Abstract
Background
The need for organic food of animal origin has increased rapidly in recent years. However, effects of organic animal husbandry on food safety have not been rigorously tested especially in meat turkey flocks. This study provides for the first time an overview on the prevalence and genetic diversity of Campylobacter species (spp.) in five organic meat turkey farms located in different regions in Germany, as well as on potential risk factors of bacterial spreading. Thirty cloacal swabs as well as water samples and darkling beetles were collected from each flock and examined for the presence of Campylobacter by conventional and molecular biological methods. The isolates were genotyped by flaA-RFLP.
Results
Campylobacter spp. were detected in cloacal swabs in all 5 turkey flocks with prevalence ranged from 90.0 to 100 %. 13 cloacal swabs collected from birds in farm III and IV were harboured mixed population of thermophilic campylobacters. In total, from 158 Campylobacter isolated from turkeys 89 (56.33 %) were identified as C. coli and 69 (43.76 %) as C. jejuni. Three Campylobacter (2 C. jejuni and 1 C. coli) were detected in drinkers of two farms and 3 C. coli were isolated from darkling beetles of one farm. No Campylobacter were isolated from main water tanks. flaA-RFLP assay showed that turkey farms can harbour more than one genotype. In a single turkey two different genotypes could be detected. The genotypes of campylobacters isolated from water samples or beetles were identical with those isolated from turkeys. No effect was found of some environmental parameters [ammonia concentration (NH3), carbon dioxide concentration (CO2), relative humidity (RH) and air temperature)] on Campylobacter prevalence in organic turkey farms. Additionally, drinking water and darkling beetles might be considered as risk factors for the spreading of Campylobacter in turkey flocks.
Conclusions
This study highlights the high prevalence and genotypic diversity of Campylobacter spp. isolated from organic turkey flocks. Further research is needed to assess other potential risk factors responsible for bacteria spreading in order to mitigate the spread of Campylobacter in organic turkey flocks by improving biosecurity control measures.
Electronic supplementary material
The online version of this article (doi:10.1186/s13099-016-0108-2) contains supplementary material, which is available to authorized users.
Keywords: Thermophilic Campylobacter, Organic turkey, Genotyping, Water, Beetles
Background
Over the last three decades, Campylobacter spp. have represented an increasing concern worldwide and appear to be the most common foodborne disease in which, consumption of poultry meat is considered as major, if not largest source of infection [1]. On the other hand, organic livestock farming has grown rapidly and the demand for organic meat consumption has increased substantially. This consideration is mainly supported by consumers’ perception of organic products as healthier and safer [2].
However, on organic farms the microbial safety risk is higher due to more contact with the environment than on conventional farms through the access of the birds to an outdoor run and contact with soil, wild birds and other animals and or their faeces [3, 4].
The pathways by which poultry flocks acquire Campylobacter are not yet fully understood in detail. The same applies for the formation of the high genetic diversity of Campylobacter which was observed in infected poultry flocks of different ages [5–7]. Horizontal transmission is generally considered to be the most significant mode of Campylobacter earning by poultry flocks [8–10]. However, the presence of a specific genotype in the environment of the birds does not in itself prove that also the birds are infected [11]. Assumed risk factors and vectors involved in the spreading are beside wild birds and their faeces insects such as darkling beetles and drinking water. Several studies have shown that beetles were only Campylobacter positive when the herd was positive, too [10, 12]. Darkling beetles can play a role in the entry of Campylobacter into a broiler flock [13, 14]. Drinking water can be an important vehicle for Campylobacter spp. transmission to the entire flock [15–17].
Most experiences with Campylobacter in organic poultry production are available from free range laying hens indicating that the access to outdoor scratching areas increases the risk of birds infection [18–21]. Although the organic flocks have access to outdoor areas, the prevalence of Campylobacter in organic and conventional broiler farms was found identical [19]. While another study in organic turkey flocks demonstrated a higher prevalence than conventional turkey flocks [22].
The prevalence, risk factors for spreading and genetic diversity of Campylobacter in organic turkey production received less attention and to best of our knowledge, no previous research was performed in Germany on Campylobacter in organic turkeys at farm level. Therefore, the aim of this study was to assess thermophilic Campylobacter prevalence and their genetic diversity in turkeys reared under organic conditions and to estimate the role of water and darkling beetles as potential risk factors for transmission of Campylobacter spp. in organic turkey flocks in Germany.
Methods
Turkey flocks
Samples were collected from five different organic turkey farms during spring and summer seasons. The farms were located in the north-eastern and western regions of Germany situated in typical rural areas surrounded by arable land. Criteria for barn selection were a usual commercial stock size, and a minimal distance of 1 km to the next livestock. The flock sizes ranged from 1000 to 2000 birds (Kelly BBB or B.U.T. 6) per flock (Table 1).
