Abstract
Aspartame is an artificial sweetener used as an alternate for sugar in several foods and beverages. The study reports that consumption of aspartame containing product could lead to cancer. However, the effect of aspartame on apoptosis process in cancer is not yet understood clearly. HeLa cells were exposed to different concentrations (0.01–0.05 mg/ml) of aspartame for 48 h. Cytotoxicity of aspartame on cancer cells was determined by SRB assay. The result indicates no significant changes on cell viability. Aspartame suppresses apoptosis process in cancer cells by down-regulation of mRNA expression of tumor suppressor gene p53, and pro-apoptotic gene bax. It up-regulates anti-apoptotic gene bcl-2 mRNA expression. In addition, Ki 67 and PCNA mRNA, and protein expressions were determined. Taking all these together, we conclude that aspartame may be a potent substance to slow-down the apoptosis process in HeLa cells. Further works are ongoing to understand the biochemical and molecular mechanism of aspartame in cancer cells.
Keywords: Aspartame, HeLa, SRB, mRNA, p53
1. Introduction
Aspartame is widely used as an artificial sweetener in several foods (Magnuson et al., 2007). It is commonly found in low calorie beverages and desserts (Oyama et al., 2002). Chemically, it is a dipeptide of aspartyl-phenylalanine methyl ester which facilitates its intestinal hydrolysis and absorption of amino acids (aspartic acid and phenylalanine) together with free methanol (Lipton et al., 1991). Aspartic acid and phenylalanine are commonly found in natural proteins, which are beneficial under normal circumstances (Woodrow, 1984). Consumption of aspartame has been linked with several neurological and behavioral disturbances (Humphries et al., 2008). However, most of the results yielded inconclusive correlations.
Aspartame is distributed under several trade names and approved by the FDA. However, the safety of aspartame was renewed by a report suggesting that an increase in the number of people with brain tumors might be associated with the introduction and use of this sweetener in the USA. A study reports that lymphomas and leukemias were induced in rats fed with very high doses of aspartame (Soffritti et al., 2005). Saccharin is another artificial sweetener which increases incidence of urinary bladder cancer at high doses in male rats. The aim of the present study was to investigate the short-term effect of aspartame on apoptosis in cancer cells.
2. Materials and methods
2.1. Materials
HeLa cells were obtained from American Type Culture Collection. DMEM and MTT were provided by Sigma–Aldrich Chemical (St Louis, Mo). Aspartame was provided by Supelco. Fetal bovine serum (FBS), penicillin–streptomycin and trypsin–EDTA were purchased from Gibco. Primers of p53, bax, bcl-2, Ki 67 and PCNA were purchased from Macro Gen (South Korea). Primary antibodies were purchased from Bioscience Technology (Seoul, South Korea).
2.2. Methods
2.2.1. SRB assay for cell viability
The effect of aspartame on HeLa cells was measured by the SRB assay (Vichai and Kirtikara, 2006). HeLa cells were seeded at a density of 2.2 × 104 cells/well into 96-well plates and allowed to adhere for 24 h at 37 °C. Then the cells were treated with aspartame at different concentrations (0.01–0.05 mg/ml) for 48 h. At the end of the treatments, cells were fixed with acetone and air dried. After fully dried, each well was added with 100% of SRB solution (0.4% w/v) and incubated for 3 h at room temperature. Microplate was washed with 1% of acetic acid and then dried under drying oven. Stained HeLa cells were photographed. Finally, 10 mm of Tris-base was added and kept overnight. Following complete dissolution of SRB in Tris-base, the absorbance was measured at 540 nm.
2.2.2. qPCR
HeLa cells were cultured in T75 flask and treated with aspartame (0.01–0.03 mg/ml) for 48 h. Total RNA was isolated from the control and aspartame treated samples (Chomczynski and Mackey, 1995). The first-strand c-DNA was synthesized from 1 μg of the total RNA using the M-MLV reverse transcriptase with the anchored oligo d(T)12–18 primer. qPCR was performed using a cDNA equivalent of 10 ng of total RNA from each sample with primers specific for p53, bax, bcl-2, Ki 67, PCNA and a housekeeping gene GAPDH. The reaction was carried out in 10 μl using SYBR Green Master Mix (Bioneer) according to the manufacturers’ instructions. Relative ratios were calculated based on the 2−ΔΔCT method (Pfaffl, 2001). PCR was monitored using the CFX96™ Real-Time System (Bio-Rad).
