Abstract
Objective . Current chronic pain treatments target nociception rather than affective “suffering” and its associated functional and psychiatric comorbidities. The left dorsolateral prefrontal cortex (DLPFC) has been implicated in affective, cognitive, and attentional aspects of pain and is a primary target of neuromodulation for affective disorders. Transcranial direct current stimulation (tDCS) can non-invasively modulate cortical activity. The present study tests whether anodal tDCS targeting the left DLPFC will increase tolerability of acute painful stimuli vs cathodal tDCS.
Methods . Forty tDCS-naive healthy volunteers received anodal and cathodal stimulation targeting the left DLPFC in two randomized and counterbalanced sessions. During stimulation, each participant performed cold pressor (CP) and breath holding (BH) tasks. We measured pain intensity with the Defense and Veterans Pain Rating Scale (DVPRS) before and after each task.
Results . Mixed ANOVA revealed no main effect of stimulation polarity for mean CP threshold, tolerance, or endurance, or mean BH time (all P > 0.27). However, DVPRS rise associated with CP was significantly smaller with anodal vs cathodal tDCS ( P = 0.024). We further observed a significant tDCS polarity × stimulation order interaction ( P = 0.042) on CP threshold, suggesting task sensitization.
Conclusions . Although our results do not suggest that polarity of tDCS targeting the left DLPFC differentially modulates the tolerability of CP- and BH-related pain distress in healthy volunteers, there was a significant effect on DVPRS pain ratings. This contrasts with our previous findings that tDCS targeting the left dorsal anterior cingulate cortex showed a trend toward higher mean CP tolerance with cathodal vs anodal stimulation. The present results may suggest tDCS-related effects on nociception or DLPFC-mediated attention, or preferential modulation of the affective valence of pain as captured by the DVPRS. Sham-controlled clinical studies are needed.
Keywords: tDCS, Transcranial Direct Current Stimulation, DLPFC, Dorsolateral Prefrontal Cortex, Neuromodulation, Pain
Introduction
Treating pain remains a significant medical challenge, and chronic pain in particular is associated with significant psychiatric symptoms, including catastrophizing about pain, avoidance of potential triggers, and eventual social withdrawal, reduced quality of life, anxiety, and depression [ 1 ]. Most current pain treatments—primarily pharmacological approaches—focus on the sensory aspect of pain by reducing nociceptive signals. Often such a treatment is sufficient for acute forms of pain, where the sensations are expected to be self-limited as an inciting injury heals. However, studies have shown a link between acute and chronic pain, noting cortical changes associated with the transition to chronic pain [ 2 , 3 ]. This suggests a failed opportunity for early interventions that could avoid at least some instances of “pain chronification.” In addition to a sensory component, chronic pain includes a cognitive-affective component representing the associated “suffering” and distress—an aspect inadequately treated by current therapies designed to treat acute pain. Specific cognitive behavioral therapy (CBT) exists to address this affective suffering and has shown clinical efficacy [ 4–7 ]. However, access to appropriately trained CBT practitioners is a treatment barrier for many patients.
Non-invasive brain stimulation encompasses several promising treatment modalities that have been investigated for a variety of neuropsychiatric disorders. Perhaps the best known is the clinical use of repetitive transcranial magnetic stimulation (rTMS) of the left dorsolateral prefrontal cortex (DLPFC) to treat major depressive disorder, which is approved by the U.S. Food and Drug Administration.
Transcranial direct current stimulation (tDCS) utilizes a portable, battery-powered stimulator to apply a fixed weak electrical current to the brain. Anodal stimulation is thought to increase the excitability of the underlying cortex, and cathodal stimulation is thought to decrease the excitability of the underlying cortex [ 8 , 9 ], achieved by subthreshold modulation of resting membrane potentials [ 9 ]. Other investigators have used tDCS to target cortical areas associated with sensation [ 10 ] and higher-order pain representations [ 11 ], and they have used imaging to demonstrate tDCS-associated changes in brain metabolism [ 12 ].
The DLPFC is part of the medial pain pathway—comprised of medial thalamus, anterior insula, anterior cingulate cortex, and posterior parietal cortex—and is implicated in the affective, cognitive, and attentional aspects of pain [ 13 ], including pain prediction and evaluation [ 14 ]. Functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies in healthy volunteers have linked the DLPFC to subjective pain processing [ 15 ], anticipated pain [ 16 ], and pain control [ 17 , 18 ]. Regarding pain control specifically, results demonstrated selective activation of anterolateral prefrontal cortex, DLPFC, and posterior parietal cortex following actual control of heat pain, whereas perceived control corresponded to activation of the orbitofrontal and mediofrontal cortex [ 18 ]. The authors theorized that this dissociation of function may reflect the DLPFC’s role in working memory processes and in the updating of expectations relating to pain relief. In a clinical population of chronic low back pain patients, DLPFC gray matter density was reduced [ 19 ] and functional connectivity altered [ 20 ], again suggesting the structure’s key role in pain processing.