Table 1.
Farm description, environmental parameters, water supply sources and system, prevalence and genotyping of Campylobacter spp. isolated from organic turkey flocks
| Flock I | Flock II | Flock III | Flock IV | Flock V | |
|---|---|---|---|---|---|
| Total number of birds/flock | 1003 | 2000 | 1400 | 1100 | 1500 |
| Age of birds (weeks) | 8 | 8 | 4 | 8 | 6 |
| Turkey-line | Kelly BBB | B.U.T. 6 | B.U.T. 6 | Kelly BBB | B.U.T. 6 |
| Water supply type | Tap water | Tap water | Well water | Tap water | Tap water |
| Type of drinkers | Cups + pendulous | Cups | Cups + drinking trough | Pendulous | Cups |
| Number of examined cloacal swabs | 30 | 30 | 30 | 30 | 30 |
| No. of positive cloacal swabs | 30 | 27 | 30a | 30a | 30 |
| Flock prevalence (%) | 100 | 90 | 100 | 100 | 100 |
| No. of isolated C. jejuni | 8 | 19 | 5 | 17 | 20 |
| No. of isolated C. coli | 22 | 8 | 30 | 19 | 10 |
| Prevalence of C. jejuni (%) | 26.67 | 70.37 | 14.29 | 47.22 | 66.67 |
| Prevalence of C. coli (%) | 73.33 | 29.63 | 85.71 | 52.78 | 33.33 |
| No. of positive water samples | 1 (C. coli) | 2 (C. jejuni) | 0 | 0 | 0 |
| No. of positive beetles sample | 0 | 3 (C. coli) | 0 | 0 | 0 |
| No. of C. jejuni genotypes | 3 | 4 | 1 | 2 | 5 |
| No. of C. coli genotypes | 2 | 1 | 2 | 4 | 4 |
| Temperature (°C) | 15.8 | 23.1 | 21.30 | 21.80 | 15.4 |
| Relative humidity (RH in %) | 63.6 | 64.2 | 74.8 | 58.3 | 56.6 |
| Ammonia (ppm) | 1 | 14 | 22 | 1 | 0 |
| CO2 (ppm) | 500 | 1400 | 800 | 350 | 400 |
aFrom the same cloacal swabs both C. coli as well as C. jejuni were isolated in 5 swabs of farm III and 8 swabs of farm IV
Ethical statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the University of Veterinary Medicine Hannover. The protocol (sampling of cloacal swabs from turkeys on farms) was approved by the Animal Welfare Officer of the University.
Isolation of Campylobacter
Isolation was performed in accordance with the ISO 10272-1 (2006) guideline [23].
Cloacal swabs
In order to estimate the prevalence of Campylobacter within the turkey flock, 30 cloacal swabs were taken from randomly selected birds (EUROTUBO®, DELTALAB, Spain). The sample size calculation was based on the assumption that the within flock prevalence in Campylobacter positive flocks would be 95 %. Samples were transported to the laboratory under cooled conditions for further laboratory investigations. Swabs were streaked directly on the farm onto modified Charcoal Cefoperazone Desoxycholate Agar (mCCDA, Oxoid, Wesel, Germany). Thereafter, each swab was placed in a tube with 9 ml Bolton Broth (Oxoid). Plates and tubes were incubated microaerobically for 4 h at 37 °C then transferred to 42 °C for 42 h. Thereafter, a loopfull from the broth was streaked onto mCCDA and further incubated.
The prevalence of Campylobacter within the flock was estimated by the ratio of Campylobacter positive birds to the total number of tested birds [24].
Drinking water
At each farm, around 3 l water samples were collected directly from the main water tank using sterile 500 ml bottles (water samples were collected from 10 cm under the water surface). Additionally, 3 l pooled water samples were taken from the drinkers in the poultry house. All water sampling bottles were contained 10 mg of sodium thiosulfate (0.1 mg per ml water) to neutralize any residual chlorine in the water.
Isolation of Campylobacter from water samples was performed using a membrane filtration technique (MFT) according to the method described by Mathewson et al. [25]. Isolation was done using two different volumes of collected water samples (one with 500 ml and other with 1 l). Samples were individually filtered through 0.45 μm sterile cellulose acetate membrane filters (Sartorius AG, Goettingen, Germany). The filter from each duplicate was inserted into tubes filled with 9 ml Bolton broth and other filter was placed on the surface of mCCDA. Plates and tubes were incubated as described above.
Darkling beetles (Alphitobius diaperinus)
Beetles were collected from 10 different places distributed inside the barns (corners and under the drinkers) by turnings over the litter with sterile small shovels. Collected beetles were placed in a sterile plastic container with perforated cover.
