Abstract
Objectives
To provide surveillance data on the susceptibility of community-acquired respiratory tract isolates from four Gulf and Near East countries from 2011 to 2013.
Methods
MICs were determined using Etests® for all antibiotics evaluated except erythromycin, where testing was by disc diffusion. Susceptibility was assessed using CLSI, EUCAST and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints.
Results
Seven hundred and twenty-six respiratory isolates comprising 265 isolates of Streptococcus pneumoniae, 125 isolates of Streptococcus pyogenes and 336 isolates of Haemophilus influenzae were collected from Bahrain, Lebanon, Oman and the United Arab Emirates (UAE). Among S. pneumoniae, susceptibility to penicillin was low in the UAE and Bahrain. Macrolide susceptibility was ∼45%–60% in the UAE and Oman but higher in Lebanon (73.7%) and Bahrain (84%–85%). Penicillin susceptibility using CLSI intravenous breakpoints was >85% in all countries. Antibiotic susceptibility of S. pneumoniae was lower in UAE and Oman. Among S. pyogenes isolates, resistance to erythromycin was highest in Oman (31.6%) but <20% in the other countries. In H. influenzae, susceptibility to most antibiotics was high, except for ampicillin in Lebanon (70.2%) and amoxicillin in Oman (95.4%). Lebanon also had a high percentage (14.9%) of β-lactamase-positive isolates with non-susceptibility to ampicillin. Amoxicillin/clavulanic acid susceptibility was >95% in all countries. Use of EUCAST versus CLSI breakpoints demonstrated profound differences for cefaclor and cefuroxime in S. pneumoniae and H. influenzae, with EUCAST showing lower susceptibility.
Conclusions
There was considerable variability in susceptibility among countries in the same region. Thus, continued surveillance is necessary to track future changes in antibiotic resistance.
Introduction
Community-acquired pneumonia (CAP) remains a significant health problem. Acute respiratory tract infections are the cause of death in 15% of children under the age of 5 years globally and in 4%–7% of children in the four countries [Bahrain, Lebanon, Oman and the United Arab Emirates (UAE)] considered here.1 CAP is also a clinical and an economic burden in the expanding ageing population.2 There are relatively few CAP epidemiology and surveillance data from these four Gulf and Near East countries in the published literature. However, a high annual incidence of invasive pneumococcal infection and a case mortality rate of 4.8% were reported among children under 5 years old in a study from Bahrain covering 1999–2003.3 Resistance to antimicrobial agents is a worldwide phenomenon, with antibiotic use being a major driver of emergence of resistance.4–6 Although some studies have not observed a correlation between mortality due to CAP and antimicrobial resistance,7 others found that penicillin resistance was related to increased mortality in hospitalized patients with pneumococcal pneumonia8 and that macrolide resistance in respiratory pathogens was related to treatment failures in children.9 In a retrospective (1997–2002) study in the UAE of inpatients with pneumonia, 22% of Streptococcus pneumoniae isolates were penicillin resistant (PRSP) and 23% of Haemophilus influenzae were resistant to amoxicillin.10 A prospective 6 year study in Lebanese hospitals found that 17.4% of S. pneumoniae isolates were non-susceptible to penicillin and 23.5% of the patients with these infections died; additionally, 10.9% of isolates were multidrug resistant.11 Because CAP is usually treated empirically, without identification and susceptibility testing of the causative agent, knowledge of local resistance patterns is especially important when treating this disease. Additionally, surveillance data can provide useful information to assist governments in controlling antimicrobial use and emergence of resistance.
The Survey of Antibiotic Resistance (SOAR) is an ongoing surveillance study of key respiratory pathogens. SOAR has been monitoring antimicrobial resistance in the Middle East, Africa, Latin America, Asia-Pacific and the Commonwealth of Independent States countries since 2002. We present an analysis of recent data from four Gulf and Near East countries to provide a picture of the current antimicrobial susceptibility situation in three major respiratory pathogens, H. influenzae, S. pneumoniae and Streptococcus pyogenes.
