Abstract
The profile of lipophilic antioxidants in different vegetative parts (leaves, shoots, buds and berries) was studied in sea buckthorn (Hippophae rhamnoides L.) male and female plants collected in the end of spring. Five lipophilic compounds, i.e. three tocopherol homologues (α, β and γ), plastochromanol-8 and β-carotene, were identified in each vegetative part of male and female sea buckthorn plants at the following concentrations: 7.25–35.41, 0.21–2.43, 0.41–1.51, 0.19–1.79 and 4.43–24.57 mg/100 g dry weight basis. Additionally, significant amounts of α-tocotrienol (1.99 mg/100 g dry weight basis) were detected in buds. The α-tocopherol and β-carotene were predominant lipophilic antioxidants in each vegetative part, accounting for 78.3–97.0% of identified compounds. The greatest amounts of lipophilic antioxidants were found in leaves, especially of female plants. Nevertheless, apart from leaves, also shoots of plants of both sexes seem to be a good source of α-tocopherol and β-carotene.
Abbreviations: DW, dry weight basis; NP-HPLC/FLD/DAD, normal phase-high-performance liquid chromatograph/fluorescence detection/diode-array detection; PC-8, plastochromanol-8; SD, standard deviation; T, tocopherol; T3, tocotrienol
Keywords: Sea buckthorn (Hippophae rhamnoides L.), Leaves, Shoots, Plant sex, Tocopherols, Plastochromanol-8, β-Carotene
1. Introduction
Sea buckthorn (Hippophae rhamnoides L.) is widely grown all over the world for its valuable berries. Nevertheless, presently not only berries, but also leaves are focused on by scientists due to the presence of nutritionally beneficial hydrophilic and lipophilic compounds (Górnaś et al., 2014d, Guan et al., 2005, Kumar et al., 2011, Sajfrtov and Sovova, 2012, Šnē et al., 2013a, Šne et al., 2013b). Moreover, Šnē et al., 2013a, Šne et al., 2013b reported that not only leaves, but also other vegetative parts, e.g. shoots, may be used as a valuable source of phenolic compounds. In the last decade an increased interest has been observed in the use of phenolic compounds from non-traditional sources (Šnē et al., 2013a, Šne et al., 2013b), but also in lipophilic antioxidants such as tocochromanols (Ciftci et al., 2011, Górnaś et al., 2014a, Górnaś et al., 2014b), β-carotene and plastochromanol-8 (Górnaś et al., 2014c, Górnaś et al., 2013). Those bio-compounds, especially tocopherols and tocotrienols, are reported to possess a wide spectrum of biological activities, such as antioxidant and inflammatory properties (Jiang et al., 2001, Nogala-Kałucka et al., 2013). Therefore, since the amount of information concerning lipophilic compounds in sea buckthorn vegetative parts (leaves, shoots and buds) is still limited or lacking, the aim of this study was to evaluate their profile.
2. Materials and methods
2.1. Reagents
Tocopherol (T) and tocotrienol (T3) homologues (α, β, γ and δ) standards (>95% of purity) were purchased from Merck (Darmstadt, Germany) and LGC Standards (Teddington, Middlesex, UK), respectively. Methanol, ethanol, n-hexane, ethyl acetate (HPLC grade), β-carotene (⩾97.0%), pyrogallol, sodium chloride and potassium hydroxide were obtained from Sigma–Aldrich (Steinheim, Germany). Since, the standard of plastochromanol‐8 (PC-8) is not commercially available, was isolated from flaxseed oil using a semi‐preparative HPLC to obtain the pure PC‐8 according to Siger et al. (2014).
2.2. Plant material
Vegetative parts of sea buckthorn (H. rhamnoides L.) were collected in Baltplant Ltd., Latvia, GPS location: N: 56°36′39″; E: 23°17′50″, at two different harvest times, in the second part of April (buds) and the middle of June (leaves, shoots and berries) 2012. Sea buckthorn vegetative parts were picked from ten randomly selected 7-year old male and female trees, grown at a 2 × 4 m spacing without fertilization and irrigation. Two female cultivars ‘Botanicheskaya Lubitelskaya’ and ‘Prozrachnaya’ (selected by T. T. Trofimov, the Moscow Botanical Garden) and male plants (open pollinated seedlings of unknown origin) were used in the experiment. About 20 ± 1 g of each vegetative part of sea buckthorn was harvested from each tree (both female and male) by sampling from the lower and upper parts around the tree circumference (between 08:30 and 09:30 a.m. local time) and transported immediately (15 min) to the laboratory. All sea buckthorn vegetative parts, separately for material collected from male and from female trees were carefully mixed, frozen in liquid nitrogen and immediately powdered using a Knifetec 1095 laboratory mill (Foss, Höganäs, Sweden).