In the laboratory only beetles identified as Alphitobius diaperinus [26] were analyzed and divided into 5 pools each containing 10 beetles and then aseptically crushed using a sterile mortar. Swabs from the crushed beetles were streaked directly on mCCDA, and then the crushed insects were aseptically transferred into 9 ml Bolton broth and handled as described above.
Identification of Campylobacter
Campylobacter-like colonies were obtained by cultivation on Columbia blood agar (Oxoid) and then phenotypically identified [24] including motility testing with phase contrast microscopy and catalase as well as oxidase reactions. Thereafter, initially positive isolates were further identified using the biochemical reaction profiles obtained by the API Campy System (BioMerieux, Germany) according to the instructions of the manufacturer.
DNA extraction
Genomic DNA was extracted from a 48 h bacterial culture on blood agar plates using High Pure PCR Template Preparation Kits (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s instructions. The DNA was eluted in 200 µl elution buffer. DNA was quantified spectrophotometrically using a Nanodrop® ND-1000 (Fisher Scientific GmbH, Schwerte, Germany).
Species confirmation and flaA-RFLP assays
The isolates were confirmed as C. jejuni or C. coli by using a multiplex PCR (mPCR) assay [17]. flaA-restriction fragment length polymorphism (RFLP) analysis was done as previously described [6]. The flaA amplicon was digested for 18 h at 37 °C with DdeI (Roche Diagnostics GmbH). The DNA segments were separated using 2.5 % agarose gels (Starlab GmbH, Hamburg, Germany) in tris-borate-EDTA buffer at 200 V for 1 h, stained with ethidium bromide and visualized under UV light. Documentation was done using a Bio Imaging System (Syngene, Cambridge, UK).
Measurements of environmental parameters
The following parameters were measured during the samplings near the bird level (between 9:00 and 11:00 a.m.). Temperature and relative humidity (RH) were measured with a thermo-hygrometer (Rotronic Date logger Hydrolog-D HygroClipSTemperatur/RH (Rotronic GmbH, Ettlingen, Germany) for about 30 min. The spot measurements of ammonia (NH3) and carbon dioxide (CO2) were carried out once during the samplings using Draegeraccuro® tube pump (Drägerwerk AG & Co. KGaA, Germany) and short term Draeger tube (Drägerwerk AG & Co. KGaA, Germany) number CH20501 for ammonia 5/a and 81 01 811 for carbon dioxide 100/a (Additional files 1, 2).
Results
Campylobacter spp. were isolated from cloacal swabs of all investigated 5 organic turkey flocks. The cloacal swabs collected from 150 birds revealed that 147 birds were identified as Campylobacter positive (Table 1). In 13 cloacal swabs collected from birds in farm III and IV, each swab harboured two types of thermophilic campylobacters. In total, from 158 Campylobacter isolated from five turkey flocks, 89 (56.3 %) isolates were identified as C. coli and 69 (43.7 %) as C. jejuni. In total three Campylobacter isolate, one C. coli and two C. jejuni were isolated from the water sample in farm I and II, respectively. Additionally, 3 C. coli were isolated from darkling beetles collected from farm II (Table 1).
Prevalence of Campylobacter isolated from turkey
The prevalence of Campylobacter was high in all 5 organic turkey farms and ranged from (90 %) in farm II to (100 %) in the other four farms (Table 1). The distribution of Campylobacter spp. varied in the different farms. C. coli was the most prominent species in three farms (I, III and IV) with shares of 73.33, 85.71 and 52.78 %, respectively. C. jejuni isolates dominated in farms II and V with prevalence of 63.33 and 66.67 %, respectively.
From 13 cloacal swabs (5 swabs from farm III and 8 from farm IV), 2 Campylobacter isolates were isolated from the same swab.
Occurrence of Campylobacter in water and darkling beetles samples
No Campylobacter spp. were detected in the water from the main tank neither with nor without enrichment (Table 1). In the water from drinkers only in farm II C. jejuni was found in 500 and 1000 ml after enrichment. In addition, C. coli could be also detected in 1000 ml drinker water after enrichment in farm I. From darkling beetles only C. coli was isolated from 3 out of 5 pools after enrichment in farm II.
flaA typing of isolated Campylobacter
The genotypes of Campylobacter spp. isolated from 5 examined turkey farms either cloacal swabs or drinking water and darkling beetles by flaA-RFLP revealed 24 different genotypes. The relatedness and genetic diversity of genotypes was presented in Fig. 1. High genetic diversity was shown in the farms I, II, IV and V (Figs. 2, 3, 4, 5). While, in farm III only one genotype was found among C. jejuni and 2 genotypes of C. coli isolates.
Fig. 1.

Dendrogram based on restriction profiles of flaA gene digested with DdeI of 163 Campylobacter isolates from 5 turkey farms (FI–FV—farm 1–5)
Fig. 2.