Materials and methods
Collaborating centres
The following four centres took part in the study: Salmaniya Medical Complex, Bahrain; Alliance for the Prudent Use of Antibiotics, Lebanon chapter (APUA), Lebanon (receiving clinical isolates from three medical centres in the country); and Sultan Qaboos University Hospital, Muscat/Oman and Super Religare Laboratories, Dubai, UAE.
Clinical isolates
During 2011–13, a total of 726 clinical respiratory isolates (250 from Bahrain; 129 from Lebanon; 187 from Oman; 160 from the UAE), comprising 265 isolates of S. pneumoniae, 125 isolates of S. pyogenes and 336 isolates of H. influenzae were analysed. Paediatric patients (≤12 years old) accounted for 409 (56.3%) isolates, adult patients (13–64 years old) for 269 (37.1%) and the elderly (≥65 years) for 48 (6.6%) isolates. All 129 isolates from Lebanon were from paediatric patients. All of the S. pyogenes isolates were from throat swabs and the other pathogens were obtained from a variety of specimen types, including blood, bronchoalveolar lavage, middle ear effusion, nasopharyngeal aspirate, pleural fluid, sputum and tracheal aspirate. Organisms were identified using conventional methods (optochin susceptibility/bile solubility for S. pneumoniae, X and V factor requirement for H. influenzae, bacitracin susceptibility for S. pyogenes). Duplicate isolates from the same patient were not accepted.
Susceptibility testing
MICs were determined by using a gradient strip (Etest®), according to the manufacturer's instructions (bioMérieux, Marcy l'Étoile, France). Although not all antimicrobial agents were tested at every centre, there was substantial overlap among countries in the panel of antimicrobial agents tested. These included penicillin, amoxicillin, amoxicillin/clavulanic acid, cefaclor, cefepime, cefixime, cefpodoxime, ceftriaxone, cefuroxime, clarithromycin, ciprofloxacin, levofloxacin and moxifloxacin against S. pneumoniae and S. pyogenes. In addition, for these two organisms erythromycin was tested by CLSI disc diffusion methodology.12 For H. influenzae, ampicillin or amoxicillin, amoxicillin/clavulanic acid, azithromycin, cefaclor, cefepime, cefixime, cefpodoxime, ceftriaxone, cefuroxime, clarithromycin, ciprofloxacin, levofloxacin and moxifloxacin were tested. The particular agents tested in each country can be found in the respective summary tables of MIC data. Quality control strains S. pneumoniae ATCC 49619, H. influenzae ATCC 49247, H. influenzae ATCC 49766 and Escherichia coli ATCC 32518 were tested concurrently with the clinical isolates. β-Lactamase production was determined for each H. influenzae isolate by a chromogenic cephalosporin (nitrocefin) disc according to the manufacturer's instructions (BD Diagnostics, Sparks, MD, USA) using E. coli ATCC 35218 and H. influenzae ATCC 49247 as the positive and negative controls, respectively.
Results of susceptibility testing were accepted if the results of the control strains were within published limits. Susceptibility to the study drugs was calculated based on CLSI breakpoints,13 EUCAST breakpoints14 and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints15 with the exception of macrolides, for which bioMérieux Etest® breakpoints for incubation at elevated CO2 tension were used. The breakpoints for all three methods of evaluation are shown in Table 1.
Table 1.
