Abstract
Myelodysplastic syndrome (MDS) is a clonal stem-cell disorder characterized by dyshematopoiesis. We report a patient who presented with cytopenias and microangiopathic hemolytic anemia. Chromosome microarray analysis (CMA), using single nucleotide polymorphism arrays, on peripheral blood revealed genomic imbalances indicative of MDS, which was confirmed by bone marrow examination. This report highlights the importance of suspecting MDS in patients with cytopenias and microangiopathic hemolytic anemia. CMA of peripheral blood may assist in the preliminary diagnosis of MDS, representing a comparatively less invasive diagnostic procedure and may aid bone marrow evaluation when an aspirate sample is insufficient for conventional cytogenetic analysis.
Keywords: Peripheral blood, Myelodysplastic syndrome, Chromosomal microarray analysis, Single nucleotide polymorphism, Whole genome scanning
Highlights
-
•
Myelodysplastic syndromes (MDS) may manifest as non-immune hemolytic anemia.
-
•
Schistocytosis may be evident on peripheral blood smear in a subset of MDS patients.
-
•
Utilization of peripheral blood DNA to detect genomic imbalances and mutations.
-
•
Genomic imbalances detection by chromosomal microarray analysis (CMA).
-
•
CMA of peripheral blood may complement diagnostics in MDS.
Introduction
Myelodysplastic syndrome (MDS) is an acquired, clonal stem-cell disorder characterized by dyshematopoiesis and stem-cell dysplasia of single or multiple blood cell lineages. MDS leads to varying degrees of cytopenias and, paradoxically, hypercellular bone marrow with potential evolution to acute myeloid leukemia (AML) or marrow failure [1], [2], [3]. As a result of dyserythropoiesis, red blood cell (RBC) morphological abnormalities, such as anisocytosis, poikilocytosis, macrocytosis and sometimes elliptocytosis, are often observed in MDS [1], [2]. Schistocytes refer to fragmented RBCs and are commonly associated with causes of microangiopathic hemolytic anemia (MAHA). However, schistocytosis with a high reticulocyte count in the peripheral blood smear is a rare and unusual manifestation of MDS [1].
Karyotypic analysis is commonly used in the diagnostic work up of various hematological diseases. Some chromosome abnormalities are specifically associated with certain diseases or disease subtypes [4], [5]. The detection of various chromosomal alterations not only assists in diagnosis, but also helps in the prognostication of patients [6]. However, due to technical limitations, such as the need for sufficient number of dividing malignant cells and the limited resolution of light microscopy, karyotypic analysis is able to detect such abnormalities in only about 50% of MDS cases [7].
Whole genome scanning technologies, such as chromosomal microarray analysis (CMA), using single nucleotide polymorphism (SNP) arrays, do not require dividing cells, have a much greater resolution than karyotypic analysis, and allow for detection of loss of heterozygosity (LOH) as well as changes in genomic copy number [7]. SNP-based CMAs also permit the detection of acquired copy-neutral LOH (cn-LOH), which is common in hematological malignancies [8], but undetectable by conventional cytogenetics [7], [9].
Here we describe severe anemia and marked schistocytosis as a manifestation of MDS in an elderly patient admitted at our cancer center. Initial bone marrow examination was inadequate for detailed histopathological evaluation and cytogenetics. However, application of SNP-based CMA on peripheral blood uncovered genomic imbalances indicative of MDS, which was confirmed upon a subsequent bone marrow examination.
Materials and methods
CMA was performed essentially as described in detail elsewhere [10]. Briefly, total genomic DNA from blood was digested with NspI restriction enzyme, ligated with an adaptor complimentary to polymerase chain reaction (PCR) primer, PCR amplified, purified using magnetic beads, fragmented, biotin labeled, and hybridized to an Affymetrix CytoScan HD array. The hybridized array was washed and scanned with a GeneChip Scanner 3000 7G. Intensities of probe hybridization were analyzed using Affymetrix GeneChip Command Console, and copy number and genotyping analyses were performed using Affymetrix Chromosome Analysis Suite software.