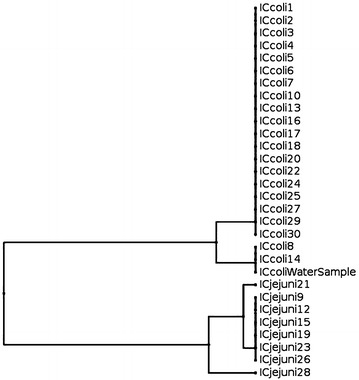
Dendrogram based on restriction profiles of flaA gene of 8 C. jejuni (7 from cloacal swabs and one from drinking water) and 22 C. coli (from cloacal swabs) isolated from farm I (isolate ICcoli21 could not be processed as it was mixed culture)
Fig. 3.

Dendrogram based on restriction profiles of flaA gene of 21 C. jejuni (19 from cloacal swabs and 2 from drinking water) and 11 C. coli (8 from cloacal swabs and 3 from dark beetles) isolated from farm II
Fig. 4.
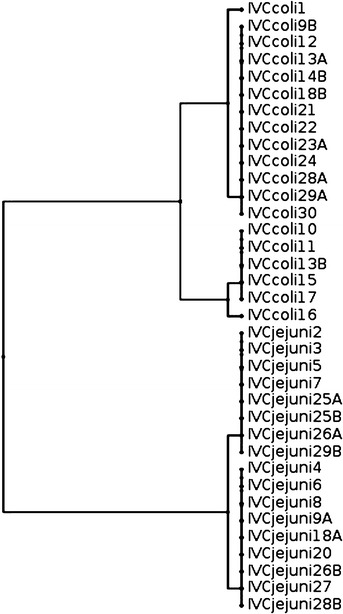
Dendrogram based on restriction profiles of flaA gene of 17 C. jejuni and 19 C. coli isolated from cloacal swabs in farm IV
Fig. 5.
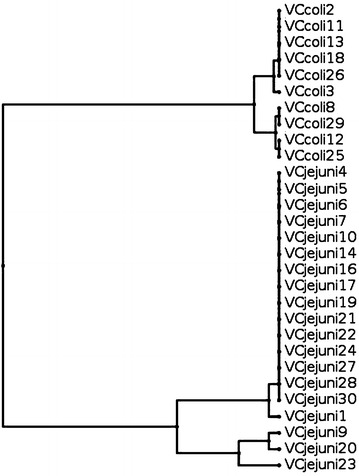
Dendrogram based on restriction profiles of flaA gene of 20 C. jejuni and 10 C. coli isolated from cloacal swabs in farm V
Two different species C. jejuni and C. coli were isolated from one bird in farms III and IV.
In farm I the genotype of C. coli isolated from drinking water was identical with that of two isolates recovered from cloacal swabs (Fig. 2). In farm II two different genotypes were detected among C. jejuni isolated from drinking water which were identical with other isolates originated from cloacal swabs from turkey in the same farm. Three C. coli isolated from darkling beetles in farm II were similar and having same genotype which was identical with all 8 C. coli isolated from cloacal swabs from the same farm (Fig. 3).
Effect of environmental parameters on occurrence of Campylobacter
No marked effects of the measured environmental parameters on the prevalence of Campylobacter spp. as temperature and atmosphere were found (Table 1). When the temperature ranged from 15.4 to 23 °C and the level of CO2 varied from 350 to 1400 ppm the Campylobacter prevalence was 90–100 % independent from both parameters. Similarly, prevalence of Campylobacter was 100 % when the RH ranged from 56.6 to 74.8 % and the ammonia concentration was between 0 and 22 ppm (Table 1).
Discussion
There was little published information about the presence of Campylobacter spp. in organic turkey flocks. The results of the presented study on 5 turkey farms indicate that Campylobacter spp. seem to be highly prevalent in organic turkey production in Germany. 90–100 % of all cloacal swab samples from 150 tested samples on the 5 farms were Campylobacter positive. This finding is in general terms consistent with previous studies, which found prevalence of Campylobacter spp. infections in organic turkey operation ranging between 6 and 100 % [22]. The results are even more in accordance with studies on broilers from France which showed that 85.7 % of faecal samples from one flock of chickens raised in a free-range system were Campylobacter positive [27] and from Denmark where 100 % of the investigated cloacal samples from organic broiler flocks were Campylobacter positive [18]. The reason for the high prevalence in organic production systems can surely be explained by the permanent access of the free-range birds to the outdoor areas, where, they easily can close contact to wild birds and theirs faeces as well as to soil and rain water. Also infectious agents transmitted by air can come more easily in contact with free range birds than housed birds. The higher risk for free range birds compared to birds reared under conventional conditions [8] is documented in several studies indicating that open environment exposure has to be considered as additional risk factor for increasing the prevalence of Campylobacter in organic poultry production [19, 28].