Pain investigators have attempted to modulate the DLPFC using both rTMS and tDCS, although the findings can be discrepant [ 13 ]. DLPFC rTMS has improved pain tolerance and analgesia of healthy volunteers [ 21–23 ]. A recent meta-analysis of tDCS targeting three superficial components of the “pain neuromatrix”—DLPFC, primary motor cortex (M1), and primary somatosensory cortex (S1)—showed that anodal tDCS of both the DLPFC and M1 reduced rated pain intensity in chronic pain patients [ 24 ]. However, the authors advised caution in interpreting their findings owing to small sample sizes and inadequate blinding in many of the included studies. A double-blinded study showed that anodal tDCS of the left DLPFC significantly reduced ratings of unpleasantness and emotional self-discomfort associated with viewing images of human pain, as compared to sham (placebo) stimulation or tDCS targeting M1 or occipital cortex (V1) [ 25 ]. Such differences may be associated with changes in EEG power, although the single-blinded study demonstrating this association was small [ 26 ]. A Chinese study using a variant of the image-rating task showed contradictory effects on emotional self-discomfort [ 27 ], suggesting possible strong influences of experimental methodology or cultural context. Additional preliminary evidence suggests lateralization of the DLPFC’s role in emotional responses to viewing painful video clips when the left and right DLPFCs were targeted with anodal and cathodal tDCS [ 28 ]. The left DLPFC has been further implicated in perceived pain control, with anodal tDCS associated with lower pain unpleasantness ratings than cathodal tDCS [ 29 ].
Given the DLPFC’s particular role in the affective, cognitive, and attentional aspects of pain—as opposed to the sensory and nociceptive aspects—and its importance for emotion regulation in general, we theorized that increasing excitability of the left DLPFC with anodal tDCS would decrease the subjective emotional distress caused by acutely painful laboratory stimuli in healthy volunteers without necessarily altering pain intensity ratings. It should be noted that this hypothesis differs from that of our prior study [ 30 ]. In that work, we theorized that cathodal (inhibitory) tDCS targeting the left dorsal anterior cingulate cortex (dACC) would decrease emotional distress from acute painful stimuli versus anodal stimulation, based partially on cingulotomy studies showing that ablation of the dACC could selectively increase pain tolerability without necessarily decreasing pain intensity [ 31–34 ]. We carried out the present study in 40 healthy volunteers, expecting anodal tDCS targeting the left DLPFC to increase tolerance to distress associated with acutely painful stimuli, as compared to cathodal stimulation. We also expected no change in pain intensity as rated with a visual analog scale.
Methods
The design of the present study was adapted from our previous study [ 30 ]. The methods are briefly reviewed, with key differences described in further detail.
Participants
Using convenience sampling via paper and online advertisements, we recruited and enrolled 40 tDCS-naive healthy participants who were at least 18 years of age, fluent in English, and right-handed; participants could be of any race, ethnicity, or sex. The study protocol was approved by the Butler Hospital Institutional Review Board, and participants provided written informed consent. Participants were tested in a dedicated, quiet, climate-controlled room at the hospital by a trained member of the research staff. Participants were financially compensated for their participation.
We screened potential participants with the Structured Clinical Interview for DSM-IV-TR (SCID) Research Version [ 35 ]. As with our prior study, the following were exclusionary: current Axis I psychiatric disorders, current use of psychotropic medications, current pain, seizure disorder, a previous history of skull trauma or intracranial surgery, presence of metal in the cranial cavity, implanted medical hardware, pregnancy, or any other condition that the investigators determined could increase tDCS-associated risks. We also requested participants to abstain from caffeine or nicotine for 3 hours prior to starting the experiment.
Study Setting
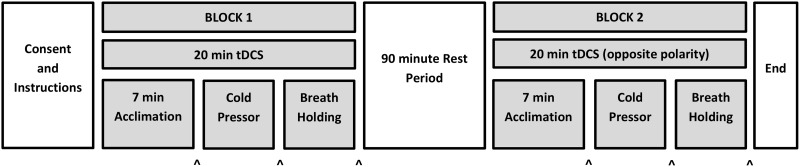
As with our prior study, two testing blocks occurred on the same day, separated by a 90-minute rest interval ( Figure 1 ). The interval length was selected based on prior work, suggesting that tDCS-associated effects can persist for up to 90 minutes post-stimulation [ 36 ] and for methodological consistency with our prior study. As previously, anodal tDCS targeting the left DLPFC occurred in one block and cathodal tDCS targeting the left DLPFC occurred in the other block; we randomized and counterbalanced the order of stimulation polarity. Participants were blinded to stimulation polarity; experimenters were not. Active tDCS occurred in both testing blocks; there was no sham condition. This within-participants design was chosen so as to 1) minimize possible difficulties maintaining tDCS sham blinding with a 2 mA active stimulation amplitude [ 37 ] and 2) examine the polarity-dependent effects of tDCS neuromodulation targeting the left DLPFC.
Figure 1.
