Abstract
Cardiovascular tissue engineering offers the promise of biologically based repair of injured and damaged blood vessels, valves, and cardiac tissue. Major advances in cardiovascular tissue engineering over the past few years involve improved methods to promote the establishment and differentiation of induced pluripotent stem cells (iPSCs), scaffolds from decellularized tissue that may produce more highly differentiated tissues and advance clinical translation, improved methods to promote vascularization, and novel in vitro microphysiological systems to model normal and diseased tissue function. iPSC technology holds great promise, but robust methods are needed to further promote differentiation. Differentiation can be further enhanced with chemical, electrical, or mechanical stimuli.
Keywords: Tissue engineering, cardiovascular, iPSCs
Introduction
Tissue engineering involves the development of functional replacements for damaged tissues or organs ( http://www.nibib.nih.gov/science-education/science-topics/tissue-engineering-and-regenerative-medicine). A common approach to produce engineered tissues is to add cells to a natural or synthetic extracellular matrix, which provides mechanical support and biochemical cues. Scaffold-free tissues are prepared by growing cells on thermally responsive polymers to facilitate the cell monolayers that form and then adding layers together or rolling the sheets. By addition of small molecules that activate specific differentiation pathways, three-dimensional organoids can be derived from human pluripotent stem cells 1. The engineered tissue may be prepared wholly or partially before implantation to activate and localize the body’s regenerative capacity to populate the implanted scaffold. Tissue engineering is a subset of the broader field of regenerative medicine, which seeks to repair or replace damaged organs. This could occur by direct injection of cells or modifying cellular processes to initiate repair and regrowth. In spite of significant research advances and insightful application of developmental cell biology, cell mechanics, and biomaterials, few products have emerged from these efforts to date, pointing to the challenges to develop truly functional tissues.
Key design goals to produce functional tissues in vitro are to reproduce the tissue structure and cell density in vivo, identify suitable sources of cells, promote growth and differentiation of cells, design a construct that reproduces the extracellular matrix with the appropriate molecular cues and suitable mechanical properties, and create a vasculature within the construct to enable oxygenation and integration with host vasculature after implantation.
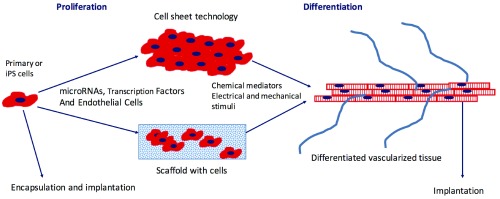
Several strategies that have emerged to address these challenges include the use of induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) that can differentiate into the cells of interest, the reprogramming of primary cells to the cell type of interest, various ways to engineer the structural support for the cells to mimic the extracellular matrix, and efforts to promote vascular network formation ( Figure 1). Over the past few years, a new use of tissue engineering has emerged in which microscale human tissue-engineered systems or microphysiological systems are used to model normal and disease states in vitro and assess drug responses.
Figure 1. Schematic of processes to produce cell-based engineered cardiac or vascular tissue.
Primary cells or induced pluripotent stem (iPS) cells undergo a period of proliferation prior to seeding into a three-dimensional scaffold or are grown as sheets on a polymer whose conformation changes in response to temperature or other stimuli, enabling detachment on the sheet. During the proliferation phase, microRNAs or transcription factors may be added to the cells to promote subsequent differentiation. To promote further differentiation, small molecules are added and/or cells are exposed to electrical or mechanical stimuli. Endothelial cells added to the tissue during formation promote vascular network formation. After the cells have reached a certain level of maturity, the engineered tissue is implanted and the host blood supply connects with the vascular network promoted by endothelial cells. Alternatively, cells may be encapsulated in a biodegradable polymer and implanted.
Recent advances in tissue engineering over the past three years were recently summarized in two reviews 2, 3. Given the breadth of tissue engineering research (13,661 publications since 1 January 2014 reported on Google Scholar), we focus this summary of recent work on cardiovascular tissue engineering as a way to demonstrate how new research results have addressed the key design challenges. Cardiovascular tissue engineering is a vibrant area of research, and applications in the cardiovascular system include cardiac patches, engineered blood vessels and heart valves, and vascular networks.
Scaffolds
Scaffold materials should match the mechanical properties of the tissue and undergo degradation or be integrated into the tissue, allowing the natural extracellular matrix to replace the original structural support. Materials used in cardiovascular tissue engineering include degradable polymers, such as polyglycolic acid 4 and polylactic acid, as well as biological hydrogels, such as collagen 5, fibrin 6, and modified hyaluronic acid 7. These scaffold materials can be modified by the addition of cell adhesion domains or sites susceptible to cleavage by matrix metalloproteinases to facilitate cell attachment and migration. Alternatively, sheets of cells can be prepared and then fabricated into different configurations such as tubes or patches. Such structures have high mechanical strength and function well in vivo 8. This approach has the advantage of not needing any synthetic polymers.