Dry weight basis (dw) in samples was measured gravimetrically according to Ruiz (2005).
2.3. Extraction of tocochromanols, PC-8 and β-carotene
Tocochromanols, PC-8 and β-carotene from the sea buckthorn vegetative parts were extracted according to the previously validated method (Górnaś et al., 2014a) with minor modifications. Since this saponification method was shown in a previous study to be the best to recover lipophilic antioxidants from sea buckthorn leaves (Górnaś et al., 2014d), it was also applied in the present study to determine the profile of lipophilic compounds in leaves, shoots, berries and buds of sea buckthorn plants. Briefly, 0.1 g of powdered sea buckthorn vegetative parts was placed in a 15 mL tube, next pyrogallol (0.05 g), ethanol (2.5 mL) and potassium hydroxide (0.25 mL, 600 g/L) were added, vortexed (10 s) and incubated in a water bath (25 min, 80 °C). During incubation in the water bath (after 10 min) the test tubes were vortexed (10 s). After the completion of incubation the samples were immediately cooled in an ice-water bath, subsequently supplemented with sodium chloride (2.5 mL, 10 g/L) and vortexed (5 s). Then n-hexane:ethyl acetate (2.5 mL, 9:1; v/v) was added, the samples were vortexed (15 s) and centrifuged (5 min, 1000g, at 4 °C). The organic layer was collected to a round bottom flask and the residues were retracted (×3) as described above. The combined organic layer fractions were evaporated using a Laborota 4000 vacuum rotary evaporator (Heidolph, Schwabach, Germany), dissolved in n-hexane (2 mL) and analysed immediately by NP-HPLC/FLD/DAD.
2.4. Chemical analysis
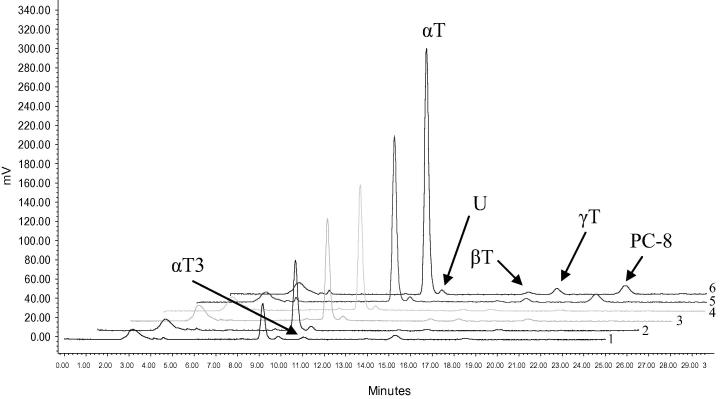
The lipophilic compounds were separated on a Water system (Milford, MA, USA) consisting of a pump (Waters 600), a fluorescence detector (Waters 474) and a diode-array detector (Waters 2998 PDA) according to Górnaś et al., 2014c. Analyses were run on a LiChrosorb Si 60, 4.6 × 250 mm, 5 μm column (Merck, Darmstadt, Germany) at the column thermostat set at 20 °C. The mobile phase was a mixture of n-hexane and 1,4-dioxane (97:3 v/v) at a flow rate of 1.5 mL/min. For tocopherol and PC-8 measurements the fluorescence detector was set at the following wavelengths, excitation λ = 295 nm and emission λ = 330 nm. β-Carotene was detected using a photo-diode array detector at a wavelength λ = 450 nm. Tocopherols and β-carotene were identified and quantified based on commercial standards whereas, PC-8 was identified using the PC-8 standard isolated from flaxseed oil and expressed as equivalents of the γ-T commercial standard. The results of chromatographic separation, with the exception of β-carotene, are presented in Fig. 1.
Figure 1.
An NP-HPLC/FLD chromatogram of tocochromanols and plastochromanol-8 in sea buckthorn vegetative parts. 1, buds (male plants); 2, shoots (male plants); 3, green berries (female plants); 4, shoots (female plants); 5, leaves (male plants); 6, leaves (female plants). αT, βT, γT and αT3 – individual homologues of tocopherol and tocotrienol; PC-8, plastochromanol-8; U, unidentified compound.