MIC breakpoints (mg/L) used for S. pneumoniae, S. pyogenes and H. influenzae isolates
| MIC breakpoints [S/I/R (mg/L)] |
|||||||
|---|---|---|---|---|---|---|---|
|
S. pneumoniae |
S. pyogenes |
H. influenzae |
All species |
||||
| Antimicrobial | CLSI | EUCAST | CLSI | EUCAST | CLSI | EUCAST | PK/PD (S only) |
| Amoxicillin | ≤2/4/≥8 | NA | NA | NA | NA | ≤2/–/≥4 | ≤2 |
| AMCa | ≤2/4/≥8 | NA | NA | NA | ≤4/–/≥8 | ≤2/–/≥4 | ≤2 (≤4) |
| Ampicillin | NA | ≤0.5/1–2/≥4 | ≤0.25/–/– | NA | ≤1/2/≥4 | ≤1/–/≥2 | NA |
| Azithromycinb | ≤4/8/≥16 | NA | NA | NA | ≤8/–/–b | NA | NA |
| Cefaclor | ≤1/2/≥4 | ≤0.03/0.06–0.5/≥1 | NA | NA | ≤8/16/≥32 | NA | ≤0.5 |
| Cefepime | ≤1/2/≥4 | ≤1/2/≥4 | ≤0.5/–/– | NA | ≤2/–/– | ≤0.25/–/≥0.5 | NA |
| Cefixime | NA | NA | NA | NA | ≤1/–/– | ≤0.12/–/≥0.25 | ≤1 |
| Cefpodoxime | ≤0.5/1/≥2 | ≤0.25/0.5/≥1 | NA | NA | ≤2/–/– | ≤0.25/0.5/≥1 | ≤0.5 |
| Ceftriaxone | ≤1/2/≥4 | ≤0.5/1–2/≥4 | ≤0.5/–/– | NA | ≤2/–/– | ≤0.12/–/≥0.25 | ≤1 |
| Cefuroximec | ≤1/2/≥4 | ≤0.25/0.5/≥1 | NA | NA | ≤4/8/≥16 | ≤0.12/0.25–1/≥2 | ≤1 |
| Ciprofloxacin | NA | ≤0.12/0.25–2/≥4 | NA | NA | ≤1/–/– | ≤0.5/–/≥1 | ≤1 |
| Clarithromycinb | ≤0.5/1/≥2 | NA | NA | NA | ≤16/32/≥64 | NA | NA |
| Levofloxacin | ≤2/4/≥8 | ≤2/–/≥4 | ≤2/4/≥8 | ≤1/2/≥4 | ≤2/–/– | ≤1/–/≥2 | ≤2 |
| Moxifloxacin | ≤1/2/≥4 | ≤0.5/–/≥1 | NA | ≤0.5/1/≥2 | ≤1/–/– | ≤0.5/–/≥1 | ≤1 |
| Penicillin (oral) | ≤0.06/0.12–1/≥2 | ≤0.06/0.12–2/≥4 | ≤0.12/–/– | ≤0.25/–/≥0.5 | NT | NT | NA |
| Penicillin (iv)d | ≤2/4/≥8 | see notee | NA | NA | NT | NT | NA |
AMC, amoxicillin/clavulanic acid; S, susceptible; I, intermediate; R, resistant; NA, not applicable; NT, not tested.
aThis agent was tested at a 2 : 1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoint based on high dose (4 g of amoxicillin with 250 mg of clavulanic acid per day for adults) shown in parentheses.
bbioMérieux Etest® breakpoints for incubation in CO2.
cBreakpoints used are for cefuroxime axetil.
dParenteral non-meningitis breakpoints. EUCAST does not indicate iv breakpoints.
eEUCAST gives iv susceptible breakpoints for pneumonia based on three doses: 1.2 g × 4 (MIC ≤0.5 mg/L = susceptible), 1.2 g × 6 or 2.4 g × 4 (MIC ≤1 mg/L = susceptible) and 2.4 g × 6 (MIC ≤2 mg/L = susceptible).
Statistical analysis
Differences in susceptibility between countries and age groups were assessed with Fisher's exact test, using XLSTAT version 2011.1.05. A P value <0.05 was considered statistically significant.
Results
Sources of isolates from all sites combined
Of the 265 S. pneumoniae isolates, 145 (54.7%) were from sputum, 53 (20.0%) from blood, 35 (13.2%) from tracheal aspirate, bronchoalveolar lavage or nasopharyngeal aspirate, and 32 (12.1%) from middle ear effusion. More than half of the isolates 139 (52.5%) were from paediatric patients, 100 (37.7%) were from adults and 26 (9.8%) were from elderly patients.