A scaffold-free cardiac patch consisting of three layers of rat cardiomyocytes was successfully engrafted onto heart tissue by overlaying the patch over a vascular supply, enhancing the ability of endothelial cells (ECs) in the patch to form a functional tubular vascular network connected to the host blood supply 9. Recently, the development of a porous patch with an electroactive polypyrrole incorporating electronics for sensing and stimulating electrophysiological activity and release of various biological molecules offers a new level of control of cardiac patches while permitting incorporation of cells attached to a bioactive scaffold 10.
While tissue-engineered blood vessels (TEBVs) constructed from natural matrix components such as collagen 11, 12 and fibrin 13, 14 have traditionally exhibited poor mechanical strength, plastic compression of collagen gels embedded with smooth muscle cells (SMCs) increases the collagen fiber density and yields rapidly producible tubular structures with high mechanical strength 15. By plastic compression of collagen, TEBVs with burst pressures exceeding 1600 mmHg can be prepared in a few hours 5. After one week of perfusion at physiological shear stresses, the medial cells exhibited differentiation and contracted in response to phenylephrine. While these TEBVs have not been studied in vivo, this rapid method of fabrication could significantly reduce the time to produce functional TEBVs.
Decellularized tissue contains many of the cues needed for cells to differentiate and responds dynamically after implantation, owing to cellular infiltration and imposed biomechanical loads. For example, after implantation of decellularized valves in sheep, collagen reorganized, responding to biomechanical stresses 16. Increased waviness of collagen corresponded to areas of greater elastin synthesis 16. Decellularization does cause loss and damage to some extracellular matrix proteins. To overcome this limitation, the addition of hyaluronic acid supplement enhanced adhesion in decellularized heart tissue 17. While decellularized tissue as thick as 1–1.5 cm can be produced, mesenchymal stem cells (MSCs) added to the decellularized constructs reached a cell density of 30 million cells/cm 3 but occupied only the outer 100 µm of the decellularized heart, suggesting that their growth was limited by oxygen levels. When the constructs were perfused, cells migrated as far as 400 µm into the decellularized tissue. The decellularized heart could support cardiomyocyte function as demonstrated by ESCs that exhibited beating three days after seeding 17.
Decellularized grafts can be modified to enhance their key functions. Immobilization of heparin to decellularized blood vessels using click chemistry reduced platelet adhesion and promoted EC attachment without altering the graft mechanical behavior 18. Selective attachment of biological molecules is preferable to passive adsorption in attempting to compensate for damage to the extracellular matrix during removal of cells. Rather than harvest and remove cells from blood vessels, the extracellular matrix synthesized by cultured SMCs can be used to create decellularized vessels in a tubular polyglycolic acid scaffold 19, thus providing a more controlled source of readily available extracellular matrix. A similar approach was used to create decellularized heart valves 20, which were repopulated with cells eight weeks after implantation and performed better than decellularized valves.
For valve leaflets, the need to have regional variations in cell types and material properties was achieved using decellularized valves or using injection molding 21 or three-dimensional bioprinting 7 to fabricate specific three-dimensional shapes. Since decellularized tissue can be formed into hydrogels 22 or electrospun, the use of different fabrication methods creates the possibility of precisely designing the tissue to be replaced.
A novel approach to generate the entire TEBV in vivo involves taking advantage of the foreign body response and implanting a mandrel subcutaneously around which a tubular tissue grows over a four-week period 23. Initially, the graft consisted of extracellular matrix and fibroblasts with a layer of M1 macrophages. After forming an end-to-end carotid anastomosis in the pig, the macrophages disappeared. After four weeks of grafting, the gene expression profile became similar to that of the carotid artery and fibroblasts adopted a contractile phenotype. The mechanical strength was very good but was less than values for actual vessels. This is a promising approach to develop engineered blood vessels, and other applications involve contracting SMCs (e.g. the bladder), which can be derived from fibroblasts involved in the foreign body response. Extending to other organ systems with specialized cells may prove difficult.
Stem cells for tissue engineering
iPSCs offer the potential to develop engineered tissues of individual human cardiovascular disease states and avoid ethical issues associated with ESCs. iPSCs can be induced to differentiate into a large number of cell types including cardiomyocytes, SMCs 24, 25, and ECs 24. The formation of teratomas 26 can be reduced using non-integrating methods 27 and immunogenicity is low 28. An exciting new development has been the creation of mouse iPSCs using small molecules that activate specific transcription factors 29, although this approach has not yet been demonstrated with human iPSCs. A challenge with the use of iPSCs in tissue engineering is that differentiation is often limited and the resulting structures do not display a mature phenotype 30, 31.