However, the general production conditions have to be taken in account. A survey in Switzerland indicated that the Campylobacter prevalence in cloacal swabs of free-range birds (69 %) was only slightly higher (not significant) than that of conventionally reared broilers (50 %) [29]. However, the samples collected from the litter showed that the presence of a genotype in the environment of the birds does not implement that also the birds are infected [11].
In this study, C. coli and C. jejuni isolates were comparable and there were only slight differences. C. coli was the predominant species isolated from the organic turkey flocks with an overall prevalence of 54.3 % of all Campylobacter isolates. This result is in contrast to earlier studies performed in both organic turkey and broiler flocks, where C. jejuni was the highly prevalent species with 66 and 72 %, respectively [22, 30]. However, the result is in agreement with findings of Smith et al. [31] who revealed that 80–90 % of isolates colonizing commercial turkey flocks were C. coli. Similar results were recently reported by Kashoma et al. [32] who found that 72.3 % of all Campylobacter isolates in commercial turkey flocks were confirmed as C. coli.
Molecular typing with flaA-RFLP considered as differentiation tool for Campylobacter [33]. From 98 % of flocks testing positive, 10 birds (6.67 %) harboured both C. jejuni and C. coli. This result was supported by previous reports which found a mixture of both Campylobacter spp. in one bird [17, 34, 35].
flaA-RFLP assay results in this study showed that single turkey farms can harbour more than one genotype in one production cycle (4 types of C. coli in farm IV, V and 5 genotypes of C. jejuni in farm V). This finding was in accordance with previous study [36].
Isolation of Campylobacter spp. from the environment is generally poor as observed in this study, it may be due to numerous ambient stressors such as low temperature, dryness, radiation and nutrition competition which can have a negative effect on the viability of Campylobacter spp. as mentioned before [37]. Enrichment in Bolton broth was very important in this study to recover Campylobacter in the water from drinkers and darkling beetles. Despite of enrichment there was no Campylobacter isolated from water either from a farm owned well or as municipal water which in agreement with previous studies [38, 39]. Moreover, other studies concluded that water considered as a primary risk factor for occurrence and spreading of Campylobacter infection within the flock [40, 41]. Furthermore, in a previous longitudinal study, Campylobacter DNA could be detected from drinkers after 6 days of stocking and before detection of infection in pullets [17]. On the other hand, studies considered that drinking water unlikely to be responsible on introduction of Campylobacter infection into poultry farms [42, 43].
Similar to the debate on the drinking water as a vector for Campylobacter transmission the role of darkling beetles is discussed. Direction of infection is not clear whether the beetles are carrying Campylobacter first and transmit it to the birds [13] or the birds excrete Campylobacter which were taken up by the beetles, acting as alternate vectors and source of infection [39, 44]. Even a single exposure of chicks to contaminated insects may be sufficient for colonization of the bird intestines as observed in a previous study [45]. These previously mentioned explanation support our findings as we detect the Campylobacter in beetles in one flock despite all flocks tested positive with high prevalence. The role of the contaminated beetles in Campylobacter transmission was discussed in previous studies [12, 14, 46] as some of these reports proved their role while others deny due to the short duration (few days) of bacterial carriage by the beetles.
In this study the molecular typing of isolated Campylobacter showed identity between genotypes detected in flocks and environmental samples which supported previous studies [10, 47].
The significance of air quality (ammonia and CO2 level) with Campylobacter occurrence in birds has not been frequently addressed. In this study, we did not found any influence of air quality on Campylobacter prevalence in examined flocks.
Conclusions
The results of this study provided new information about the Campylobacter prevalence in German organic turkey production and pointed out some potential sources of Campylobacter spreading for this kind of rearing system. This study showed that the water and darkling beetles considered as risk factor for presence of Campylobacter in organic turkey farm that should be taken into account during cleaning and disinfection of farm. Moreover, an influence of air quality on Campylobacter prevalence was not found in the sporadic and short time measurement and need further investigation.
Authors’ contributions
MA, HE, HH, JH and HMH participated in the conception and design of the study and MA, HE and HH performed the farm and laboratory work. MA, HE, HH, JH, HT, HN and HMH analyzed the data and wrote the manuscript. MA, HE, HH, JH, HT, HN and HMH were contributed to the analysis and helped in the manuscript discussion. All authors read and approved the final manuscript.
Acknowledgements
We thank B. Hofmann at Friedrich-Loeffler-Institut, Jena, Germany for her excellent technical assistance.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are additional files included within the article.
Funding
The project was funded from Institute for Animal Hygiene, Animal Welfare and Farm Animal Behaviour, University of Veterinary Medicine Hannover, Foundation, Germany and Friedrich-Loeffler-Institut, Institute of Bacterial Infections and Zoonoses, Germany.