2.5. Statistical analysis
The results are presented as means ± standard deviation from three replicates of each experiment. A p-value <0.05 was used to denote significant differences between mean values determined by the analysis of variance (ANOVA). The homogeneity of variance was verified by Levene’s test. Homogeneous groups were determined by the Bonferroni post hoc test with the assistance of Statistica 10.0 (StatSoft, Tulsa, OK, USA) software.
3. Results and discussion
Five lipophilic compounds, i.e. α-T, β-T, γ-T, PC-8 and β-carotene, were identified in each vegetative part of sea buckthorn (leaves, shoots, berries and buds) male and female plants. Additionally, significant amounts of α-T3 (1.99 mg/100 g dw) were detected in buds. In each vegetative part of both male and female sea buckthorn plants α-T was a predominant homologue of tocopherol (81.9–96.9%). However, when the concentration of α-T was considered in relation to all identified lipophilic antioxidants, it accounted for 57.6–74.0% and 39.2–65.0% in vegetative parts of female and male plants, respectively. The smaller percentage of α-T in parts collected from male plants is due to a higher concentration of β-carotene and lower of α-T when compared to those collected from female plants. In contrast, in most of the fruit seeds and kernels γ-T is a predominant homologue (Górnaś et al., 2015a, Górnaś et al., 2015b, Górnaś et al., 2015c) with some exceptions, for instance Chaenomeles japonica (Górnaś et al., 2014c, Górnaś et al., 2013) or fenugreek (Trigonella foenum-graecum) (Ciftci et al., 2011), in which, similarly as in leaves, α-T is the predominant form of tocopherol. β-Carotene accounts for 19.4–33.1% and 29.5–46.3% of total identified lipophilic antioxidants in vegetative parts of female and male sea buckthorn plants, respectively. The contribution of β-T, γ-T and PC-8 was within a similar range of 0.7–4.0% in all vegetative parts of sea buckthorn, with the exception of γ-T (6.8%) and α-T3 (10.8%) in buds. Significant differences (p < 0.05) were detected between analysed lipophilic compounds in various sea buckthorn parts. The highest concentration of α-T was detected in female leaves, while it was lowest in buds (35.41 and 7.25 mg/100 g dw, respectively) (Table 1). Approximately a twofold lower amount of α-T was recorded in shoots and berries of female and shoots of male plants when compared with their counterparts in leaves. However, the concentration of α-T in shoots of female plants was almost twofold higher than in shoots of male plants (19.56 and 10.87 mg/100 g dw, respectively). The concentration of α-T in the present study is slightly lower when compared to the previous study; however, vegetative parts of sea buckthorn were collected two weeks earlier and as it was shown previously, the amount of α-T increases during leaf development (Górnaś et al., 2014d). Nevertheless, the trend towards a higher concentration of α-T in leaves of female plants than male plants has been maintained in both studies. The highest concentration of β-carotene was recorded in leaves of male and female plants (24.57 and 20.33 mg/100 g dw, respectively). These data are comparable with the previous study (Górnaś et al., 2014d). A significant concentration of β-carotene in sea buckthorn leaves was also reported by Guan et al. (2005) and Sajfrtov and Sovova (2012). Much lower levels of β-carotene when compared to leaves (3–4 times) were recorded in the other vegetative parts of sea buckthorn. Differences in the concentration of β-carotene in shoots of female and buds of male as well as in berries of female and shoots of male plants were not statistically significant (6.46 and 7.23, and 4.43 and 4.94 mg/100 g dw, respectively). The highest concentrations of β-T, γ-T and PC-8 were detected in leaves, especially from female plants (2.43, 1.51 and 1.79 mg/100 g dw, respectively), while they were lowest in shoots of plants of both sexes (0.21–0.22, 0.41–0.44 and 0.19–0.26 mg/100 g dw, respectively). Andersson et al., 2008, Andersson et al., 2009 when studying tocopherols, tocotrienols and carotenoids in sea buckthorn berries during ripening found higher amounts of tocopherols and β-carotene when compared to the present study; however, the harvesting time was markedly later (end of July and the middle of June, respectively). To the best of our knowledge this is the first report on the lipophilic antioxidant composition in sea buckthorn shoots and buds, therefore, a direct comparison is not possible. As it was pointed out by Shunguang et al. (2003), sea buckthorn leaves and shoots have a great potential in food processing due to the biologically active substances; therefore, at the beginning of their development, when shoots are still green, tender and non-woody, it seems to be a valuable source of lipophilic antioxidants. The presented study confirms this statement, proving that not only leaves of sea buckthorn, but also its shoots are a good source of α-T.