Vascular cells can be obtained from iPSCs or ESCs by first activating the Wnt signaling pathway. Early activation of Wnt and β-catenin by inhibition of glycogen synthase kinase 3 (GSK3) before differentiation on surfaces with serum produces cardiomyocytes 32. Following Wnt pathway activation with GSK3 inhibitors, ECs can be obtained by addition of vascular endothelial growth factor (VEGF) and forskolin, while SMCs can be obtained using platelet-derived growth factor-BB (PDGF-BB) and ActivinA 25. Interestingly, a recent report indicated that by culturing murine iPSCs on gelatin-coated polycaprolactone nanofibrous scaffolds, Wnt/β-catenin can be transiently activated to induce differentiation towards cardiomyocytes 33. Combining GSK3 inhibitors with specific modification of substrate properties may lead to more robust differentiation.
One week of electrical stimulation at 0.5, 1, or 2 Hz of human ESCs or iPSCs in three-dimensional engineered tissues facilitates differentiation to cardiomyocytes by producing hypertrophy, an increase in connexin-43 gap junctions, and increased expression of hERG, the potassium channel which regulates cardiomyocyte repolarization 34. Some connexin-40 is expressed, indicating that rapidly conducting cells can be stimulated; however, it is not yet possible to regulate the relative expression of the various connexins though selection of a specific stimulation protocol. The stimulated cells responded to chronotropic drugs and the cells maintained synchrony to the rate of applied stimulation for two weeks after the stimulation ended.
Cardiomyocytes generated by selecting for Nkx2-5-positive cells among mouse iPSCs exhibit a number of markers found in mature cardiomyocytes, and the resting membrane potential approaches physiological levels 35. Three-dimensional engineered tissues produced aligned cardiomyocytes that exhibited adherens and gap junctions, although the electrophysiological responses were similar to those exhibited by fetal cardiomyocytes 35.
Human ESCs in three-dimensional patches showed extensive maturation and exhibited β-adrenergic responses in the physiological range 6. Engineered cardiac tissue derived from human ESCs integrated into damaged mouse myocardium and formed a vasculature connected to the host blood supply after 28 days but did not improve heart function owing to extensive cell loss 36. Alternatively, partial reprogramming of cardiac fibroblasts can be done using viral transfection of transcription factors, a cocktail of small molecules or microRNAs that activate key transcription factors (e.g. Mef2c, myocardin, and serum response factor). These approaches have yielded some success in producing spontaneously contracting cells, although the frequency of these cells among the population is low 37.
Culturing human cardiac myocytes derived from iPSCs on polydimethylsiloxane (PDMS) membranes coated with Matrigel for one week led to significant maturation of the cardiac cells in which the action potential upstroke velocity increased and conduction velocities were twice the value found when the cells were grown on Matrigel-coated glass coverslips 38, although this value was still about 57% of the in vivo value. This increased maturation was due to a substantial increase in increased inward rectifier potassium and sodium inward current densities, elevated connexin-43 protein expression, hypertrophy of the cardiomyocytes, and increased cardiac troponin β 1 integrin and focal adhesion kinase. The elastic modulus of the PDMS is approximately 4 MPa, much lower than the modulus of glass (~50 GPa), and the PDMS modulus is much greater than the modulus of cardiac tissue (0.1 MPa) 39, suggesting that substrates with lower elastic modulus might enhance differentiation further. Modulating the substrate stiffness together with mechanical loading and electrical stimulation, which promote physiological force-frequency and force-length relations 40, could produce cardiomyocytes with in vivo electrical and mechanical properties.
The use of small molecules to differentiate iPSCs has been used to create highly differentiated ECs that model the high transport resistance of brain ECs 41. A number of cardiac disease models have been generated using iPSC technology and could replicate the response to cardiotoxic drugs using cells from various individuals 42. The technology can also be used to assess the adaptive response to dilated cardiomyopathy. For example, cardiomyocytes derived from iPSCs of healthy individuals using small molecules exhibited many of the molecules involved in β-adrenergic signaling and isoproterenol treatment induced inotropic and chronotropic regulation of contractile function 43. However, cardiomyocytes derived from iPSCs of individuals with dilated cardiomyopathy exhibited abnormal sarcomere structure and deficits in contractile force, calcium handling, and beat frequency after treatment with isoproterenol that was traced to overexpression of phosphodiesterases 2 and 3a 43.