Diagram of experimental protocol, adapted from authors’ prior study [ 30 ]. The two testing blocks were separated by a 90-minute rest interval. The polarity of tDCS received during block 1 was randomized and counterbalanced; the opposite polarity was delivered during block 2. Within each testing block, there was a 7-minute tDCS acclimation period (see text) followed by cold pressor and breath holding tasks. As the cold pressor was the key metric of interest in the present study, it was always presented before breath holding. Arrowheads at the bottom of the figure indicate when DVPRS ratings were obtained.
tDCS
To target the left DLPFC, the stimulating electrode was placed on the scalp at F 3 per the 10–20 electroencephalography (EEG) system. The return electrode was placed over the contralateral mastoid process. Electrodes were carbon rubber and enclosed in 3 × 5 cm sponge pockets saturated in normal saline (0.9% NaCl) and held on the scalp with rubber headbands adjusted to fit snugly but comfortably. No hair was shaved. To enhance connection, conductive gel (Microlyte, Coulbourn Instruments, Whitehall, PA, USA) was applied to the scalp beneath the sponge pockets. Electrodes were then connected to a battery-powered Soterix 1X1 bipolar tDCS device (Soterix Medical Inc., New York, NY, USA). Before stimulation, impedance of the electrode-to-scalp interface was checked with the device to ensure that it remained at or below a predetermined safety cutoff level. The amplitude of the tDCS was ramped up to 2 mA over 30 seconds and then maintained for 20 minutes before being ramped back down to 0 mA. The current was otherwise lowered by the tDCS staff only in the event of poor tolerability. We began tDCS 7 minutes before the first experimental task to acclimate participants fully to stimulation; similar studies of tDCS-associated effects on cognitive task performance [ 38 , 39 ] used acclimation periods of 4–5 minutes.
Tasks
As in our prior study, pain intensity was measured with the validated and self-rated Defense and Veterans Pain Rating Scale (DVPRS) [ 40 ]. With this 11-point visual analog scale, 0 indicates no pain and 10 indicates the most severe pain. Ratings were obtained after the first 7 minutes of tDCS in each testing block and immediately after each of the experimental tasks.
For the present study, we wished to focus on the cold pressor (CP) task [ 41 ] since in our prior study it had exhibited trend-level effects suggesting possible modulation by cathodal tDCS targeting the left dACC [ 30 ]. To that end, in each testing block we administered the CP task first, followed by the breath-holding (BH) task [ 42 , 43 ]. We omitted the pressure algometer because of significant sensitization effects in our prior study. In no instance did the preprogrammed 20 minutes of tDCS elapse before all tasks were completed. The CP task involved immersing the participant’s dominant (right) hand and arm in an ice water bath using our previously described apparatus [ 30 ]. Briefly, our apparatus consisted of an insulated vessel with a cylindrical mesh screen to separate the ice from the remainder of the water bath, thereby preventing direct contact between the ice and the participant’s skin. This design made it possible to maintain good temperature uniformity to 33 ± 1°F without a water circulator. As previously, threshold was the total time elapsed from task onset until the participant reported pain, tolerance was the total time elapsed from task onset until the participant removed his or her hand (maximum 5 minutes for safety), and endurance was the difference between tolerance and threshold [ 44 , 45 ]. If participants reached the 5-minute safety limit for threshold and/or tolerance, they were asked to remove their hand from the CP’s ice water bath and a 300-second threshold and/or tolerance time was recorded. For BH, we measured the total time a participant was able to hold a deeply inspired breath. If a participant completed both tasks before the entire 20 minutes of preprogrammed tDCS had elapsed, the stimulation was manually ended after completion of the tasks.
Data Analysis
As previously, the difference between post-task and baseline DVPRS ratings was used for statistical analyses. For the CP task, endurance was calculated as the difference between tolerance and threshold; therefore, it was not an independent variable. Statistical analysis comprised mixed ANOVA and t-tests, common for these types of psychological tasks [ 41 , 44–50 ], as well as Shapiro–Wilk tests of data set normality. Stimulation polarity was the within-participants factor; stimulation order (i.e., cathodal-first vs anodal-first) was the between-participants factor. We used the usual two-tailed P < 0.05 significance level. All analyses were performed with R 3.0.0 ( http://www.r-project.org /) using the open-source RKWard 0.6.1 graphical user interface and the rk.anova 0.01–17 plug-in ( https://rkward.kde.org /), as well as SPSS 21.0 (IBM, New York, NY, USA); both software packages gave identical results.
Results
Participants
Study participants had a mean age of 31.7 years (standard deviation 13.0, range 18–65) and a median age of 25.5 years. Twenty-four of 40 participants (60%) were female.
Sensations from tDCS were mild, typically consisting of feelings of heat, tingling, or itching. One participant reported mild dizziness. Another participant reported severe itching that did not improve with adjustment of the electrodes but did resolve when stimulation current was reduced from 2.0 to 1.8 mA for the second testing block only. All other participants were able to tolerate the full 2.0 mA stimulation amplitude without difficulty.