Table 1.
Contents of tocochromanols, plastochromanol-8 and β-carotene in sea buckthorn vegetative parts.
| Plant sex | Vegetative part | Tocopherol (mg/100 g dw) |
PC-8 (mg/100 g dw) | β-Carotene (mg/100 g dw) | ||
|---|---|---|---|---|---|---|
| α-T | β-T | γ-T | ||||
| Female | ||||||
| Leaves | 35.41 ± 0.59f | 2.43 ± 0.14d | 1.51 ± 0.07c | 1.79 ± 0.08c | 20.33 ± 0.45c | |
| Shoots | 19.56 ± 0.38d | 0.21 ± 0.02a | 0.41 ± 0.03a | 0.19 ± 0.02a | 6.46 ± 0.35b | |
| Berries | 16.90 ± 0.36c | 0.46 ± 0.04b | 0.58 ± 0.04b | 0.46 ± 0.04b | 4.43 ± 0.21a | |
| Male | ||||||
| Leaves | 23.96 ± 0.35e | 1.69 ± 0.04c | 1.27 ± 0.05c | 1.61 ± 0.04c | 24.57 ± 0.44c | |
| Shoots | 10.87 ± 0.25b | 0.22 ± 0.02a | 0.44 ± 0.03a | 0.26 ± 0.01a | 4.94 ± 0.27a | |
| Buds⁎ | 7.25 ± 0.15a | 0.34 ± 0.02b | 1.26 ± 0.06c | 0.42 ± 0.03b | 7.23 ± 0.19b | |
Results are expressed as the mean ± standard deviation (n = 3). Different letters in the same column indicate statistically significant difference at p < 0.05. T, tocopherol; T3, tocotrienol; PC-8, plastochromanol-8; dw, dry weight basis.
In the sample of male buds α-T3 was detected (1.99 ± 0.13 mg/100 g dw).
4. Conclusions
The presented study shows that α-T and β-carotene are predominant lipophilic antioxidants, accounting for 78.3–97.0% of identified compounds in different parts of sea buckthorn (H. rhamnoides L.) plants. A significant effect on concentrations of α-T and β-carotene was found not only for the vegetative part, but also plant sex. Leaves of female plants were richest in lipophilic antioxidants. Reported information concerning lipophilic antioxidants, which play a significant role in protection against oxidative stress, should improve the nutritional value of sea buckthorn vegetative parts and promote their use in food and/or pharmaceutical industries.
Conflict of interest
None.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Acknowledgement
This research was supported by the Latvian Council of Science, collaborative research project nr. 672/2014: “Scientific and technological developments for sustainable cultivation and comprehensive use of sea buckthorn”.
Footnotes
Peer review under responsibility of King Saud University.