TEBVs fabricated with SMCs derived from murine 44 and human 45 iPSCs maintained their differentiated phenotype after subcutaneous implantation for two weeks. Contractile TEBVs with SMCs differentiated from iPSCs developed from human foreskin fibroblasts and MSCs demonstrated intermediate and late SMC proteins 46. While TEBVs derived from karyotypically normal human iPSC clones function well and express mid-differentiation markers SM-22α and calponin and secreted extracellular matrix, those derived from karyotypically abnormal clones exhibit senescence, shortened telomeres, and calcification 4.
Human blood-derived ECs can be reprogrammed to SMCs by activating myocardin using a lentivirus system 47. Functional TEBVs were produced with these cells that exhibited flow-mediated vasodilation and vasoconstriction in the presence of 1 µM phenylephrine 47. While the vasoactivity was somewhat less than that of primary cells 5, the results do show that TEBVs can be recreated with cells from a single donor.
Vascularization
The density and thickness of engineered tissues is limited by the transport of nutrients to the cells. Oxygen is often the limiting nutrient, since it is consumed at the highest rate and is critical for producing the energy needed for normal cell function. In vivo, capillary distances range from 15–50 µm depending on the cell density and the metabolic demands 48. In vitro, cell densities are lower, but uniform cell densities can be achieved only for thicknesses of about 100 µm owing to consumption of oxygen in the engineered tissue. Perfusion can lead to somewhat thicker tissues. However, without its own microvascular network that could integrate with the host network after implantation, only thin tissue-engineered constructs can remain viable after implantation.
While addition of VEGF can initiate the formation of new capillaries or branches from existing capillaries in vitro, the resulting structures are unstable and last at most a few days. New vessel formation involves several discrete stages. Initially, exogenous VEGF causes the release of matrix metalloproteinases, which degrade the extracellular matrix, enabling migration of the newly forming vessel buds. The newly forming vessel secretes growth factors to recruit mural cells, such as fibroblasts, pericytes, or SMCs, which interact with the newly formed microvessels, stabilizing them.
Although MSCs can stabilize EC networks in vitro and exhibit pericyte-like behavior 49, the heterogeneity of MSCs from various sources or by different isolation methods leads to variable responses 50. The cells must be characterized and tested for their ability to stabilize networks when developing a system to create microvascular networks. iPSCs could provide a ready source of pericytes 51, although SMCs or fibroblasts derived from iPSCs may be suitable.
A potentially useful model system to identify conditions that promote vascularization of tissue-engineered systems involves creating microvascular networks in a synthetic extracellular matrix hydrogel. The hydrogel contains matrix metalloproteinase degradation sites and peptide sequences of extracellular matrix proteins to elicit specific cell binding 52. Photopolymerization of polyethylene glycol gels enables straightforward incorporation of cells, and the networks are robust and sensitive to perfusion in the extracellular space 52. The direction of flow is very critical for improving mass transfer and enabling microvessels to stabilize 53. The extracellular matrix peptide sequences provided influence the extent of network formation, with addition of cell binding sequences from both fibronectin (RGD) and laminin (YIGSR) producing the most robust network formation in the hydrogel 54. Adding macrophages enhanced new vessel formation in synthetic hydrogels, consistent with their role in vivo 55. Other factors to enhance microvessel network formation in hydrogels involve regulating growth factor delivery 56 and a hypoxic environment 57.
Several approaches have been taken to incorporate vascular networks into tissue-engineered constructs for implantation. When ECs were added with MSCs to decellularized heart tissue, vascular networks formed and enabled cell growth further into the construct than could be accomplished with MSCs alone 17. The resulting network may have facilitated more effective fluid and nutrient transport throughout the decellularized tissue.
EC cords show promise as a method to create functional microvascular networks in engineered constructs 58, 59. The cords are formed by mixing ECs and mural cells in collagen. After shrinkage by 50% in diameter over four hours, the cords are encased in fibrin and integrated into the tissue-engineered construct 58. After implantation of cords into mice, capillaries formed within seven days and matured by 14 days. Red cells were observed in the lumen and an EC monolayer formed, defining the capillary border 58. The capillaries involved both donor and host ECs. When the EC and MSC cords were added with hepatic construct, they improved key hepatocyte functions 58. Cord diameters of 25, 75, and 250 µm all produced functional capillary networks, although smaller cords produced a higher density of vessels and the larger cords led to more dispersed vessels 59. Mural cells were not necessary to form functioning capillaries after implantation, possibly owing to the involvement of host mural cells 59. This approach can be used to control the density and geometry of the microvascular network, two properties that vary based on demand and function of the tissue.