CP and BH Performance
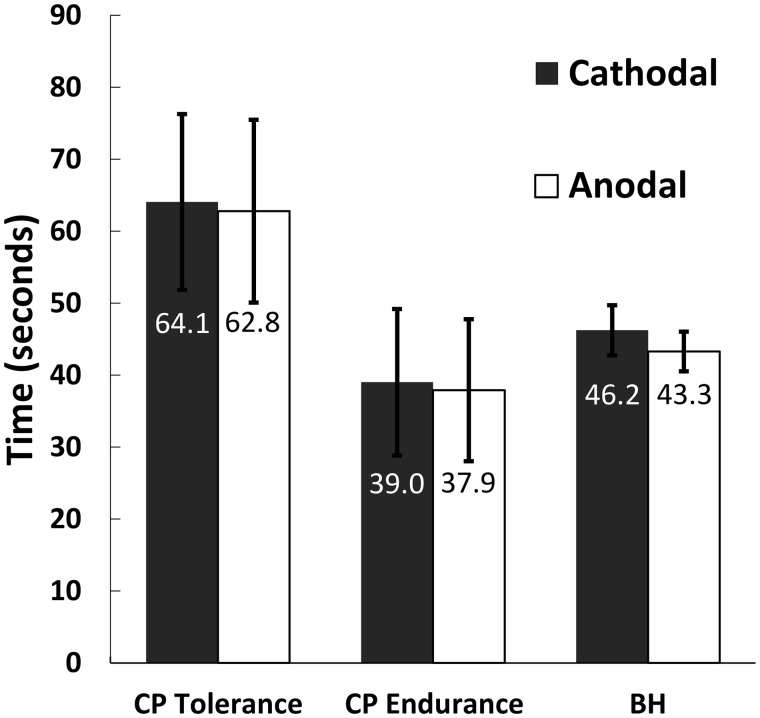
Mixed ANOVA on the full 40-participant data set revealed ( Figure 2 ) no significant main effect of stimulation polarity for mean CP threshold, mean CP tolerance, mean CP endurance, or mean BH time (all F1,38 < 1.23, all P > 0.27). There was a significant stimulation polarity × order (anodal-first vs cathodal-first) interaction for the mean CP threshold ( F1,38 = 4.44, P = 0.042). The mean threshold was greater for the first testing block (26.6 seconds vs 23.4 seconds for the second block), which may suggest task-related sensitization. There was also a significant main effect of stimulation order on mean CP endurance only ( F1,38 = 4.54, P = 0.040). Mean endurance was greater for anodal-first trials (57.5 seconds) than for cathodal-first trials (19.4 seconds); this result may be driven by mean CP threshold sensitization as endurance is a calculated variable (tolerance − threshold).
Figure 2.
There was no significant effect of stimulation polarity on mean CP threshold (not graphed), CP tolerance, CP endurance, or BH time (all F1,38 < 1.23, all P > 0.27). Mean values by polarity are below each error bar. Each participant received both polarities of stimulation in this within-participants design. Error bars represent ±1 standard error of the mean (SEM).
Exactly one participant achieved the 5-minute (300 second) CP safety limit for threshold and tolerance in both testing blocks (i.e., with both anodal and cathodal tDCS). Excluding this participant and re-performing the mixed ANOVA described previously with the resulting 39-participant data set did not significantly alter our results, so subsequent analyses were performed using the full 40-participant data set.
Defense and Veterans Pain Rating Scale
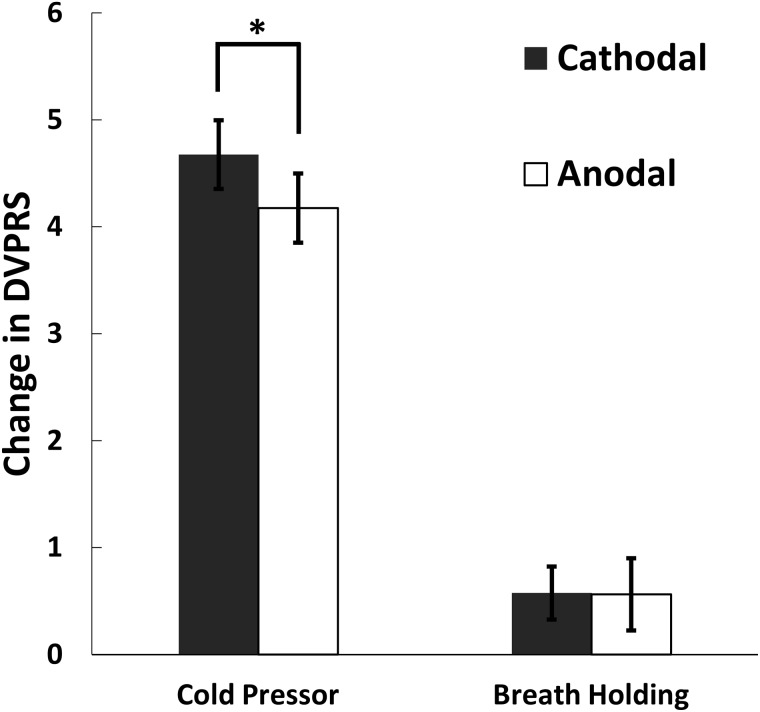
For all experimental trials, baseline DVPRS ratings had a mean ± standard error of the mean (SEM) of 1.28 ± 0.12. DVPRS ratings expectedly rose from baseline after each painful task (by 4.43 units for CP and 0.57 units for BH). Paired two-tailed t-tests indicated that these increases were significant for both tasks (all t > 2.7, all P < 0.008). Parsing further, for CP trials, ratings rose by a mean ± SEM of 4.68 ± 0.32 for cathodal tDCS and 4.18 ± 0.32 for anodal tDCS. For BH trials, ratings rose by 0.58 ± 0.25 and 0.56 ± 0.34 for cathodal and anodal tDCS, respectively.
Mixed ANOVA revealed a significant main effect of stimulation polarity (within-participants factor) on CP DVPRS rise ( Figure 3 ), with a smaller mean increase (by 0.50 points) with anodal stimulation ( F1,38 = 5.56, P = 0.024). There were no other significant main or interaction effects for CP or BH. For rigor, we performed Shapiro–Wilk normality tests for all CP and BH DVPRS data (i.e., pooled anodal and cathodal trials). Although these tests suggested rejection of the null hypothesis that the data were normally distributed, re-analysis of the CP DVPRS rise data with the non-parametric, paired, two-tailed, exact Wilcoxon signed-rank test provided equivalent statistically significant results ( W S = 360.5, P = 0.023).