All 125 S. pyogenes isolates were from throat swabs. The majority, 70 (56.0%), were from paediatric patients, 54 (43.2%) of isolates were from adult patients and there was a single isolate (0.8%) from an elderly patient.
The majority, 195 (58.0%), of the 336 H. influenzae isolates came from sputum, 63 (18.8%) isolates were from middle ear effusions, 59 (17.6%) were from tracheal aspirates or bronchoalveolar lavage, 16 (4.8%) from nasopharyngeal aspirates, 2 (0.6%) from blood and 1 (0.3%) from pleural fluid. Paediatric patients accounted for 159 (47.3%), adults for 156 (46.4%) and elderly patients for 21 (6.3%) isolates.
S. pneumoniae susceptibility in individual countries
Bahrain
Summary MIC and susceptibility data for 100 S. pneumoniae isolates from Bahrain are shown in Table 2. Complete MIC distribution data are shown in Table 3. Using the intravenous (iv) breakpoint, 100% of the isolates from Bahrain were susceptible to penicillin (PSSP), whereas only 60% were PSSP according to the CLSI oral breakpoint and the EUCAST criteria. Using these two breakpoints, 37% and 40%, respectively, were scored as intermediate (PISP) and 3% and zero isolates, respectively, were penicillin resistant (PRSP) (Table 2).
Table 2.
MIC and susceptibility results for all S. pneumoniae isolates from Bahrain
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| Amoxicillin | 100 | ≤0.015 | 1 | ≤0.015 | 4 | 98.0 | 2.0 | 0 | 98.0 | NA | NA | NA |
| AMCa | 100 | ≤0.015 | 1 | ≤0.015 | 4 | 98.0 | 2.0 | 0 | 98.0 (100) | NA | NA | NA |
| Azithromycin | 100 | 0.5 | 32 | 0.06 | >256 | 85.0 | 1.0 | 14.0 | NA | NA | NA | NA |
| Cefaclor | 100 | 1 | 128 | 0.5 | >256 | 61.0 | 2.0 | 37.0 | 40.0 | 0 | 40.0 | 60.0 |
| Cefepime | 100 | 0.12 | 1 | ≤0.015 | 1 | 100.0 | 0 | 0 | NA | 100.0 | 0 | 0 |
| Cefixime | 100 | 1 | 8 | 0.06 | 32 | NA | NA | NA | 51.0 | NA | NA | NA |
| Cefpodoxime | 100 | 0.12 | 2 | ≤0.015 | 8 | 71.0 | 18.0 | 11.0 | 71.0 | 63.0 | 8.0 | 29.0 |
| Ceftriaxone | 100 | 0.06 | 0.25 | 0.004 | 1 | 100 | 0 | 0 | 100 | 99.0 | 1.0 | 0 |
| Cefuroxime | 100 | 0.06 | 2 | ≤0.015 | 8 | 85.0 | 13.0 | 2.0 | 85.0 | 65.0 | 5.0 | 30.0 |
| Ciprofloxacin | 100 | 1 | 2 | 0.25 | 4 | NA | NA | NA | 78.0 | 0 | 99.0 | 1.0 |
| Clarithromycin | 100 | 0.12 | 8 | ≤0.015 | >256 | 84.0 | 0 | 16.0 | NA | NA | NA | NA |
| Levofloxacin | 100 | 1 | 2 | 0.5 | 2 | 100.0 | 0 | 0 | 100 | 100.0 | 0 | 0 |
| Moxifloxacin | 100 | 0.25 | 0.25 | 0.06 | 0.25 | 100.0 | 0 | 0 | 100 | 100.0 | 0 | 0 |
| Penicillin (oral) | 100 | ≤0.015 | 1 | ≤0.015 | 2 | 60.0 | 37.0 | 3.0 | NA | 60.0 | 40.0 | 0 |
| Penicillin (iv) | 100 | ≤0.015 | 1 | ≤0.015 | 2 | 100.0 | 0 | 0 | NA | 86–100.0 | NA | NA |
| Erythromycinb | 100 | NT | NT | NT | NT | 84.0 | 2.0 | 14.0 | NA | 84.0 | 0.0 | 16.0 |
min, minimum; max, maximum; AMC, amoxicillin/clavulanate; S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, no breakpoint data available (NA for azithromycin and clarithromycin by EUCAST because Etest® breakpoints in CO2 not available).