Case summary and results
A 70-year-old woman was admitted with a 2-month history of exertional dyspnea associated with weakness and fatigue, decreased appetite and weight loss. Her complaints had been worsening during the previous 2 weeks, and she had also noticed purple lesions on both knees at that time. She denied any history of hematemesis, hemoptysis, melena, hematochezia or hematuria.
Past medical history was significant for right-sided triple negative breast cancer, treated by lumpectomy followed by local radiation therapy ~11 years prior to this encounter. She also had a history of stage 1A uterine cancer, for which she underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy, followed by 3 cycles of chemotherapy with carboplatin and paclitaxel with radiation therapy to the pelvis 3 years prior to this visit.
Physical examination showed no apparent distress. Other than conjunctival pallor and palpable purpuric lesions on both knees, no bruises or petechiae were noted on any other part of her body. The rest of the systemic examinations were unremarkable.
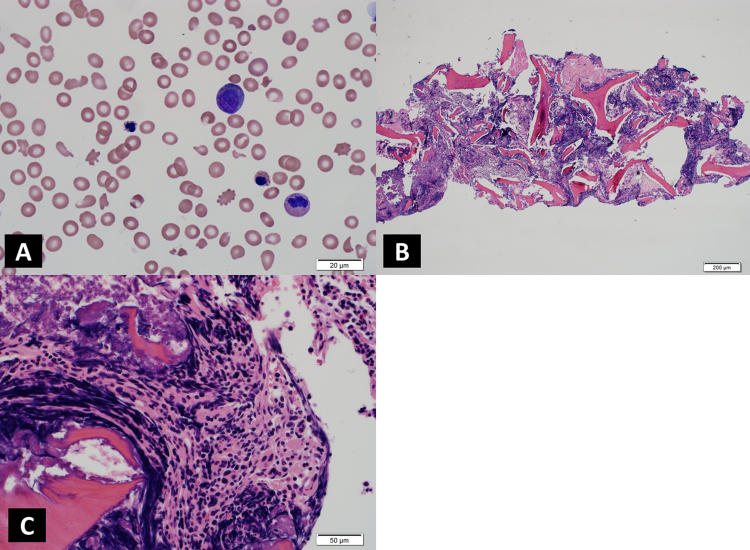
Blood work on presentation showed pancytopenia (hemoglobin: 6.2 g/dL, leukocytes: 3.5×109/L and platelets: 57×109/L) with reticulocyte count of 10.15% and hyperbilirubinemia with significant schistocytosis, elliptocytosis, tear drop cells and basophilic stippling, abnormal immature myeloid cells, hyposegmented hypogranular neutrophils and abnormal nucleated RBCs (Fig. 1A), pointing towards hemolysis. A work up for hemolytic anemia was ordered. Her blood counts had continued to fall. Her coagulation work up was normal. Direct and indirect Coombs tests were negative. Bilirubin was increased to 3.7 mg/dL, with normal direct bilirubin and normal liver enzymes. LDH 683 U/L, haptoglobin was <10 mg/dL, and her ESR was 100 mm. Serum folate, B12, iron and total iron binding capacity were within normal limits. Erythropoietin was elevated: 49.9 mU/mL. Chest and abdominal CT of lungs and liver ruled out metastasis from previous malignancies that could account for shortness of breath and elevated bilirubin levels. Paroxysmal nocturnal haemoglobinuria was also excluded based on normal CD55 and CD59 expression on flow cytometry.
Fig. 1.
A) Peripheral blood film showing marked schistocytosis, anisocytosis and nucleated red blood cells as well as myelocytes and dysplastic neutrophils. B) Bone marrow biopsy with severe crush artifacts. C) Bone marrow biopsy with severe crush artifacts showing cluster of dysplastic megakaryocytes.