Figure 3.
There was a significant main effect of stimulation polarity on the change in DVPRS following the CP task ( F1,38 = 5.56, P = 0.024). There was a smaller rise in mean CP DVPRS by 0.5 points with anodal stimulation. Each participant received both polarities of stimulation in this within-participants design. Error bars represent ±1 SEM.
Discussion
Our results do not initially suggest that the polarity of tDCS targeting the left DLPFC differentially modulates the tolerability of pain-related distress from CP or BH tasks in healthy volunteers. This contrasts with our prior pilot study of tDCS targeting the left dACC in another cohort of 40 healthy volunteers that showed a non-significant trend toward increased mean CP tolerance with cathodal versus anodal stimulation and no significant effects on DVPRS [ 30 ].
However, in the present study we found a robustly statistically significant reduction in the amount by which DVPRS pain ratings rose following the CP task with anodal versus cathodal tDCS targeting the left DLPFC in healthy volunteers. This relative attenuation in CP DVPRS rise could reflect direct modulation of nociception by tDCS, potentially by increasing pain thresholds, as suggested by a study using similar stimulation parameters and electric finger shock as the painful stimulus [ 51 ]. Given the DLPFC’s role in the medial pain pathway [ 13 ], anodal tDCS may also enhance a shifting of attentional focus as compared to cathodal tDCS. Alternatively, the DVPRS may capture some affective valence of the acutely painful stimuli, and that affective component may be preferentially modulated by anodal vs cathodal tDCS targeting the left DLPFC. Note that the DVPRS uses facial cartoons, colors, and text descriptions as anchors for the various rating levels [ 40 ]. Such a design would naturally encourage participants to consider mood and anxiety factors, potentially altering cognitive evaluation of the pain [ 14 ] and the subsequent rating. A future study could characterize this further by comparing ratings for identical painful stimuli separately obtained with the DVPRS and a numeric-only Likert scale.
The present study has several limitations, some of which are similar to those of our prior study. The present study had no sham control; instead, it compared two active stimulation conditions. This was done to simplify a complex experimental design, maximize the statistical power of our sample size, and avoid blinding difficulties noted with 2 mA tDCS amplitudes [ 37 ]. However, this choice made it impossible to separate polarity-dependent effects of tDCS from polarity-independent general effects of stimulation. Furthermore, the choice of a 90-minute rest interval between testing blocks may be problematic—even though our study demonstrated no significant DVPRS polarity × order interactions—in light of recent work suggesting that tDCS-related effects could persist for up to 120 minutes post-stimulation [ 52 ]. Future studies should consider a longer rest interval or a multi-day study design.
It is also not possible to state with certainty the volume of cortex activated by the chosen tDCS electrode montage. The lack of focality with bipolar tDCS virtually guarantees that areas adjacent to the left DLPFC were co-activated. Future studies employing empirically validated computer modeling or pairing imaging (e.g., EEG source localization or fMRI) with simultaneous tDCS are needed to demonstrate cortical target engagement.
The laterality effects of brain stimulation must also be explored. The left DLPFC is the usual target for clinical rTMS for major depressive disorder, and there is evidence that rTMS of the left DLPFC also has anti-nociceptive [ 21 ] and analgesic effects, perhaps by modulating the endogenous opioid system [ 23 ]. However, other investigators have found evidence that rTMS of the right DLPFC may preferentially modify the tolerability of cold pain, although these studies did not control for handedness [ 22 , 53 ]. Comparisons of rTMS and tDCS studies should be interpreted with caution, as rTMS is considered a depolarizing stimulus, in contrast to the much weaker subthreshold modulation thought to occur with tDCS [ 9 ]. The complexity of the current experiment’s design precluded comparison of left- and right-sided tDCS targeting the left DLPFC, but future studies could better characterize the effects of different stimulation modalities and of stimulation laterality.
Participants in this study were healthy volunteers receiving only one day’s worth of tDCS. This is likely an insufficient dose for a clinical response in a chronic pain population. Such patients may require multiple sessions of tDCS over several weeks, similar to how rTMS is typically prescribed for depression. Although engaging a different cortical target, various pain studies using tDCS targeting the motor cortex over multiple (5, 10, or 20) treatment sessions have shown long-lasting beneficial effects persisting for up to 12 weeks [ 10 , 12 , 54–59 ]. We caution against extrapolating our results obtained in healthy volunteers to clinical populations, as numerous studies have demonstrated cortical structural and functional connectivity changes associated with chronic pain [ 2 , 3 , 19 , 60 ], although there is evidence that DLPFC abnormalities can reverse with pain treatment [ 7 , 20 ]. Nonetheless, the action and effects of tDCS may differ between healthy and clinical groups, and future studies must take this into account.
Despite these open questions, the DLPFC remains a popular target for non-invasive neuromodulation. Recently, investigators have begun exploring the role of the DLPFC in placebo analgesia [ 61 ]. Current tDCS efforts targeting the DLPFC attempt to modulate placebo analgesia [ 62 ] and fear vigilance [ 63 ], with the hope that these approaches will yield novel pain treatments. For these and other approaches, rigorous double-blinded, sham-controlled studies will be needed in chronic pain populations, using tDCS designed to engage a variety of cortical targets associated with the affective, cognitive, and attentional aspects of pain.