Microphysiological systems
High-throughput screens for function or to test drugs are being developed by integrating tissue engineering, microfluidics, and advanced methods of sensing. The National Center for Advancing Translational Science (NCATS) at NIH and the Defense Advanced Research Programs Association have led an effort to advance individual microphysiological systems and examine interactions among different organ systems. Microphysiological systems have been developed for the heart 34, 60, blood vessels 5, microcirculation 53, kidney 61, gut 62, lung 63, liver 64, skeletal muscle 65, and female reproductive tract 66. The small size of the systems reduces or eliminates mass transfer limitations, and function can be monitored with sensors or reporter systems. These systems have been developed using a combination of primary human cells and iPSCs. iPSCs provide the ability to create patient-specific cardiovascular disease models owing to their ability to maintain the disease phenotype post-differentiation 67, 68. Gene editing makes feasible isogenic controls for in vitro studies 69.
Microphysiological systems based on the cardiovascular system reproduce key functions and known drug responses. Human endothelialized TEBVs with inner diameters of 500–800 µm exhibit a dose-dependent contraction in response to phenylephrine and a dose-dependent relaxation following exposure to acetylcholine over five weeks in culture 5. The TEBVs elicited reversible activation to acute inflammatory stimulation by TNF-α, which was blocked by pre-treating the TEBVs with statins 5 and consistent with the pleotropic effect that statins exert on ECs 70.
Several different microphysiological systems have been developed to model cardiac function. In one, cardiomyocytes are grown on poly(N-isopropylacrylamide) (PIPAAm) in a microfluidic chamber 71. Forces exerted by contracting cardiomyocytes are determined from deformation of PIPAAm. As many as 28 PIPAAm cantilevers can be incorporated in one chip, and the fluidics enable easy exchange after drug or agonist exposure. This system was used to study Barth syndrome, an X-linked mutation of an acyltransferase essential for modification of cardiolipin. Individuals with this syndrome die within a year of birth due to heart failure and/or infection. This cardiac microphysiological system showed reduced contractile stresses by cardiomyocytes derived from Barth syndrome iPSCs 72. Contractile stresses returned to normal for cells treated with a modified RNA that corrected the mutation and was improved after treatment with linoleic acid 72, suggesting a novel treatment. An alternative approach to quantify contraction involves measuring strains using digital image correlation software to analyze the deformation of the engineered muscle 34. To convert to stress, the stress-strain behavior of the muscle is needed.
Another system provided short transport distances and confined cardiomyocytes at high cell density in a microfluidic chamber 60. The confinement barrier mimics the diffusive resistance of an endothelial monolayer but lacks the biochemical signals that arise from EC-cardiomyocyte interactions. Aligned and synchronously beating human cardiomyocytes derived from iPSCs were produced over a period of seven days. Cardiac cell motion was analyzed using custom software and was found to accurately represent cardiac cell responses to calcium channel and hERG blockers and β-adrenergic agonists and antagonists. The system can easily integrate the measurement of reporter fluorescence assays and analysis of the media after perfusion 60.
Summary and future directions
New technologies to promote cell differentiation and vascularize engineered constructs address key challenges in making viable engineered tissues that can be implanted. At the same time, decellularized tissues, either derived from organs and tissues or fabricated in the lab, make available an alternative approach to tissue engineering in which the implanted matrix serves as a substrate to guide cell repopulation and differentiation after implantation. Both approaches have aided our understanding of the complex interactions between cells and the extracellular matrix in producing a functional tissue.