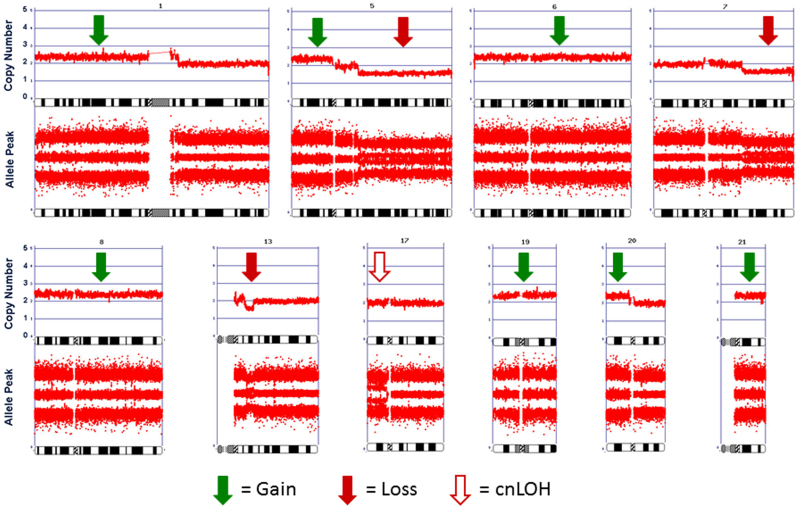
Initial bone marrow biopsy was inadequate in size for detailed examination and had severe crush artifact. The preserved focus of marrow showed hypercellularity with large, multinucleated, dysplastic megakaryocytes and megaloblastoid changes in erythroid series (Fig. 1B and C). There was no increase in reticulin fiber content in the marrow biopsy, making a myelophthisic process as the underlying cause of schistocytosis unlikely. Blood vessels were not appreciated in the bone marrow biopsy due to severe crush artifact. A skin biopsy showed deep subcutaneous hemorrhage and no evidence of microangiopathic changes. The marrow aspirate was also insufficient for further analysis by flow cytometry or cytogenetics. CMA of peripheral blood revealed multiple genomic imbalances in a mosaic state, including losses of portions of chromosome arms 5q, 7q and 13q, isodisomy of 17p, and gains of chromosomes/chromosomal segments 1p, 5p, 6, 8, 19, 20p, 21 (Fig. 2), findings consistent with the diagnosis of MDS. Conventional cytogenetic analysis performed on a repeat bone marrow sample revealed an abnormal karyotype: 47-50,X,-X,der(?)t(?;1)(?;q21),+add(1)(q12)x2, del(5)(q14),+6,add(7)(q22),+8,+9,−13,+19,−20,+21,+0-2mar[cp15]/46,XX. The findings were similar to those of the peripheral blood CMA and confirmed the diagnosis of therapy-related MDS with complex cytogenetics.
Fig. 2.
Chromosomal microarray analysis showing multiple genomic imbalances in a mosaic state, including losses of 13q12.3q14.11 and portions of chromosome arms 5q and 7q, as well as gains of chromosomes or chromosome segments 1p, 5p, 6, 8, 19, 20p, and 21 as well as isodisomy of 17p.
Discussion
This is a case report of therapy-associated MDS and MAHA, a rare manifestation of MDS. The initial presentation was low platelet count and anemia, elevated bilirubin and schistocytosis in the peripheral blood smear. The patient presented with non-immune hemolytic anemia. Hemolytic anemias are common occurrences in patients with hematologic malignancies, particularly AML and chronic myeloid leukemia (CML), but are rarely observed in MDS, with only a few cases reported in the literature [11].
Abnormal RBC morphologies have previously been reported in cases of MDS. These include elliptocytosis [1], [2], [12], spherocytosis [13], acanthocytosis [14] and basophilic stippling [1], [2], [3]. However, marked schistocytosis by itself is rare. To the best of our knowledge, only 3 cases of schistocytosis with underlying MDS have been reported in the English literature [1], [12]. In 1984, Hartz et al. reported a 78-year-old man who presented with refractory anemia with peripheral schistocytes [12]. The patient was subsequently diagnosed with MDS upon further work up. Rummens et al. described two cases of MDS (ages 59 and 60) who presented with schistocytosis on peripheral blood film [1]. Schistocytosis has also been reported in other malignant hematologic disorders. For example, Atkins and Muss described a series of 12 patients with erythroleukemia [13]. All of their patients had evidence of schistocytosis and elliptocytosis in peripheral blood. RBC fragmentation has been described as a general observation in patients with leukemia [13], [15]. Our patient showed other morphologic abnormalities of RBCs, including occasional elliptocytes and basophilic stippling, which are commonly observed with MDS [1], [2], [3].