References
- Andersson S.C., Olsson M.E., Johansson E., Rumpunen K. Carotenoids in sea buckthorn (Hippophae rhamnoides L.) berries during ripening and use of pheophytin a as a maturity marker. J. Agric. Food Chem. 2009;57:250–258. doi: 10.1021/jf802599f. [DOI] [PubMed] [Google Scholar]
- Andersson S.C., Rumpunen K., Johansson E., Olsson M.E. Tocopherols and tocotrienols in sea buckthorn (Hippophae rhamnoides L.) berries during ripening. J. Agric. Food Chem. 2008;56:6701–6706. doi: 10.1021/jf800734v. [DOI] [PubMed] [Google Scholar]
- Ciftci O.N., Przybylski R., Rudzinska M., Acharya S. Characterization of fenugreek (Trigonella foenum-graecum) seed lipids. J. Am. Oil Chem. Soc. 2011;88:1603–1610. [Google Scholar]
- Górnaś P., Mišina I., Grāvīte I., Soliven A., Kaufmane E., Segliņa D. Tocochromanols composition in kernels recovered from different apricot varieties: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Nat. Prod. Res. 2015;45:12–15. doi: 10.1080/14786419.2014.997727. [DOI] [PubMed] [Google Scholar]
- Górnaś P., Mišina I., Lāce B., Lācis G., Segliņa D. Tocochromanols composition in seeds recovered from different pear cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. LWT – Food Sci. Technol. 2015;62:104–107. [Google Scholar]
- Górnaś P., Mišina I., Ruisa S., Rubauskis E., Lācis G., Segliņa D. Composition of tocochromanols in kernels recovered from different sweet cherry (Prunus avium L.) cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Eur. Food Res. Technol. 2015;240:663–667. [Google Scholar]
- Górnaś P., Pugajeva I., Segliņa D. Seeds recovered from by-products of selected fruit processing as a rich source of tocochromanols: RP-HPLC/FLD and RP-UPLC-ESI/MSn study. Eur. Food Res. Technol. 2014;239:519–524. [Google Scholar]
- Górnaś P., Segliņa D., Lācis G., Pugajeva I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ) LWT – Food Sci. Technol. 2014;59:211–214. [Google Scholar]
- Górnaś P., Siger A., Juhņeviča K., Lācis G., Šnē E., Segliņa D. Cold-pressed Japanese quince (Chaenomeles japonica (Thunb.) Lindl. ex Spach) seed oil as a rich source of α-tocopherol, carotenoids and phenolics: a comparison of the composition and antioxidant activity with nine other plant oils. Eur. J. Lipid Sci. Technol. 2014;116:563–570. [Google Scholar]
- Górnaś P., Siger A., Segliņa D. Physicochemical characteristics of the cold-pressed Japanese quince seed oil: New promising unconventional bio-oil from by-products for the pharmaceutical and cosmetic industry. Ind. Crops Prod. 2013;48:178–182. [Google Scholar]
- Górnaś P., Šnē E., Siger A., Segliņa D. Sea buckthorn (Hippophae rhamnoides L.) leaves as valuable source of lipophilic antioxidants: The effect of harvest time, sex, drying and extraction methods. Ind. Crops Prod. 2014;60:1–7. [Google Scholar]
- Guan T.T., Cenkowski S., Hydamaka A. Effect of drying on the nutraceutical quality of sea buckthorn (Hippophae rhamnoides L. ssp. sinensis) leaves. J. Food Sci. 2005;70:514–518. [Google Scholar]
- Jiang Q., Christen S., Shigenaga M.K., Ames B.N. Γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- Kumar M., Dutta R., Prasad D., Misra K. Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides) leaves for the comparative evaluation of antioxidant activity. Food Chem. 2011;127:1309–1316. doi: 10.1016/j.foodchem.2011.01.088. [DOI] [PubMed] [Google Scholar]
- Nogala-Kałucka M., Dwiecki K., Siger A., Górnaś P., Polewski K., Ciosek S. Antioxidant synergism and antagonism between tocotrienols, quercetin and rutin in model system. Acta Aliment. 2013;42:360–370. [Google Scholar]
- Ruiz R.P. In: Handbook of Food Analytical Chemistry, Water, Proteins, Enzymes, Lipids, and Carbohydrates. Wrolstad R.E., Decker E.A., Schwartz S.J., Sporns P., editors. John Wiley & Sons Inc.; New Jersey: 2005. pp. 7–8. [Google Scholar]
- Sajfrtov M., Sovova H. Solute-matrix and solute-solute interactions during supercritical fluid extraction of sea buckthorn leaves. Proc. Eng. 2012;42:1682–1691. [Google Scholar]
- Siger A., Kachlicki P., Czubiński J., Polcyn D., Dwiecki K., Nogala-Kalucka M. Isolation and purification of plastochromanol-8 for HPLC quantitative determinations. Eur. J. Lipid Sci. Technol. 2014;116:413–422. [Google Scholar]
- Šnē, E., Galoburda, R., Segliņa, D., 2013a. Sea buckthorn vegetative parts – a good source of bioactive compounds. In: Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences, pp. 101–108.
- Šne, E., Segliņa, D., Galoburda, R., Krasnova, I., 2013b. Content of Phenolic Compounds in Various Sea Buckthorn Parts. In: Proceedings of the Latvian Academy of Sciences. Section B. Natural, Exact, and Applied Sciences, pp. 411–415.
- Shunguang L, Zhengping J, Xiufeng W, Keqin W, Dengming L, In: Proceedings of the 1st Congress of the International Seabuckthorn Association 2003, pp. 36–46.