Abbreviations
- spp.
species
- NH3
ammonia
- CO2
carbon dioxide
- RH
relative humidity
- MFT
membrane filtration technique
- mCCDA
modified Charcoal Cefoperazone Desoxycholate Agar
- mPCR
multiplex PCR
- RFLP
restriction fragment length polymorphism
Additional files
10.1186/s13099-016-0108-2 Bird and environmental samples identification with conventional and molecular method and flock description data.
10.1186/s13099-016-0108-2 The within flock prevalence of Campylobacter isolated from 30 cloacal swabs in 5 different organic turkey flocks and C. jejuni, C. coli genotypes and Campylobacter positive environmental samples (water tank, water at birds and darkling beetles) in 5 different organic turkey flocks.
Contributor Information
Marwa Fawzy El Metwaly Ahmed, Email: mrw_fwzy@yahoo.com.
Hosny El-Adawy, Email: hosny.eladawy@fli.bund.de.
Helmut Hotzel, Email: helmut.hotzel@fli.bund.de.
Herbert Tomaso, Email: herbert.tomaso@fli.bund.de.
Heinrich Neubauer, Email: heinrich.neubauer@fli.bund.de.
Nicole Kemper, Email: nicole.kemper@tiho-hannover.de.
Joerg Hartung, Email: joerg.hartung@tiho-hannover.de.
Hafez Mohamed Hafez, Email: hafez@vetmed.fu-berlin.
References
- 1.EFSA The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2012. EFSA J. 2015;12:3547. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Commission, Directorate-General for Agriculture and Rural Development. An analysis of the EU organic sector. Brussels; 2010.
- 3.Kijlstra A, Meerburg B, Bos A. Food safety in free-range and organic livestock systems: risk management and responsibility. J Food Prot. 2009;72(12):2629–2637. doi: 10.4315/0362-028x-72.12.2629. [DOI] [PubMed] [Google Scholar]
- 4.Bull S, Allen V, Domingue G, Jørgensen F, Frost J, Ure R, et al. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Appl Environ Microbiol. 2006;72(1):645–652. doi: 10.1128/AEM.72.1.645-652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson D, Rathinam V, Qi W, Wick L, Landgraf J, Bell J, et al. Genetic diversity in Campylobacter jejuni is associated with differential colonization of broiler chickens and C57BL/6J IL10-deficient mice. Microbiology. 2010;156(7):2046–2057. doi: 10.1099/mic.0.035717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Adawy H, Hotzel H, Tomaso H, Neubauer H, Taboada E, Ehricht R, et al. Detection of genetic diversity in Campylobacter jejuni isolated from a commercial turkey flock using flaA typing, MLST analysis and microarray assay. PLoS One. 2013;8(2):e51582. doi: 10.1371/journal.pone.0051582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wieczorek K, Denis E, Osek J. Comparative analysis of antimicrobial resistance and genetic diversity of Campylobacter from broilers slaughtered in Poland. Int J Food Microbiol. 2015;210:24–32. doi: 10.1016/j.ijfoodmicro.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Newell D, Fearnley C. Sources of Campylobacter colonization in broiler chickens. Appl Environ Microbiol. 2003;69(8):4343–4351. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandeplas S. Prevalence and sources of Campylobacter spp. contamination in free-range broiler production in the southern part of Belgium. Biotechnol Agron Soc Environ. 2010;14:279–288. [Google Scholar]
- 10.Agunos A, Waddell L, Léger D, Taboada E. A systematic review characterizing on-farm sources of Campylobacter spp. for broiler chickens. PLoS One. 2014;9(8):e104905. doi: 10.1371/journal.pone.0104905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colles F, Jones T, McCarthy N, Sheppard S, Cody A, Dingle K, et al. Campylobacter infection of broiler chickens in a free-range environment. Environ Microbiol. 2008;10(8):2042–2050. doi: 10.1111/j.1462-2920.2008.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Templeton J, De Jong A, Blackall P, Miflin J. Survival of Campylobacter spp. in darkling beetles (Alphitobius diaperinus) and their larvae in Australia. Appl Environ Microbiol. 2006;72(12):7909–7911. doi: 10.1128/AEM.01471-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates C, Hiett KL, Stern NJ. Relationship of Campylobacter isolated from poultry and from darkling beetles in New Zealand. Avian Dis. 2004;48(1):138–147. doi: 10.1637/7082. [DOI] [PubMed] [Google Scholar]