2.2.3. Western blot analysis
HeLa cells were cultured in T75 flask and treated with aspartame (0.03 mg/ml) for 48 h. Control and aspartame treated HeLa cells were washed three times with ice-cold PBS and lysed with 10 mM Tris–HCl (pH 7.5), 100 mM NaCl, 1% NP-40, 50 mM NaF, 2 mM EDTA (pH 8.0), 1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin. Equal amounts of lysate protein samples were run on SDS polyacrylamide gel, and then transferred onto a PVDF membrane. Non-specific binding was blocked by soaking the membrane in Tris buffered saline-Tween (TBST) buffer that contained 5% nonfat dry milk for 1 h. The membrane was probed overnight with an antibody against Ki 67 and PCNA. Following washing with TBST buffer, the membrane was incubated for 1 h with horseradish peroxidase conjugated goat anti-rabbit IgG. The protein levels of Ki 67 and PCNA were determined by using enhanced chemiluminescence kit (Muthuraman et al., 2014).
3. Results and discussion
Aspartame is one of the artificial sweeteners, used in a wide variety of foods and beverages. It was first approved by the US Food and Drug Administration (FDA) for use in solid food in 1981 (FDA, 1981), and extended to soft drinks (FDA, 1983). The acceptable daily intake of aspartame is 50 mg/kg body weight (BW) in the United States, and 40 mg/kg BW in the European Union. Even though it is widely used in several foods and beverages, the intake of aspartame was associated with visual impairments, high aspartate transaminase, ear buzzing, loss of equilibrium, muscle aches, pancreatitis, depression and high blood pressure (Woodrow, 1984).
HeLa cells were exposed to different concentrations of aspartame for 48 h. Aspartame in concentrations of 0.01–0.05 mg/ml showed no significant changes in cancer cells validated by the SRB assay (Fig. 1). HeLa cells exposed to different concentrations of aspartame for 48 h showed significant changes in the mRNA expression of apoptotic genes such as p53, bax, and bcl-2.
Figure 1.
Effects of various concentrations of aspartame on cell viability. HeLa cells were seeded at seeding densities of 2 × 104 cells/ml into 96 well micro plates and allowed to adhere for 24 h and treated with 0.01–0.05 mg/ml of aspartame for 48 h.
The mRNA expression level of tumor suppressor gene p53 and pro-apoptotic gene bax was significantly down-regulated. The mRNA expression of p53 was significantly reduced 0.17, 0.31 and 0.45 folds at 0.01, 0.02 and 0.03 mg/ml of aspartame treatment respectively. The mRNA expression of bax was significantly reduced 0.09, 0.2 and 0.38 folds at 0.01, 0.02 and 0.03 mg/ml of aspartame treatment respectively, whereas bcl-2 mRNA expression significantly upregulated 0.14, 0.29 and 0.44 folds at 0.01, 0.02 and 0.03 mg/ml of aspartame treatment respectively (Fig. 2, *p < 0.05). The mRNA expression of Ki 67 was significantly upregulated 0.12, 0.29 and 0.54 folds at 0.01, 0.02 and 0.03 mg/ml of aspartame treatment respectively. The mRNA expression of PCNA was significantly upregulated 0.26, 0.52 and 0.63 folds at 0.01, 0.02 and 0.03 mg/ml of aspartame treatment respectively (Fig. 3, *p < 0.05). Protein expression of Ki 67 and PCNA was significantly upregulated 0.33 and 0.2 folds at 0.03 mg/ml of aspartame treatment respectively (Fig. 4, *p < 0.05).
Figure 2.