aPK/PD susceptibility at high dose shown in parentheses.
bUsing S/I/R zone diameters (mm) of CLSI (≤15/16–20/≥21) and EUCAST (≤18/19–21/≥22).
Table 3.
Distribution of MICs for all S. pneumoniae isolates from Bahrain
| Isolates susceptible at MIC (mg/L) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | n | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | >256 |
| Amoxicillin | 100 | 0 | 0 | 53 | 0 | 6 | 1 | 5 | 5 | 13 | 14 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Amoxicillin/clavulanic acid | 100 | 0 | 0 | 51 | 0 | 8 | 1 | 5 | 6 | 14 | 12 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Azithromycin | 100 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 6 | 47 | 26 | 0 | 1 | 1 | 2 | 4 | 1 | 0 | 0 | 7 |
| Cefaclor | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 21 | 2 | 1 | 0 | 5 | 9 | 9 | 6 | 1 | 6 |
| Cefepime | 100 | 0 | 0 | 15 | 0 | 26 | 3 | 9 | 14 | 19 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefixime | 100 | 0 | 0 | 0 | 0 | 0 | 2 | 16 | 13 | 13 | 7 | 17 | 12 | 12 | 6 | 2 | 0 | 0 | 0 | 0 |
| Cefpodoxime | 100 | 0 | 0 | 16 | 0 | 22 | 6 | 7 | 12 | 8 | 18 | 10 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceftriaxone | 100 | 4 | 20 | 0 | 19 | 4 | 17 | 8 | 18 | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefuroxime | 100 | 0 | 0 | 38 | 0 | 6 | 8 | 11 | 2 | 5 | 15 | 13 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 18 | 56 | 21 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Clarithromycin | 100 | 0 | 0 | 1 | 0 | 2 | 13 | 66 | 2 | 0 | 0 | 0 | 4 | 3 | 2 | 0 | 0 | 0 | 0 | 7 |
| Levofloxacin | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 57 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moxifloxacin | 100 | 0 | 0 | 0 | 0 | 0 | 7 | 31 | 62 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillin | 100 | 0 | 0 | 51 | 0 | 6 | 3 | 6 | 6 | 14 | 11 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
For both amoxicillin and amoxicillin/clavulanic acid, 98% of the isolates were susceptible by both CLSI and PK/PD criteria; 100% of isolates were susceptible to high-dose amoxicillin/clavulanic acid (PK/PD). Among cephalosporins tested, 100% of isolates were susceptible to cefepime by both CLSI and EUCAST criteria, and susceptibility to ceftriaxone was 100%, 99% and 100% by CLSI, EUCAST and PK/PD criteria, respectively. In contrast, cefaclor, cefpodoxime and cefuroxime showed non-susceptibility (intermediate and resistant) in 39%, 29% and 15% of isolates, respectively, with the CLSI breakpoints. With EUCAST breakpoints, non-susceptibility was observed in 100%, 37% and 35% of the isolates and with PK/PD breakpoints 60%, 29% and 15% were non-susceptible. For cefixime, only 51% of isolates were susceptible using PK/PD breakpoints, the only available criterion for this agent.