Acknowledgements
I appreciate the many helpful conversations with my colleagues Nenad Bursac and William Kraus and students Cristina Fernandez and Leigh Atchison.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Irving H Zucker, Department of Cellular and Integrative Physiology, University of Nebraska Medical Center, Omaha, NE, 68198, USA
Gordana Vunjak-Novakovic, Departments of Biomedical Engineering and Medicine, Columbia University, New York, NY, USA
David Schaffer, Department of Bioengineering, Department of Chemical and Biomolecular Engineering and Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA, 94720-3220, USA
Funding Statement
This work was supported, in part, by NIH grant UH3TR000505.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Jones JR, Zhang SC: Engineering human cells and tissues through pluripotent stem cells. Curr Opin Biotechnol. 2016;40:133–8. 10.1016/j.copbio.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrison RH, St-Pierre JP, Stevens MM: Tissue engineering and regenerative medicine: a year in review. Tissue Eng Part B Rev. 2014;20(1):1–16. 10.1089/ten.TEB.2013.0668 [DOI] [PubMed] [Google Scholar]
- 3. Wobma H, Vunjak-Novakovic G: Tissue Engineering and Regenerative Medicine 2015: A Year in Review. Tissue Eng Part B Rev. 2016;22(2):101–13. 10.1089/ten.TEB.2015.0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sundaram S, One J, Siewert J, et al. : Tissue-engineered vascular grafts created from human induced pluripotent stem cells. Stem Cells Transl Med. 2014;3(12):1535–43. 10.5966/sctm.2014-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez CE, Yen RW, Perez SM, et al. : Human Vascular Microphysiological System for in vitro Drug Screening. Sci Rep. 2016;6: 21579. 10.1038/srep21579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang D, Shadrin IY, Lam J, et al. : Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34(23):5813–20. 10.1016/j.biomaterials.2013.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duan B, Kapetanovic E, Hockaday LA, et al. : Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014;10(5):1836–46. 10.1016/j.actbio.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. L'Heureux N, Dusserre N, Konig G, et al. : Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12(3):361–5. 10.1038/nm1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sekine H, Shimizu T, Sakaguchi K, et al. : In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4: 1399. 10.1038/ncomms2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feiner R, Engel L, Fleischer S, et al. : Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat Mater. 2016. 10.1038/nmat4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weinberg CB, Bell E: A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231(4736):397–400. 10.1126/science.2934816 [DOI] [PubMed] [Google Scholar]
- 12. Boccafoschi F, Rajan N, Habermehl J, et al. : Preparation and characterization of a scaffold for vascular tissue engineering by direct-assembling of collagen and cells in a cylindrical geometry. Macromol Biosci. 2007;7(5):719–26. 10.1002/mabi.200600242 [DOI] [PubMed] [Google Scholar]
- 13. Isenberg BC, Tranquillo RT: Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng. 2003;31(8):937–49. 10.1114/1.1590662 [DOI] [PubMed] [Google Scholar]
- 14. Syedain ZH, Meier LA, Bjork JW, et al. : Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2011;32(3):714–22. 10.1016/j.biomaterials.2010.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghezzi CE, Risse PA, Marelli B, et al. : An airway smooth muscle cell niche under physiological pulsatile flow culture using a tubular dense collagen construct. Biomaterials. 2013;34(8):1954–66. 10.1016/j.biomaterials.2012.11.025 [DOI] [PubMed] [Google Scholar]
- 16. Ghazanfari S, Driessen-Mol A, Sanders B, et al. : In Vivo Collagen Remodeling in the Vascular Wall of Decellularized Stented Tissue-Engineered Heart Valves. Tissue Eng Part A. 2015;21(15–16):2206–15. 10.1089/ten.TEA.2014.0417 [DOI] [PubMed] [Google Scholar]
- 17. Sarig U, Nguyen EB, Wang Y, et al. : Pushing the envelope in tissue engineering: ex vivo production of thick vascularized cardiac extracellular matrix constructs. Tissue Eng Part A. 2015;21(9–10):1507–19. 10.1089/ten.tea.2014.0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dimitrievska S, Cai C, Weyers A, et al. : Click-coated, heparinized, decellularized vascular grafts. Acta Biomater. 2015;13:177–87. 10.1016/j.actbio.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dahl SL, Kypson AP, Lawson JH, et al. : Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3(68):68ra9. 10.1126/scitranslmed.3001426 [DOI] [PubMed] [Google Scholar]
- 20. Weber B, Dijkman PE, Scherman J, et al. : Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials. 2013;34(30):7269–80. 10.1016/j.biomaterials.2013.04.059 [DOI] [PubMed] [Google Scholar]