Conclusions
This pilot study generated the unanticipated result that anodal tDCS targeting the left DLPFC significantly attenuated the increase in DVPRS pain intensity ratings following the CP task as compared to cathodal stimulation. This finding may reflect a reduction in nociception because tDCS shifts attentional focus away from the painful task. Alternatively, tDCS may selectively reduce the affective valence of the painful task. This latter interpretation could be seen as consistent with the use of neuromodulation of the left DLPFC with rTMS to treat major depressive disorder. The promising results of the present study underscore the need for future sham-controlled studies of non-invasive neuromodulation in clinical chronic pain populations engaging the DLPFC and other cortical targets.
Acknowledgments
The authors thank Nicole McLaughlin, PhD, Benjamin Jacobson, MD, and Ganaelle Joseph for their assistance during the research. NM assisted with study logistics and supervision of student volunteers in the laboratory. BJ contributed to the initial conception and design of the study. GJ assisted with participant recruitment, screening, and data collection.
Funding sources: This work was supported by internal funding from Butler Hospital, NIMH R25 MH101076, the Brown Institute for Brain Science, and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service, and the Center of Excellence for Neurorestoration and Neurotechnology at the Providence VA Medical Center.
Disclosure and conflicts of interest: The authors report no conflicts of interest or other disclosures. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1. Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, Karoly P. Fear-avoidance model of chronic pain: The next generation . Clin J Pain 2012. ; 28 ( 6 ): 475 – 83 . [DOI] [PubMed] [Google Scholar]
- 2. Baliki MN, Petre B, Torbey S , et al. . Corticostriatal functional connectivity predicts transition to chronic back pain . Nat Neurosci 2012. ; 15 ( 8 ): 1117 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mansour AR, Baliki MN, Huang L , et al. . Brain white matter structural properties predict transition to chronic pain . Pain 2013. ; 154 ( 10 ): 2160 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions . Psychol Bull 2007. ; 133 ( 4 ): 581 – 624 . [DOI] [PubMed] [Google Scholar]
- 5. Kerns RD, Sellinger J, Goodin BR. Psychological treatment of chronic pain . Annu Rev Clin Psychol 2011. ; 7 : 411 – 34 . [DOI] [PubMed] [Google Scholar]
- 6. Reid MC, Otis J, Barry LC, Kerns RD. Cognitive-behavioral therapy for chronic low back pain in older persons: A preliminary study . Pain Med 2003. ; 4 ( 3 ): 223 – 30 . [DOI] [PubMed] [Google Scholar]
- 7. Seminowicz DA, Shpaner M, Keaser ML , et al. . Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain . J Pain 2013. ; 14 ( 12 ): 1573 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brunoni AR, Nitsche MA, Bolognini N , et al. . Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions . Brain Stimulat 2012. ; 5 ( 3 ): 175 – 95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nitsche MA, Cohen LG, Wassermann EM , et al. . Transcranial direct current stimulation: State of the art 2008 . Brain Stimulat 2008. ; 1 ( 3 ): 206 – 23 . [DOI] [PubMed] [Google Scholar]
- 10. Fregni F, Boggio PS, Lima MC , et al. . A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury . Pain 2006. ; 122 ( 1–2 ): 197 – 209 . [DOI] [PubMed] [Google Scholar]
- 11. Mendonca ME, Santana MB, Baptista AF , et al. . Transcranial DC stimulation in fibromyalgia: Optimized cortical target supported by high-resolution computational models . J Pain 2011. ; 12 ( 5 ): 610 – 7 . [DOI] [PubMed] [Google Scholar]
- 12. Yoon EJ, Kim YK, Kim H-R , et al. . Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: A mechanistic PET study . Neurorehabil Neural Repair 2014. ; 28 ( 3 ): 250 – 9 . [DOI] [PubMed] [Google Scholar]
- 13. Mylius V, Borckardt JJ, Lefaucheur J-P. Noninvasive cortical modulation of experimental pain . Pain 2012. ; 153 ( 7 ): 1350 – 63 . [DOI] [PubMed] [Google Scholar]
- 14. Atlas LY, Lindquist MA, Bolger N, Wager TD. Brain mediators of the effects of noxious heat on pain . Pain 2014. ; 155 ( 8 ): 1632 – 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilcox CE, Mayer AR, Teshiba TM , et al. . The subjective experience of pain: An FMRI study of percept-related models and functional connectivity . Pain Med 2015. , doi: 10.1111/pme.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lobanov OV, Zeidan F, McHaffie JG, Kraft RA, Coghill RC. From cue to meaning: Brain mechanisms supporting the construction of expectations of pain . Pain 2014. ; 155 ( 1 ): 129 – 36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation . Brain J Neurol 2003. ; 126 ( Pt 5 ): 1079 – 91 . [DOI] [PubMed] [Google Scholar]