CLSI guidelines indicate that isolates susceptible to penicillin G (MIC ≤0.06 mg/L) can be reported as susceptible to amoxicillin, amoxicillin/clavulanic acid, ceftriaxone, cefaclor, cefepime, cefpodoxime and cefuroxime. Data from this study confirmed this, as in most cases PSSP were also susceptible to the β-lactams listed above. The exception was one penicillin-susceptible isolate that was cefaclor resistant. However, the reverse was not always found. Of the 40 penicillin non-susceptible isolates, all were ceftriaxone susceptible, 38 (95.0%) were amoxicillin or amoxicillin/clavulanic acid susceptible, 28 (70%) were cefepime susceptible, 25 (62.5%) were cefuroxime susceptible and 11 (27.5%) were cefpodoxime susceptible. However, only two penicillin-non-susceptible S. pneumoniae were cefaclor susceptible (5.0%). A similar ‘expert rule’ is provided by EUCAST but for penicillins only, i.e. amoxicillin/clavulanic acid (amoxicillin) in this study. However, unlike CLSI, individual breakpoints are not provided by EUCAST for amoxicillin/clavulanic acid to make this comparison.
All 100 isolates were susceptible to the fluoroquinolones levofloxacin and moxifloxacin by all three evaluation criteria. Ciprofloxacin was less effective: by EUCAST criteria, no strains were susceptible, 99% were intermediate and 1% (1 isolate) was resistant. Using the PK/PD breakpoint, 78% of strains were susceptible. There are no CLSI breakpoints for ciprofloxacin against S. pneumoniae. The three macrolides tested had similar activity: for azithromycin and clarithromycin (CLSI breakpoints in CO2 only), 85% and 84% of isolates, respectively, were susceptible; 84% of isolates were susceptible to erythromycin by both CLSI and EUCAST disc diffusion criteria.
Lebanon
Summary MIC and susceptibility data for 57 S. pneumoniae isolates from Lebanon are shown in Table 4. The complete MIC distribution data are given in Table 5. Using the CLSI iv breakpoint 100% of isolates were PSSP, whereas using the CLSI oral and the EUCAST breakpoints only 59.6% were PSSP; 40.4% were scored as PISP with these two criteria, and no isolate was PRSP (Table 4).
Table 4.
MIC and susceptibility results for all S. pneumoniae isolates from Lebanon
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| Amoxicillin | 57 | 0.015 | 0.06 | 0.015 | 0.06 | 100.0 | 0 | 0 | 100.0 | NA | NA | NA |
| AMCa | 57 | 0.015 | 1 | ≤0.015 | 4 | 96.5 | 3.5 | 0 | 96.5 (100) | NA | NA | NA |
| Azithromycin | 57 | 0.5 | >256 | ≤0.015 | >256 | 73.7 | 0 | 26.3 | NA | NA | NA | NA |
| Cefaclor | 57 | 0.25 | 8 | 0.06 | 8 | 79.0 | 7.0 | 14.0 | 71.9 | 0 | 71.9 | 28.1 |
| Cefepime | 57 | 0.03 | 0.03 | 0.03 | 0.03 | 100.0 | 0 | 0 | NA | 100.0 | 0 | 0 |
| Cefixime | 57 | 0.25 | 0.5 | 0.25 | 0.5 | NA | NA | NA | 100.0 | NA | NA | NA |
| Cefpodoxime | 57 | 0.03 | 2 | ≤0.015 | 4 | 79.0 | 7.0 | 14.0 | 79.0 | 79.0 | 0 | 21.1 |
| Ceftriaxone | 57 | 0.03 | 0.06 | 0.03 | 0.06 | 100.0 | 0 | 0 | 100.0 | 100.0 | 0 | 0 |
| Cefuroxime | 57 | 0.03 | 1 | ≤0.015 | 4 | 91.2 | 5.3 | 3.5 | 91.2 | 86.0 | 1.8 | 12.3 |
| Ciprofloxacin | 57 | 0.03 | 0.5 | 0.015 | 2 | NA | NA | NA | 96.5 | 71.9 | 28.1 | 0 |
| Clarithromycin | 57 | 0.03 | >256 | ≤0.015 | >256 | 73.7 | 0 | 26.3 | NA | NA | NA | NA |
| Levofloxacin | 57 | 0.25 | 4 | 0.12 | 4 | 100.0 | 0 | 0 | 100.0 | 100.0 | — | 0 |
| Moxifloxacin | 57 | 0.015 | 0.03 | 0.015 | 0.5 | 100.0 | 0 | 0 | 100.0 | 100.0 | — | 0 |
| Penicillin (oral) | 57 | 0.015 | 0.03 | 0.015 | 0.06 | 59.6 | 40.4 | 0 | NA | 59.6 | 40.4 | 0 |
| Penicillin (iv) | 57 | 0.03 | 0.5 | 0.004 | 1 | 100.0 | 0 | 0 | NA | 94.7–100.0 | NA | NA |
| Erythromycinb | 57 | NT | NT | NT | NT | 73.7 | 0 | 26.3 | NA | 73.7 | 0 | 26.3 |
min, minimum; max, maximum; AMC, amoxicillin/clavulanate; S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, no breakpoint data available (NA for azithromycin and clarithromycin by EUCAST because Etest® breakpoints in CO2 not available).