Karyotypic analysis has been invaluable for detection of chromosomal rearrangements specific to certain diseases, such as t(9;22) in CML and t(15;17) in acute promyelocytic leukemia [4], [5], and can predict sensitivity to molecularly targeted drugs, such as imatinib in CML [16]. Cytogenetics have been part of the revised International Prognostic Scoring System (IPSS-R) and shown to have important prognostic implications in MDS patients, with detection of 5q- syndrome being a prominent example [6].
Whole genome scanning studies, such as CMA using SNP arrays, eliminate the need for dividing cells, have a much higher level of resolution than karyotypic analysis, and allow for detection of both DNA copy number and LOH throughout the genome in a single experiment [7]. CMA also uncovers acquired cn-LOH, which is indicative of somatic uniparental isodisomy. While cn-LOH occurs commonly in hematological malignancies [8], it is undetectable by classical cytogenetics [7], [9]. Such was the case in our patient, in which CMA of peripheral blood demonstrated isodisomy of 17p, a site encompassing the TP53 tumor suppressor gene. Notably, recurrent mutations of TP53 have been previously described in myeloid malignancies [8].
While this report demonstrates that CMA with SNP arrays is useful for the analysis of genomic imbalances in the peripheral blood of patients with suspected MDS, this technique is not readily available at many medical centers and many institutions perform fluorescence in situ hybridization to detect cytogenetic abnormalities. Hence, it is important to consider/discuss other diagnostic methods which can utilize peripheral blood (such as high-throughput sequencing technologies) in this context [17]. Mutation analysis based on Sanger sequencing, PCR, and next-generation sequencing (NGS) technologies has been introduced in many centers and is able to detect MDS-related mutations in bone marrow and may be used to advance classification and prognostication of MDS. In recent years, the field of molecular diagnostics has been significantly advanced with the introduction of NGS. There are now multiple commercially available NGS assays, each with a unique method of template preparation, sequencing and imaging and different approaches for the analysis of data [18], [19], [20].
In conclusion, our case highlights the importance of considering a diagnosis of MDS in a patient with cytopenias and MAHA, especially in the presence of predisposing factors such as prior chemotherapy and radiation therapy. Our studies indicate that CMA analysis of peripheral blood can assist in the initial diagnosis of MDS. Peripheral blood CMA can be a useful investigation if MDS is suspected, representing a comparatively less invasive diagnostic procedure that may precede bone marrow examination. Prospective studies with large sample sizes will help determine its sensitivity and specificity as a potential ‘screening test’ in the diagnostic workup of patients suspected to have MDS.
Author contributions
MFZ and ED performed the pathological assessment of the patient and wrote the manuscript. NK took care of the patient in a clinician's capacity and critically revised the clinical case summary of the patient in the manuscript. JP and JRT performed the CMA, interpreted the genomic findings, and assisted in the description of the Methodology and Discussion sections. All authors contributed to writing the manuscript and read and approved the final copy of the manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This study was supported in part by National Cancer Institute Grant CA-06927 and an appropriation from the Commonwealth of Pennsylvania. The Fox Chase Cancer Center Genomics Facility was used in the course of this work. The study sponsors had no involvement in the study design, collection, analysis and interpretation of data.