- 14.Hazeleger W, Bolder N, Beumer R, Jacobs-Reitsma W. Darkling beetles (Alphitobius diaperinus) and their larvae as potential vectors for the transfer of Campylobacter jejuni and Salmonellaenterica serovar paratyphi B variant java between successive broiler flocks. Appl Environ Microbiol. 2008;74(22):6887–6891. doi: 10.1128/AEM.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronowski C, James C, Winstanley C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol Lett. 2014;356(1):8–19. doi: 10.1111/1574-6968.12488. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Boto D, García-Peña F, Abad-Moreno J, Hurtado-Pizarro M, Pérez-Cobo I, Aurora Echeita M. Drinking water as the source of Campylobacter coli infection in grandparent heavy breeders. Avian Pathol. 2010;39(6):483–487. doi: 10.1080/03079457.2010.518138. [DOI] [PubMed] [Google Scholar]
- 17.El-Adawy H, Hotzel H, Tomaso H, Neubauer H, Hafez H. Elucidation of colonization time and prevalence of thermophilic Campylobacter species during turkey rearing using multiplex polymerase chain reaction. Poult Sci. 2012;91(2):454–459. doi: 10.3382/ps.2010-01810. [DOI] [PubMed] [Google Scholar]
- 18.Heuer O, Pedersen K, Andersen J, Madsen M. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Lett Appl Microbiol. 2001;33(4):269–274. doi: 10.1046/j.1472-765X.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- 19.Van Overbeke I, Duchateau L, Zutter L, Albers G, Ducatelle R. A comparison survey of organic and conventional broiler chickens for infectious agents affecting health and food safety. Avian Dis. 2006;50(2):196–200. doi: 10.1637/7448-093005R.1. [DOI] [PubMed] [Google Scholar]
- 20.Rodenburg T, Hulst-van Arkel M, van der Kwakkel R. Campylobacter and Salmonella infections on organic broiler farms. NJAS Wagening J Life Sci. 2004;52:101–108. doi: 10.1016/S1573-5214(04)80006-X. [DOI] [Google Scholar]
- 21.Tuyttens F, Heyndrickx M, De Boeck M, Moreels A, Van Nuffel A, Van Poucke E, et al. Broiler chicken health, welfare and fluctuating asymmetry in organic versus conventional production systems. Livest Sci. 2008;113(2–3):123–132. doi: 10.1016/j.livsci.2007.02.019. [DOI] [Google Scholar]
- 22.Luangtongkum T, Morishita T, Ison A, Huang S, McDermott P, Zhang Q. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl Environ Microbiol. 2006;72(5):3600–3607. doi: 10.1128/AEM.72.5.3600-3607.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ISO. Microbiology of food and animal feeding stuffs—horizontal method for detection and enumeration of Campylobacter spp. Part 1: Detection method. Geneva. International Organization for Standardization; [ISO 10272-1:2006] 2006.
- 24.Ahmed M, Schulz J, Hartung J. Air samplings in a Campylobacter jejuni positive laying hen flock. Ann Agric Environ Med. 2013;20(1):16–20. [PubMed] [Google Scholar]
- 25.Mathewson J, Keswick B, DuPont H. Evaluation of filters for recovery of Campylobacter jejuni from water. Appl Environ Microbiol. 1983;46(5):985–987. doi: 10.1128/aem.46.5.985-987.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagstrum D, Klejdysz T, Subramanyam B, Nawrot J. Stored-product insect. In: Atlas of stored-product insects and mites: AACC International Press; 2009. p. 3–8.
- 27.Rivoal K, Denis M, Salvat G, Colin P, Ermel G. Molecular characterization of the diversity of Campylobacter spp. isolates collected from a poultry slaughterhouse: analysis of cross-contamination. Lett Appl Microbiol. 1999;29(6):370–374. doi: 10.1046/j.1472-765X.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- 28.Hald B, Knudsen K, Lind P, Madsen M. Study of the infectivity of saline-stored Campylobacter jejuni for day-old chicks. Appl Environ Microbiol. 2001;67(5):2388–2392. doi: 10.1128/AEM.67.5.2388-2392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittwer M, Keller J, Wassenaar T, Stephan R, Howald D, Regula G, et al. Genetic diversity and antibiotic resistance patterns in a Campylobacter population isolated from poultry farms in Switzerland. Appl Environ Microbiol. 2005;71(6):2840–2847. doi: 10.1128/AEM.71.6.2840-2847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright S, Carver D, Siletzky R, Romine S, Morrow W, Kathariou S. Longitudinal study of prevalence of Campylobacter jejuni and Campylobacter coli from turkeys and swine grown in close proximity. J Food Prot. 2008;71(9):1791–1796. doi: 10.4315/0362-028x-71.9.1791. [DOI] [PubMed] [Google Scholar]
- 31.Smith K, Reimers N, Barnes J, Lee B, Siletzky RM, Kathariou S. Campylobacter colonization of sibling turkey flocks reared under different management conditions. J Food Prot. 2004;67(7):1463–1468. doi: 10.4315/0362-028x-67.7.1463. [DOI] [PubMed] [Google Scholar]