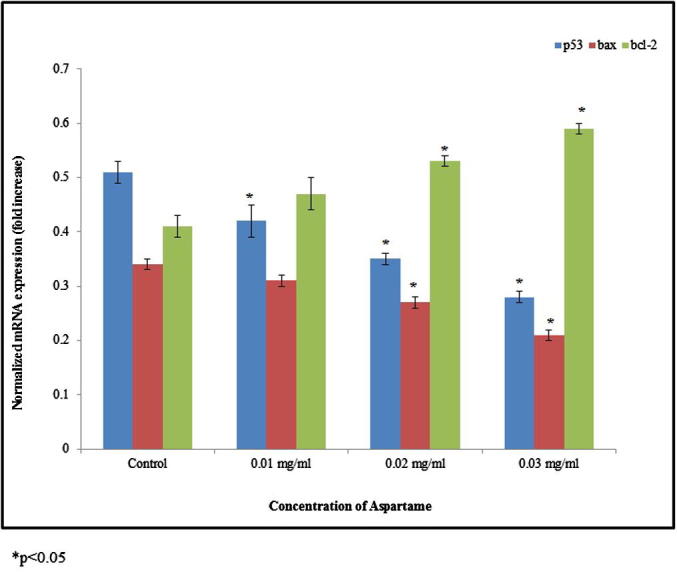
Expression of p53, bax and bcl-2 in the HeLa cells. HeLa cells were treated with 0.01–0.03 mg/ml of aspartame for 48 h. Expressions of p53, bax and bcl-2 mRNA are related to GAPDH and presented as fold. Relative expression values are the normalized mean ± SEM, n = 6. *p < 0.05.
Figure 3.
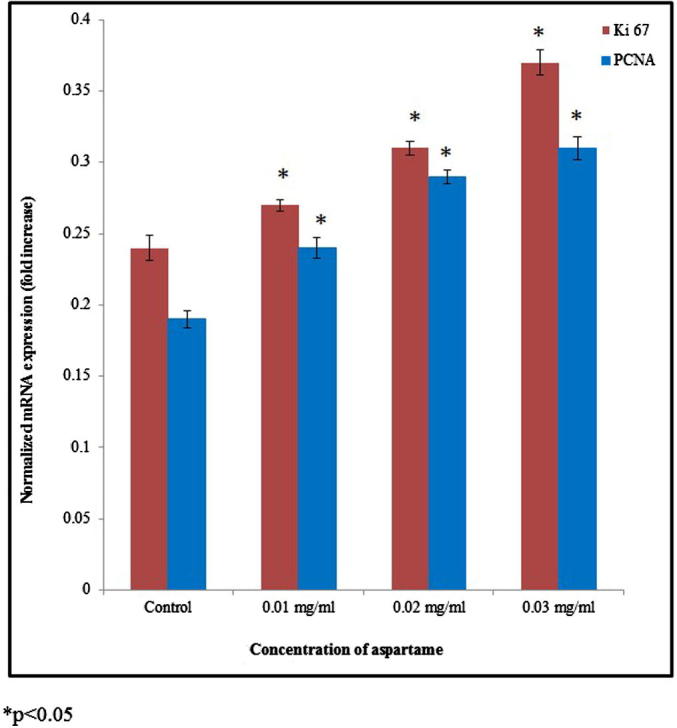
Expression of Ki 67 and PCNA in the HeLa cells. HeLa cells were treated with 0.01-0.03 mg/ml of aspartame for 48 h. Expressions of Ki 67 and PCNA mRNA are related to GAPDH and presented as fold. Relative expression values are the normalized mean ± SEM, n = 6. *p < 0.05.
Figure 4.
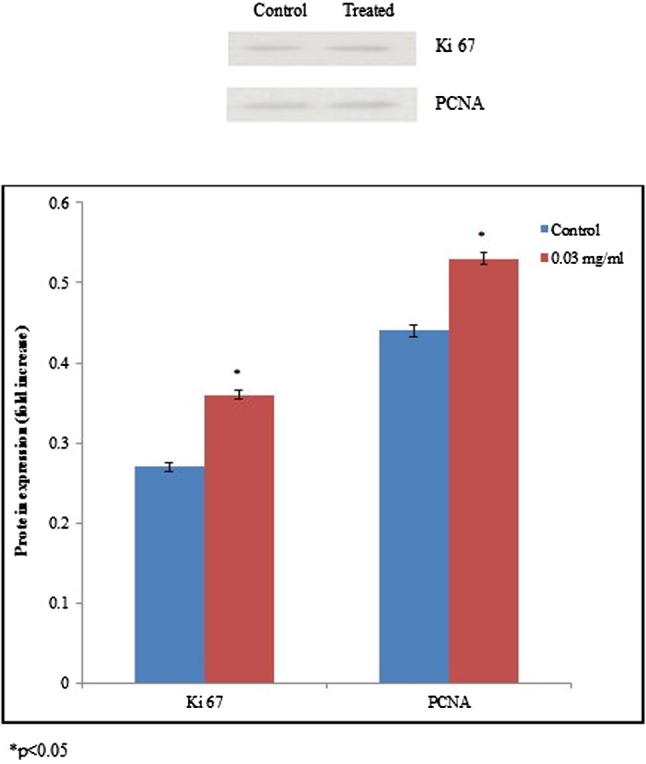
Expression of Ki 67 and PCNA proteins by aspartame treatment in the HeLa cancer cells. Western blot analysis of HeLa cancer cell extracts probed with anti-Ki 67 and PCNA. Quantitation analysis was carried out using densitometry. Values are expressed means ± SEM, n = 6. *p < 0.05.