- 21. Weber M, Gonzalez de Torre I, Moreira R, et al. : Multiple-Step Injection Molding for Fibrin-Based Tissue-Engineered Heart Valves. Tissue Eng Part C Methods. 2015;21(8):832–40. 10.1089/ten.TEC.2014.0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang RM, Christman KL: Decellularized myocardial matrix hydrogels: In basic research and preclinical studies. Adv Drug Deliv Rev. 2016;96:77–82. 10.1016/j.addr.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rothuizen TC, Damanik FF, Lavrijsen T, et al. : Development and evaluation of in vivo tissue engineered blood vessels in a porcine model. Biomaterials. 2016;75:82–90. 10.1016/j.biomaterials.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 24. Bao X, Lian X, Dunn KK, et al. : Chemically-defined albumin-free differentiation of human pluripotent stem cells to endothelial progenitor cells. Stem Cell Res. 2015;15(1):122–9. 10.1016/j.scr.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patsch C, Challet-Meylan L, Thoma EC, et al. : Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol. 2015;17(8):994–1003. 10.1038/ncb3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herberts CA, Kwa MS, Hermsen HP: Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. 10.1186/1479-5876-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schlaeger TM, Daheron L, Brickler TR, et al. : A comparison of non-integrating reprogramming methods. Nat Biotechnol. 2015;33(1):58–63. 10.1038/nbt.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neofytou E, O'Brien CG, Couture LA, et al. : Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest. 2015;125(7):2551–7. 10.1172/JCI80575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hou P, Li Y, Zhang X, et al. : Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–4. 10.1126/science.1239278 [DOI] [PubMed] [Google Scholar]
- 30. Gieseck RL, 3rd, Hannan NR, Bort R, et al. : Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS One. 2014;9(1):e86372. 10.1371/journal.pone.0086372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang H, Xiong ZM, Cao K: Mechanisms controlling the smooth muscle cell death in progeria via down-regulation of poly(ADP-ribose) polymerase 1. Proc Natl Acad Sci U S A. 2014;111(22):E2261–70. 10.1073/pnas.1320843111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lian X, Hsiao C, Wilson G, et al. : Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109(27):E1848–57. 10.1073/pnas.1200250109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen Y, Zeng D, Ding L, et al. : Three-dimensional poly-(ε-caprolactone) nanofibrous scaffolds directly promote the cardiomyocyte differentiation of murine-induced pluripotent stem cells through Wnt/β-catenin signaling. BMC Cell Biol. 2015;16:22. 10.1186/s12860-015-0067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eng G, Lee BW, Protas L, et al. : Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nat Commun. 2016;7: 10312. 10.1038/ncomms10312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Christoforou N, Liau B, Chakraborty S, et al. : Induced pluripotent stem cell-derived cardiac progenitors differentiate to cardiomyocytes and form biosynthetic tissues. PLoS One. 2013;8(6):e65963. 10.1371/journal.pone.0065963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riegler J, Tiburcy M, Ebert A, et al. : Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ Res. 2015;117(8):720–30. 10.1161/CIRCRESAHA.115.306985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Monaghan MG, Holeiter M, Layland SL, et al. : Cardiomyocyte generation from somatic sources - current status and future directions. Curr Opin Biotechnol. 2016;40:49–55. 10.1016/j.copbio.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 38. Herron TJ, Rocha AM, Campbell KF, et al. : Extracellular Matrix-Mediated Maturation of Human Pluripotent Stem Cell-Derived Cardiac Monolayer Structure and Electrophysiological Function. Circ Arrhythm Electrophysiol. 2016;9(4): pii: e003638. 10.1161/CIRCEP.113.003638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mathur AB, Collinsworth AM, Reichert WM, et al. : Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J Biomech. 2001;34(12):1545–53. 10.1016/S0021-9290(01)00149-X [DOI] [PubMed] [Google Scholar]
- 40. Godier-Furnémont AF, Tiburcy M, Wagner E, et al. : Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials. 2015;60:82–91. 10.1016/j.biomaterials.2015.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lippmann ES, Al-Ahmad A, Azarin SM, et al. : A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci Rep. 2014;4: 4160. 10.1038/srep04160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karakikes I, Ameen M, Termglinchan V, et al. : Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ Res. 2015;117(1):80–8. 10.1161/CIRCRESAHA.117.305365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu H, Lee J, Vincent LG, et al. : Epigenetic Regulation of Phosphodiesterases 2A and 3A Underlies Compromised β-Adrenergic Signaling in an iPSC Model of Dilated Cardiomyopathy. Cell Stem Cell. 2015;17(1):89–100. 10.1016/j.stem.2015.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie C, Hu J, Ma H, et al. : Three-dimensional growth of iPS cell-derived smooth muscle cells on nanofibrous scaffolds. Biomaterials. 2011;32(19):4369–75. 10.1016/j.biomaterials.2011.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Y, Hu J, Jiao J, et al. : Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials. 2014;35(32):8960–9. 10.1016/j.biomaterials.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bajpai VK, Mistriotis P, Loh YH, et al. : Functional vascular smooth muscle cells derived from human induced pluripotent stem cells via mesenchymal stem cell intermediates. Cardiovasc Res. 2012;96(3):391–400. 10.1093/cvr/cvs253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ji H, Atchison L, Chen Z, et al. : Transdifferentiation of human endothelial progenitors into smooth muscle cells. Biomaterials. 2016;85:180–94. 10.1016/j.biomaterials.2016.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Truskey GA, Yuan F, Katz DF: Transport Phenomena in Biological Systems. 2nd ed. Upper Saddle River: Pearson Prentice Hall;2009. Reference Source [Google Scholar]