- 32.Kashoma I, Kumar A, Sanad Y, Gebreyes W, Kazwala R, Garabed R, et al. Phenotypic and genotypic diversity of thermophilic Campylobacter spp. in commercial turkey flocks: a longitudinal study. Foodborne Pathog Dis. 2014;11(11):850–860. doi: 10.1089/fpd.2014.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen L, Newell DG. The ability of fla-typing schemes to discriminate between strains of Campylobacter jejuni. J Appl Microbiol. 2001;91(2):217–224. doi: 10.1046/j.1365-2672.2001.01383.x. [DOI] [PubMed] [Google Scholar]
- 34.Anderson J, Horn BJ, Gilpin BJ. The prevalence and genetic diversity of Campylobacter spp. in domestic ‘backyard’ poultry in Canterbury, New Zealand. Zoonoses Public Health. 2012;59(1):52–60. doi: 10.1111/j.1863-2378.2011.01418.x. [DOI] [PubMed] [Google Scholar]
- 35.Noormohamed A, Fakhr M. Prevalence and antimicrobial susceptibility of Campylobacter spp. in Oklahoma conventional and organic retail poultry. Open Microbiol J. 2014;8:130–137. doi: 10.2174/1874285801408010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bokkers E, de Boer I. Economic, ecological, and social performance of conventional and organic broiler production in the Netherlands. Brit Poult Sci. 2009;50(5):546–557. doi: 10.1080/00071660903140999. [DOI] [PubMed] [Google Scholar]
- 37.Ridley A, Allen V, Sharma M, Harris J, Newell D. Real-time PCR approach for detection of environmental sources of Campylobacter strains colonizing broiler flocks. Appl Environ Microbiol. 2008;74(8):2492–2504. doi: 10.1128/AEM.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphrey T, Henley A, Lanning D. The colonization of broiler chickens with Campylobacter jejuni: some epidemiological investigations. Epidemiol Infect. 1993;110(3):601–607. doi: 10.1017/S0950268800051025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs-Reitsma W, van de Giessen A, Bolder N, Mulder R. Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol Infect. 1995;114(3):413–421. doi: 10.1017/S0950268800052122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerin M, Martin W, Reiersen J, Berke O, McEwen S, Bisaillon J, et al. A farm-level study of risk factors associated with the colonization of broiler flocks with Campylobacter spp. in Iceland, 2001–2004. Acta Vet Scand. 2007;49(18):1–12. doi: 10.1186/1751-0147-49-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki Y, Tsujiyama Y, Tanaka H, Yoshida S, Goshima T, Oshima K, et al. Risk factors for Campylobacter colonization in broiler flocks in Japan. Zoonoses Public Health. 2011;58(5):350–356. doi: 10.1111/j.1863-2378.2010.01370.x. [DOI] [PubMed] [Google Scholar]
- 42.Miflin J, Templeton J, More S. Epidemiological studies on Campylobacter colonization in broiler flock in south east Queensland. In: Proceedings of the Australia Poulty Science Symposium, vol. 13; 2001. p. 140–3.
- 43.Patriarchi A, Maunsell B, O’Mahony E, Fox Á, Fanning S, Buckley J, et al. Prevalence of Campylobacter spp. in a subset of intensive poultry flocks in Ireland. Lett Appl Microbiol. 2009;49(3):305–310. doi: 10.1111/j.1472-765X.2009.02658.x. [DOI] [PubMed] [Google Scholar]
- 44.Gregory E, Barnhart H, Dreesen D, Stern N, Corn J. Epidemiological study of Campylobacter spp. in broilers: source, time of colonization, and prevalence. Avian Dis. 1997;41(4):890–898. doi: 10.2307/1592343. [DOI] [PubMed] [Google Scholar]
- 45.Strother K, Steelman C, Gbur E. Reservoir competence of lesser mealworm (Coleoptera: Tenebrionidae) for Campylobacter jejuni (Campylobacterales: Campylobacteraceae) J Med Entomol. 2005;42(1):42–47. doi: 10.1603/0022-2585(2005)042[0042:RCOLMC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Neubauer C, Bibl D, Szolgyenyi W, Jauk V, Schmidt M, Gabler C, et al. Epidemiological investigation of Campylobacter spp. in Austrian broiler flocks: prevalence and risk factors. Wien Tierarztliche Mon. 2005;92:4–10. [Google Scholar]
- 47.Zimmer M, Barnhart H, Idris U, Lee M. Detection of Campylobacter jejuni strains in the water lines of a commercial broiler house and their relationship to the strains that colonized the chickens. Avian Dis. 2003;47:101–107. doi: 10.1637/0005-2086(2003)047[0101:DOCJSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are additional files included within the article.