- 18. Mohr C, Leyendecker S, Petersen D, Helmchen C. Effects of perceived and exerted pain control on neural activity during pain relief in experimental heat hyperalgesia: A fMRI study . Eur J Pain 2012. ; 16 ( 4 ): 496 – 508 . [DOI] [PubMed] [Google Scholar]
- 19. Ivo R, Nicklas A, Dargel J , et al. . Brain structural and psychometric alterations in chronic low back pain . Eur Spine J 2013. ; 22 ( 9 ): 1958 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Čeko M, Shir Y, Ouellet JA , et al. . Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment . Hum Brain Mapp 2015. ; 36 ( 6 ): 2075 – 92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brighina F, De Tommaso M, Giglia F , et al. . Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex . J Headache Pain 2011. ; 12 ( 2 ): 185 – 91 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graff-Guerrero A, González-Olvera J, Fresán A , et al. . Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain . Brain Res Cogn Brain Res 2005. ; 25 ( 1 ): 153 – 60 . [DOI] [PubMed] [Google Scholar]
- 23. Taylor JJ, Borckardt JJ, George MS. Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia . Pain 2012. ; 153 ( 6 ): 1219 – 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vaseghi B, Zoghi M, Jaberzadeh S. Does anodal transcranial direct current stimulation modulate sensory perception and pain? A meta-analysis study . Clin Neurophysiol 2014. ; 125 ( 9 ): 1847 – 58 . [DOI] [PubMed] [Google Scholar]
- 25. Boggio PS, Zaghi S, Fregni F. Modulation of emotions associated with images of human pain using anodal transcranial direct current stimulation (tDCS) . Neuropsychologia 2009. ; 47 ( 1 ): 212 – 7 . [DOI] [PubMed] [Google Scholar]
- 26. Maeoka H, Matsuo A, Hiyamizu M, Morioka S, Ando H. Influence of transcranial direct current stimulation of the dorsolateral prefrontal cortex on pain related emotions: A study using electroencephalographic power spectrum analysis . Neurosci Lett 2012. ; 512 ( 1 ): 12 – 6 . [DOI] [PubMed] [Google Scholar]
- 27. Wang J, Wang Y, Hu Z, Li X. Transcranial direct current stimulation of the dorsolateral prefrontal cortex increased pain empathy . Neuroscience 2014. ; 281C : 202 – 7 . [DOI] [PubMed] [Google Scholar]
- 28. Rêgo GG, Lapenta OM, Marques LM , et al. . Hemispheric dorsolateral prefrontal cortex lateralization in the regulation of empathy for pain . Neurosci Lett 2015. ; 594 : 12 – 6 . [DOI] [PubMed] [Google Scholar]
- 29. Naylor JC, Borckardt JJ, Marx CE , et al. . Cathodal and anodal left prefrontal tDCS and the perception of control over pain . Clin J Pain 2014. ; 30 ( 8 ): 693 – 700 . [DOI] [PubMed] [Google Scholar]
- 30. Mariano TY, van’t Wout M, Jacobson BL , et al. . Effects of transcranial direct current stimulation (tDCS) on pain distress tolerance: A preliminary study . Pain Med 2015. ; 16 ( 8 ): 1580 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aghion DM, Rees Cosgrove G. Chapter 5 - Surgical Interventions for Pain . In: Saab CY , editor. Chronic Pain and Brain Abnormalities [Internet] . San Diego: : Academic Press; ; 2014. : 75 – 93 . Available at: http://www.sciencedirect.com/science/article/pii/B9780123983893000054 (accessed September 3, 2015). [Google Scholar]
- 32. Ballantine HT, Cassidy WL, Flanagan NB, Marino R. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain . J Neurosurg 1967. ; 26 ( 5 ): 488 – 95 . [DOI] [PubMed] [Google Scholar]
- 33. Foltz EL, White LE. Pain “relief” by frontal cingulumotomy . J Neurosurg 1962. ; 19 : 89 – 100 . [DOI] [PubMed] [Google Scholar]
- 34. Spangler WJ, Cosgrove GR, Ballantine HT , et al. . Magnetic resonance image-guided stereotactic cingulotomy for intractable psychiatric disease . Neurosurgery 1996. ; 38 ( 6 ): 1071 – 6; discussion 1076–8. [PubMed] [Google Scholar]
- 35. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition With Psychotic Screen. (SCID-I/P W/PSYCHOTIC SCREEN, 1/2007 revision) . New York, NY: : Biometrics Research Department, New York State Psychiatric Institute; ; 2007. . [Google Scholar]
- 36. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans . Neurology 2001. ; 57 ( 10 ): 1899 – 901 . [DOI] [PubMed] [Google Scholar]
- 37. O’Connell NE, Cossar J, Marston L , et al. . Rethinking clinical trials of transcranial direct current stimulation: Participant and assessor blinding is inadequate at intensities of 2mA . PLoS One 2012. ; 7 ( 10 ): e47514 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fecteau S, Knoch D, Fregni F , et al. . Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: A direct current stimulation study . J Neurosci 2007. ; 27 ( 46 ): 12500 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Knoch D, Nitsche MA, Fischbacher U , et al. . Studying the neurobiology of social interaction with transcranial direct current stimulation–The example of punishing unfairness . Cereb Cortex 2008. ; 18 ( 9 ): 1987 – 90 . [DOI] [PubMed] [Google Scholar]
- 40. Buckenmaier CC, Galloway KT, Polomano RC , et al. . Preliminary validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a military population . Pain Med 2013. ; 14 ( 1 ): 110 – 23 . [DOI] [PubMed] [Google Scholar]