A study reports that aspartame added to feed caused a statistically significant increase in lymphomas and leukemia in female rats (Soffritti et al., 2005). The ability of cancer cells to avoid apoptosis and propagate is the fundamental hallmark of cancer and is a major target of cancer therapy. The key finding of our study demonstrates that aspartame slows down the apoptosis process in cancer cells.
4. Conclusion
HeLa cells remain viable at all the concentrations of aspartame. Aspartame significantly down-regulates mRNA expression of tumor suppressor gene p53 and apoptotic gene bax in the cancer cells. The mRNA expression of bcl-2 was significantly up-regulated in the HeLa cells. The mRNA and protein expression of Ki 67 and PCNA were significantly upregulated. However, further studies are required to define the detailed mechanism of aspartame on apoptosis and cancer cells. Further works are ongoing to understand the molecular mechanism of aspartame in cancer cells.
Acknowledgements
This paper work was supported by research funds of KU Research Professor Program of Konkuk University, Republic of Korea.
Footnotes
Peer review under responsibility of King Saud University.
References
- Chomczynski P., Mackey K. Short technical report. Modification of the TRIZOL reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- FDA Aspartame commissioner’s final decision. Fed. Reg. 1981;46:38285–38308. [Google Scholar]
- FDA Food additive permitted for direct addition to food for human consumption:aspartame. Fed. Reg. 1983;48:31376–31382. [Google Scholar]
- Humphries P., Pretorius E., Naude H. Direct and indirect cellular effects of aspartame on the brain. Eur. J. Clin. Nutr. 2008;62:451–462. doi: 10.1038/sj.ejcn.1602866. [DOI] [PubMed] [Google Scholar]
- Lipton W.E., Li Y.N., Younoszai M.K., Stegink L.D. Metabolism. 1991;40:1337–1345. doi: 10.1016/0026-0495(91)90040-4. [DOI] [PubMed] [Google Scholar]
- Magnuson B.A., Burdock G.A., Doull J., Kroes R.M., Marsh G.M., Pariza M.W., Spencer P.S., Waddell W.J., Walker R., Williams G.M. Aspartame: a safety elevation based on current use levels, regulations, and toxicological and epidemiological studies. Crit. Rev. Toxicol. 2007;37:629–727. doi: 10.1080/10408440701516184. [DOI] [PubMed] [Google Scholar]
- Muthuraman P., Jeongeun P., Eunjung K. Aspartame downregulates 3T3-L1 differentiation. In Vitro Cell. Dev. Biol. Anim. 2014;50:851–857. doi: 10.1007/s11626-014-9789-3. [DOI] [PubMed] [Google Scholar]
- Oyama Y., Sakai H., Arata T., Okano Y., Akaike N., Sakai K., Noda K. Cytotoxic effects of methanol, formaldehyde and formate on dissociated rat thymocytes: a possibility of aspartame toxicity. Cell Biol. Toxicol. 2002;18:43–50. doi: 10.1023/a:1014419229301. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffritti M., Belpoggi F., Esposti D.D., Lambertini L. Aspartame induces lymphomas and leukaemias in rats. Eur. J. Oncol. 2005;10:107–116. [Google Scholar]
- Woodrow C. Monte and methanol. J. Appl. Nutr. 1984;36:1–15. [Google Scholar]
- Vichai V., Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]