- 49. Peters EB, Christoforou N, Moore E, et al. : CD45+ Cells Present Within Mesenchymal Stem Cell Populations Affect Network Formation of Blood-Derived Endothelial Outgrowth Cells. Biores Open Access. 2015;4(1):75–88. 10.1089/biores.2014.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blocki A, Wang Y, Koch M, et al. : Not all MSCs can act as pericytes: functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem Cells Dev. 2013;22(17):2347–55. 10.1089/scd.2012.0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kusuma S, Facklam A, Gerecht S: Characterizing human pluripotent-stem-cell-derived vascular cells for tissue engineering applications. Stem Cells Dev. 2015;24(4):451–8. 10.1089/scd.2014.0377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cuchiara MP, Gould DJ, McHale MK, et al. : Integration of Self-Assembled Microvascular Networks with Microfabricated PEG-Based Hydrogels. Adv Funct Mater. 2012;22(21):4511–8. 10.1002/adfm.201200976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alonzo LF, Moya ML, Shirure VS, et al. : Microfluidic device to control interstitial flow-mediated homotypic and heterotypic cellular communication. Lab Chip. 2015;15(17):3521–9. 10.1039/c5lc00507h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ali S, Saik JE, Gould DJ, et al. : Immobilization of Cell-Adhesive Laminin Peptides in Degradable PEGDA Hydrogels Influences Endothelial Cell Tubulogenesis. Biores Open Access. 2013;2(4):241–9. 10.1089/biores.2013.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hsu CW, Poché RA, Saik JE, et al. : Improved Angiogenesis in Response to Localized Delivery of Macrophage-Recruiting Molecules. PLoS One. 2015;10(7):e0131643. 10.1371/journal.pone.0131643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park KM, Gerecht S: Harnessing developmental processes for vascular engineering and regeneration. Development. 2014;141(14):2760–9. 10.1242/dev.102194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chan XY, Black R, Dickerman K, et al. : Three-Dimensional Vascular Network Assembly From Diabetic Patient-Derived Induced Pluripotent Stem Cells. Arterioscler Thromb Vasc Biol. 2015;35(12):2677–85. 10.1161/ATVBAHA.115.306362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baranski JD, Chaturvedi RR, Stevens KR, et al. : Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci U S A. 2013;110(19):7586–91. 10.1073/pnas.1217796110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chaturvedi RR, Stevens KR, Solorzano RD, et al. : Patterning vascular networks in vivo for tissue engineering applications. Tissue Eng Part C Methods. 2015;21(5):509–17. 10.1089/ten.TEC.2014.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mathur A, Loskill P, Shao K, et al. : Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep. 2015;5: 8883. 10.1038/srep08883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ligresti G, Nagao RJ, Xue J, et al. : A Novel Three-Dimensional Human Peritubular Microvascular System. J Am Soc Nephrol. 2015; pii: ASN.2015070747. 10.1681/ASN.2015070747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zachos NC, Kovbasnjuk O, Foulke-Abel J, et al. : Human Enteroids/Colonoids and Intestinal Organoids Functionally Recapitulate Normal Intestinal Physiology and Pathophysiology. J Biol Chem. 2016;291(8):3759–66. 10.1074/jbc.R114.635995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Benam KH, Villenave R, Lucchesi C, et al. : Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13(2):151–7. 10.1038/nmeth.3697 [DOI] [PubMed] [Google Scholar]
- 64. Vernetti LA, Senutovitch N, Boltz R, et al. : A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp Biol Med (Maywood). 2016;241(1):101–14. 10.1177/1535370215592121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Madden L, Juhas M, Kraus WE, et al. : Bioengineered human myobundles mimic clinical responses of skeletal muscle to drugs. eLife. 2015;4:e04885. 10.7554/eLife.04885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Arslan SY, Yu Y, Burdette JE, et al. : Novel three dimensional human endocervix cultures respond to 28-day hormone treatment. Endocrinology. 2015;156(4):1602–9. 10.1210/en.2014-1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang J, Lian Q, Zhu G, et al. : A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell. 2011;8(1):31–45. 10.1016/j.stem.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 68. Biel NM, Santostefano KE, DiVita BB, et al. : Vascular Smooth Muscle Cells From Hypertensive Patient-Derived Induced Pluripotent Stem Cells to Advance Hypertension Pharmacogenomics. Stem Cells Transl Med. 2015;4(12):1380–90. 10.5966/sctm.2015-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Santostefano KE, Hamazaki T, Biel NM, et al. : A practical guide to induced pluripotent stem cell research using patient samples. Lab Invest. 2015;95(1):4–13. 10.1038/labinvest.2014.104 [DOI] [PubMed] [Google Scholar]
- 70. Blum A, Shamburek R: The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203(2):325–30. 10.1016/j.atherosclerosis.2008.08.022 [DOI] [PubMed] [Google Scholar]
- 71. Agarwal A, Goss JA, Cho A, et al. : Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13(18):3599–608. 10.1039/c3lc50350j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang G, McCain ML, Yang L, et al. : Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20(6):616–23. 10.1038/nm.3545 [DOI] [PMC free article] [PubMed] [Google Scholar]