- 41. Compton MA. Cold-pressor pain tolerance in opiate and cocaine abusers: Correlates of drug type and use status . J Pain Symptom Manage 1994. ; 9 ( 7 ): 462 – 73 . [DOI] [PubMed] [Google Scholar]
- 42. Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts . J Abnorm Psychol 2002. ; 111 ( 1 ): 180 – 5 . [PubMed] [Google Scholar]
- 43. Brown RA, Lejuez CW, Strong DR , et al. . A prospective examination of distress tolerance and early smoking lapse in adult self-quitters . Nicotine Tob Res 2009. ; 11 ( 5 ): 493 – 502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hezel DM, Riemann BC, McNally RJ. Emotional distress and pain tolerance in obsessive-compulsive disorder . J Behav Ther Exp Psychiatry 2012. ; 43 ( 4 ): 981 – 7 . [DOI] [PubMed] [Google Scholar]
- 45. Zettle R, Hocker T, Mick K , et al. . Differential strategies in coping with pain as a function of level of experiential avoidance . Psychol Rec [Internet] 2005. ; 55 ( 4 ): Available at: http://opensiuc.lib.siu.edu/tpr/Vol01704/iss4/1 (accessed July 31, 2015). [Google Scholar]
- 46. Hodes RL, Howland EW, Lightfoot N, Cleeland CS. The effects of distraction on responses to cold pressor pain . Pain 1990. ; 41 ( 1 ): 109 – 14 . [DOI] [PubMed] [Google Scholar]
- 47. Hooley JM, Delgado ML. Pain insensitivity in the relatives of schizophrenia patients . Schizophr Res 2001. ; 47 ( 2-3 ): 265 – 73 . [DOI] [PubMed] [Google Scholar]
- 48. Pud D, Golan Y, Pesta R. Hand dominancy–A feature affecting sensitivity to pain . Neurosci Lett 2009. ; 467 ( 3 ): 237 – 40 . [DOI] [PubMed] [Google Scholar]
- 49. Ren Z-Y, Shi J, Epstein DH, Wang J, Lu L. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving . Psychopharmacology 2009. ; 204 ( 3 ): 423 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vigil JM, Rowell LN, Alcock J, Maestes R. Laboratory personnel gender and cold pressor apparatus affect subjective pain reports . Pain Res Manage 2014. ; 19 ( 1 ): e13 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers . Eur J Neurol 2008. ; 15 ( 10 ): 1124 – 30 . [DOI] [PubMed] [Google Scholar]
- 52. Batsikadze G, Moliadze V, Paulus W, Kuo M-F, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans . J Physiol 2013. ; 591 ( Pt 7 ): 1987 – 2000 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nahmias F, Debes C, de Andrade DC, Mhalla A, Bouhassira D. Diffuse analgesic effects of unilateral repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers . Pain 2009. ; 147 ( 1-3 ): 224 – 32 . [DOI] [PubMed] [Google Scholar]
- 54. Antal A, Terney D, Kühnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition . J Pain Symptom Manage 2010. ; 39 ( 5 ): 890 – 903 . [DOI] [PubMed] [Google Scholar]
- 55. Auvichayapat P, Janyacharoen T, Rotenberg A , et al. . Migraine prophylaxis by anodal transcranial direct current stimulation, a randomized, placebo-controlled trial . J Med Assoc Thail Chotmaihet Thangphaet 2012. ; 95 ( 8 ): 1003 – 12 . [PubMed] [Google Scholar]
- 56. Fregni F, Gimenes R, Valle AC , et al. . A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia . Arthritis Rheum 2006. ; 54 ( 12 ): 3988 – 98 . [DOI] [PubMed] [Google Scholar]
- 57. Kim YJ, Ku J, Kim HJ , et al. . Randomized, sham controlled trial of transcranial direct current stimulation for painful diabetic polyneuropathy . Ann Rehabil Med 2013. ; 37 ( 6 ): 766.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mori F, Codecà C, Kusayanagi H , et al. . Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis . J Pain 2010. ; 11 ( 5 ): 436 – 42 . [DOI] [PubMed] [Google Scholar]
- 59. Valle A, Roizenblatt S, Botte S , et al. . Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: Results of a randomized, sham-controlled longitudinal clinical trial . J Pain Manage 2009. ; 2 ( 3 ): 353 – 61 . [PMC free article] [PubMed] [Google Scholar]
- 60. Zhuo M. Long-term potentiation in the anterior cingulate cortex and chronic pain . Philos Trans R Soc Lond B Biol Sci 2014. ; 369 ( 1633 ): 20130146 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sevel LS, O’Shea AM, Letzen JE , et al. . Effective connectivity predicts future placebo analgesic response: A dynamic causal modeling study of pain processing in healthy controls . NeuroImage 2015. ; 110 : 87 – 94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Egorova N, Yu R, Kaur N , et al. . Neuromodulation of conditioned placebo/nocebo in heat pain: Anodal vs cathodal transcranial direct current stimulation to the right dorsolateral prefrontal cortex . Pain 2015. ; 156 ( 7 ): 1342 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ironside M, O’Shea J, Cowen PJ, Harmer CJ. Frontal cortex stimulation reduces vigilance to threat: implications for the treatment of depression and anxiety . Biol Psychiatry [Internet] 2015. . Available at: http://www.biologicalpsychiatryjournal.com/article/S0006322315004928/abstract (accessed July 24, 2015). [DOI] [PubMed] [Google Scholar]