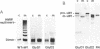Abstract
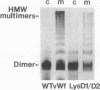
von Willebrand factor (vWf) is synthesized as a large precursor that dimerizes in the endoplasmic reticulum and forms multimers in the trans- and post-Golgi compartments of megakaryocytes and endothelial cells. The disulfide-bonded multimers are stored in alpha granules of platelets and Weibel-Palade bodies of endothelial cells. The prosequence, composed of two homologous D domains, is required for vWf multimerization and storage. Each D domain contains vicinal cysteines (159Cys-Gly-Leu-162Cys and 521Cys-Gly-Leu-524Cys) that are similar to those at the active site of disulfide isomerases that catalyze thiol protein disulfide interchange. As in disulfide isomerases, a positively charged amino acid (lysine) is also found in close proximity to the vicinal cysteines. Although conserved, the lysine present in thioredoxin was shown not to be essential for its redox activity. We investigated the role of the vicinal cysteines and the lysine residue in the vWf propolypeptide by site-directed mutagenesis and expression of the resulting constructs in mammalian cells. Insertion of an extra glycine between the vicinal cysteines in either D domain inhibited multimerization of dimers, whereas alteration of lysine to glycine in both domains (residues 157 and 519) had no effect. This suggests the importance of the vicinal cysteines but not the lysines in vWf multimerization. Expression of the mutant with an additional glycine in the D1 domain in AtT-20 cells, a mouse pituitary cell line that can store vWf, led to the storage of the resulting dimers. This demonstrates that the mutation did not effect the capacity of the propolypeptide to direct vWf storage while its ability to promote interchain disulfide bonding was eliminated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boniface J. J., Reichert L. E., Jr Evidence for a novel thioredoxin-like catalytic property of gonadotropic hormones. Science. 1990 Jan 5;247(4938):61–64. doi: 10.1126/science.2104678. [DOI] [PubMed] [Google Scholar]
- Bonthron D., Orr E. C., Mitsock L. M., Ginsburg D., Handin R. I., Orkin S. H. Nucleotide sequence of pre-pro-von Willebrand factor cDNA. Nucleic Acids Res. 1986 Sep 11;14(17):7125–7127. doi: 10.1093/nar/14.17.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid N. J., Freedman R. B. Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 1988 Oct 13;335(6191):649–651. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Foster P. A., Fulcher C. A., Marti T., Titani K., Zimmerman T. S. A major factor VIII binding domain resides within the amino-terminal 272 amino acid residues of von Willebrand factor. J Biol Chem. 1987 Jun 25;262(18):8443–8446. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gleason F. K., Lim C. J., Gerami-Nejad M., Fuchs J. A. Characterization of Escherichia coli thioredoxins with altered active site residues. Biochemistry. 1990 Apr 17;29(15):3701–3709. doi: 10.1021/bi00467a016. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Glutaredoxin from Escherichia coli and calf thymus. Methods Enzymol. 1985;113:525–540. doi: 10.1016/s0076-6879(85)13071-5. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Söderberg B. O., Eklund H., Brändén C. I. Three-dimensional structure of Escherichia coli thioredoxin-S2 to 2.8 A resolution. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2305–2309. doi: 10.1073/pnas.72.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. 6. The amino acid sequence of the protein from escherichia coli B. Eur J Biochem. 1968 Dec 5;6(4):475–484. doi: 10.1111/j.1432-1033.1968.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Joelson T., Sjöberg B. M., Eklund H. Modifications of the active center of T4 thioredoxin by site-directed mutagenesis. J Biol Chem. 1990 Feb 25;265(6):3183–3188. [PubMed] [Google Scholar]
