antimicrobial activity of Murraya Koenigii. World J. Pharm. Pharm. Sci. 2014;3(12):1635–1645. [Google Scholar]
- Gunalan S., Sivaraj R., Rajendran V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012;22(6):693–700. org/10.1016/j.pnsc.2012.11.015. [Google Scholar]
- Gupta A., Srivastava P., Bahadur L., Amalnerkar D.P., Chauhan R. Comparison of physical and electrochemical properties of ZnO prepared via different surfactant-assisted precipitation routes. Appl. Nanosci. 2014 doi: 10.1007/s13204-014-0379-1. [DOI] [Google Scholar]
- Kolekar T.V., Bandgar S.S., Shirguppikar S.S., Ganachari V.S. Synthesis and characterization of ZnO nanoparticles for efficient gas sensors. Arch. Appl. Sci. Res. 2013;5(6):20–28. [Google Scholar]
- Kumar S.S., Venkateswarlu P., Rao V.R., Rao G.N. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 2013;3:30. doi: 10.1186/2228-5326-3-30. [DOI] [Google Scholar]
- Jha A.K., Kumar V., Prasad K. Biosynthesis of metal and oxide nanoparticles using orange juice. J. Bionanosci. 2011;5(2):162–166. doi: 10.1166/jbns.2011.1053. [DOI] [Google Scholar]
- Lakshmi J.V., Sharath R., Chandraprabha M.N., Neelufar E., Hazra Abhishikta, Patra Malyasree. Synthesis, characterization and evaluation of antimicrobial activity of zinc oxide nanoparticles. J. Biochem. Technol. 2012;3(5):S151–S154. [Google Scholar]
- Liu J., Burdette J.E., Xu H., Gu C., van Breemen R.B., Bhat K.P., Booth N., Constantinou A.I., Pezzuto J.M., Fong H.H., Farnsworth N.R., Bolton J.L. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J. Agric. Food Chem. 2001;49:2472–2479. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- Mahanty A., Mishra S., Bosu R., Maurya U.K., Netam S.P., Sarkar B. Phytoextracts-synthesized silver nanoparticles inhibit bacterial fish pathogen Aeromonas hydrophila. Indian J. Microbiol. 2013;53(4):438–446. doi: 10.1007/s12088-013-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhiuto F., Palumbo D.R., Samperi S., Zangla G., Pino A., De Pasquale R., Circosta C. The isoflavones mixture from Trifolium pretense L. protects HCN 1-A neurons from oxidative stress. Phytother. Res. 2009;23:192–196. doi: 10.1002/ptr.2584. [DOI] [PubMed] [Google Scholar]
- Prakasham R.S., Kumar B.S., Kumar Y.S., Kumar K.P. Production and characterization of protein encapsulated silver nanoparticles by marine isolate Streptomyces parvulus SSNP11. Indian J. Microbiol. 2014;54(3):329–336. doi: 10.1007/s12088-014-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajiv P., Rajeshwari S., Venckatesh R. Rambutan peels promoted biomimetic synthesis of bioinspired zinc oxide nanochains for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013;112:384–387. doi: 10.1016/j.saa.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Ramesh P., Rajendran A., Meenakshisundaram M. Green synthesis of zinc oxide nanoparticles using flower extract Cassia Auriculata. J NS NT. 2014;1(1):41–45. February |Pp 41-45| ISSN 2279 – 0381. [Google Scholar]
- Rouhi J., Mahmud S., Naderi N., Ooi C.R., Mahmood M.R. Physical properties of fish gelatin-based bio-nanocomposite films incorporated with ZnO nanorods. Nanoscale Res. Lett. 2013;8:364. doi: 10.1186/1556-276X-8-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam A.H., Sivaraj R., Venckatesh R. Green synthesis and characterization of zinc oxide nanoparticles from Ocimum basilicum L. var. purpurascens Benth.-Lamiaceae leaf extract. Mater. Lett. 2014;131:16–18. doi: 10.1016/j.matlet.2014.05.033. [DOI] [Google Scholar]
- Sangeetha G., Rajeshwari S., Venckatesh R. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: structure and optical properties. Mater. Res. Bull. 2011;46:2560–2566. [Google Scholar]
- Samat N.A., Nor R.M. Sol–gel synthesis of zinc oxide nanoparticles using Citrus aurantifolia extracts. Ceram. Int. 2013;39:S545–S548. doi: 10.1016/j.ceramint.2012.10.132. [DOI] [Google Scholar]
- Senthilkumar S.R., Sivakumar T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZNO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci. 2014;6:461–465. [Google Scholar]
- Vijayalakshmi R., Rajendran V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012;4(2):1183–1190. [Google Scholar]
- Vijayakumar S., Vinoj G., Malaikozhundan B., Shanthi S., Vaseeharan B. Plectranthus amboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larva. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;137:886–891. doi: 10.1016/j.saa.2014.08.064. [DOI] [PubMed] [Google Scholar]
- Yuvakkumar R., Suresh J., Saravanakumar B., Nathanaeld A.J., Honga S.I., Rajendran Rambutan peels promoted biomimetic synthesis of bioinspired zinc oxide nanochains for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;137:250–258. doi: 10.1016/j.saa.2014.08.022. [DOI] [PubMed] [Google Scholar]