References
- 1.Rummens J.L., Verfaillie C., Criel A., Hidajat M., Vanhoof A., Van den Berghe H. Elliptocytosis and schistocytosis in myelodysplasia: report of two cases. Acta Haematol. 1986;75:1747. doi: 10.1159/000206114. [DOI] [PubMed] [Google Scholar]
- 2.Ishida F., Shimodaira S., Kobayashi H., Saito H., Kaku M., Kanzaki A. Elliptocytosis in myelodysplastic syndrome associated with translocation (1;5)(p10;q10) and deletion of 20q. Cancer Genet. Cytogenet. 1999;108:162–165. doi: 10.1016/s0165-4608(98)00135-6. [DOI] [PubMed] [Google Scholar]
- 3.Aleem A., Murray J.A. Spherocytosis preceding the development of myelodysplasia. Clin. Lab. Haematol. 2001;23:249–251. doi: 10.1046/j.1365-2257.2001.00385.x. [DOI] [PubMed] [Google Scholar]
- 4.de Thé H., Chomienne C., Lanotte M., Degos L., Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor α gene to a novel transcribed locus. Nature. 1990;347:558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 5.Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature, 1973, 43, pp. 290–293. In: Landmarks in Medical Genetics: Classic Papers with Commentaries 2004, 51, p. 104. [DOI] [PubMed]
- 6.Greenberg P.L., Tuechler H., Schanz J., Sanz G., Garcia-Manero G., Solé F. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiu R.V., Gondek L.P., O’Keefe C.L., Elson P., Huh J., Mohamedali A. Prognostic impact of SNP array karyotyping in myelodysplastic syndromes and related myeloid malignancies. Blood. 2011;117:4552–4560. doi: 10.1182/blood-2010-07-295857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jasek M., Gondek L.P., Bejanyan N., Tiu R., Huh J., Theil K.S. TP53 mutations in myeloid malignancies are either homozygous or hemizygous due to copy number-neutral loss of heterozygosity or deletion of 17p. Leukemia. 2010;24:216. doi: 10.1038/leu.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maciejewski J.P., Tiu R.V., O’Keefe C. Application of array-based whole genome scanning technologies as a cytogenetic tool in haematological malignancies. Br. J. Haematol. 2009;146:479–488. doi: 10.1111/j.1365-2141.2009.07757.x. [DOI] [PubMed] [Google Scholar]
- 10.Roberts J.L., Buckley R.H., Luo B., Pei J., Lapidus A., Peri S. CD45-deficient severe combined immunodeficiency caused by uniparental disomy. Proc. Natl. Acad. Sci. USA. 2012;109:10456–10461. doi: 10.1073/pnas.1202249109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin J.T., Wang W.S., Yen C.C., Chiou T.J., Liu J.H., Hsiao L.T. Myelodysplastic syndrome complicated by autoimmune hemolytic anemia: remission of refractory anemia following mycophenolate mofetil. Ann. Hematol. 2002;81:723–736. doi: 10.1007/s00277-002-0539-3. [DOI] [PubMed] [Google Scholar]
- 12.Hartz J.W., Buss D.H., White D.R., Bond M.G., Scharyj M. Marked elliptocytosis and schistocytosis in hematopoietic dysplasia. Am. J. Clin. Pathol. 1984;82:354–359. doi: 10.1093/ajcp/82.3.354. [DOI] [PubMed] [Google Scholar]
- 13.Atkins J.N., Muss H.B. Schistocytes in erythroleukemia. Am. J. Med. Sci. 1985;289:110–113. doi: 10.1097/00000441-198503000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Doll D.C., List A.F., Dayhoff D.A., Loy T.S., Ringenberg Q.S., Yarbro J.W. Acanthocytosis associated with myelodysplasia. J. Clin. Oncol. 1987;7:1569–1572. doi: 10.1200/JCO.1989.7.10.1569. [DOI] [PubMed] [Google Scholar]
- 15.Visudhiphan S., Piankijagum A., Sathayapraseart P., Mitrchai N. Erythrocyte fragmentation in disseminated intravascular coagulation and other diseases. N. Engl. J. Med. 1983;309:113. doi: 10.1056/NEJM198307143090217. [DOI] [PubMed] [Google Scholar]
- 16.Druker B.J., Guilhot F., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 17.Kohlmann A., Bacher U., Schnittger S., Haferlach T. Perspective on how to approach molecular diagnostics in acute myeloid leukemia and myelodysplastic syndromes in the era of next-generation sequencing. Leuk. Lymphoma. 2014;55:1725–1734. doi: 10.3109/10428194.2013.856427. [DOI] [PubMed] [Google Scholar]
- 18.Metzker M.L. Sequencing technologies – the next generation. Nat. Rev. Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 19.Margulies M., Egholm M., Altman W.E. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bentley D.R., Balasubramanian S., Swerdlow H.P. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–59. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]