- Kallis G. B., Holmgren A. Differential reactivity of the functional sulfhydryl groups of cysteine-32 and cysteine-35 present in the reduced form of thioredoxin from Escherichia coli. J Biol Chem. 1980 Nov 10;255(21):10261–10265. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert N., Freedman R. B. Kinetics and specificity of homogeneous protein disulphide-isomerase in protein disulphide isomerization and in thiol-protein-disulphide oxidoreduction. Biochem J. 1983 Jul 1;213(1):235–243. doi: 10.1042/bj2130235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyte A., Voorberg J., Van Schijndel H. B., Duim B., Pannekoek H., Van Mourik J. A. The pro-polypeptide of von Willebrand factor is required for the formation of a functional factor VIII-binding site on mature von Willebrand factor. Biochem J. 1991 Feb 15;274(Pt 1):257–261. doi: 10.1042/bj2740257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti T., Rösselet S. J., Titani K., Walsh K. A. Identification of disulfide-bridged substructures within human von Willebrand factor. Biochemistry. 1987 Dec 15;26(25):8099–8109. doi: 10.1021/bi00399a013. [DOI] [PubMed] [Google Scholar]
- Mayadas T. N., Wagner D. D. In vitro multimerization of von Willebrand factor is triggered by low pH. Importance of the propolypeptide and free sulfhydryls. J Biol Chem. 1989 Aug 15;264(23):13497–13503. [PubMed] [Google Scholar]
- Mayadas T. N., Wagner D. D. von Willebrand factor biosynthesis and processing. Ann N Y Acad Sci. 1991;614:153–166. doi: 10.1111/j.1749-6632.1991.tb43700.x. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Oikarinen J. A rapid and sensitive assay for protein disulphide isomerase activity. J Biochem Biophys Methods. 1983 Feb;7(2):115–121. doi: 10.1016/0165-022x(83)90045-3. [DOI] [PubMed] [Google Scholar]
- Pigiet V. P., Schuster B. J. Thioredoxin-catalyzed refolding of disulfide-containing proteins. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7643–7647. doi: 10.1073/pnas.83.20.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest. 1980 Jun;65(6):1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg B. M., Holmgren A. Studies on the structure of T4 thioredoxin. II. Amino acid sequence of the protein and comparison with thioredoxin from Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8063–8068. [PubMed] [Google Scholar]
- Sporn L. A., Marder V. J., Wagner D. D. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986 Jul 18;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Diergaarde P. J., Hart M., Pannekoek H. Full-length von Willebrand factor (vWF) cDNA encodes a highly repetitive protein considerably larger than the mature vWF subunit. EMBO J. 1986 Aug;5(8):1839–1847. doi: 10.1002/j.1460-2075.1986.tb04435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., Hart M., Pannekoek H. Expression of variant von Willebrand factor (vWF) cDNA in heterologous cells: requirement of the pro-polypeptide in vWF multimer formation. EMBO J. 1987 Oct;6(10):2885–2890. doi: 10.1002/j.1460-2075.1987.tb02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorberg J., Fontijn R., van Mourik J. A., Pannekoek H. Domains involved in multimer assembly of von willebrand factor (vWF): multimerization is independent of dimerization. EMBO J. 1990 Mar;9(3):797–803. doi: 10.1002/j.1460-2075.1990.tb08176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Fay P. J., Sporn L. A., Sinha S., Lawrence S. O., Marder V. J. Divergent fates of von Willebrand factor and its propolypeptide (von Willebrand antigen II) after secretion from endothelial cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1955–1959. doi: 10.1073/pnas.84.7.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells. Identification of a large precursor polypeptide chain. J Biol Chem. 1983 Feb 25;258(4):2065–2067. [PubMed] [Google Scholar]
- Wagner D. D., Olmsted J. B., Marder V. J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982 Oct;95(1):355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Saffaripour S., Bonfanti R., Sadler J. E., Cramer E. M., Chapman B., Mayadas T. N. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell. 1991 Jan 25;64(2):403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]
- Wise R. J., Dorner A. J., Krane M., Pittman D. D., Kaufman R. J. The role of von Willebrand factor multimers and propeptide cleavage in binding and stabilization of factor VIII. J Biol Chem. 1991 Nov 15;266(32):21948–21955. [PubMed] [Google Scholar]
- Wise R. J., Pittman D. D., Handin R. I., Kaufman R. J., Orkin S. H. The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell. 1988 Jan 29;52(2):229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- Zhang R. M., Snyder G. H. Dependence of formation of small disulfide loops in two-cysteine peptides on the number and types of intervening amino acids. J Biol Chem. 1989 Nov 5;264(31):18472–18479. [PubMed] [Google Scholar]