aPK/PD susceptibility at high dose is shown in parentheses.
bUsing S/I/R zone diameters (mm) of CLSI (≤15/16–20/≥21) and EUCAST (≤18/19–21/≥22).
Table 5.
Distribution of MICs for all S. pneumoniae isolates from Lebanon
| Isolates susceptible at MIC (mg/L) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | n | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 128 | 256 | >256 |
| Amoxicillin | 57 | 0 | 0 | 0 | 30 | 0 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AMC | 57 | 0 | 0 | 27 | 2 | 2 | 8 | 1 | 7 | 2 | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Azithromycin | 57 | 0 | 0 | 5 | 0 | 0 | 0 | 8 | 13 | 16 | 0 | 0 | 0 | 0 | 2 | 0 | 13 |
| Cefaclor | 57 | 0 | 0 | 0 | 0 | 0 | 12 | 9 | 9 | 11 | 4 | 4 | 1 | 7 | 0 | 0 | 0 |
| Cefepime | 57 | 0 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefixime | 57 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 39 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefpodoxime | 57 | 0 | 0 | 17 | 7 | 6 | 4 | 6 | 5 | 0 | 4 | 6 | 2 | 0 | 0 | 0 | 0 |
| Ceftriaxone | 57 | 0 | 0 | 0 | 0 | 51 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefuroxime | 57 | 0 | 0 | 19 | 5 | 8 | 4 | 5 | 8 | 1 | 2 | 3 | 2 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 57 | 0 | 0 | 0 | 20 | 14 | 2 | 5 | 0 | 13 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| Clarithromycin | 57 | 0 | 0 | 5 | 2 | 25 | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 14 |
| Levofloxacin | 57 | 0 | 0 | 0 | 31 | 23 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moxifloxacin | 57 | 0 | 0 | 0 | 51 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillin | 57 | 3 | 9 | 3 | 9 | 9 | 1 | 6 | 9 | 5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
All 57 isolates were susceptible to amoxicillin based on CLSI and PK/PD breakpoints. They were also susceptible to cefepime, ceftriaxone, levofloxacin and moxifloxacin based on CLSI and EUCAST breakpoints and to cefixime, ceftriaxone, levofloxacin and moxifloxacin based on PK/PD breakpoints. On initial testing two isolates showed intermediate susceptibility to amoxicillin/clavulanic acid, but it was not possible to repeat the test to assess the validity of this finding. Using CLSI, EUCAST and PK/PD breakpoints, 91.2%, 86.0% and 91.2% of isolates, respectively, were susceptible to cefuroxime. By all three criteria, 79.0% of strains were susceptible to cefpodoxime. For cefaclor, susceptibilities by CLSI and PK/PD breakpoints were 79.0% and 71.9%, respectively. As observed in other countries, no isolate was susceptible to cefaclor by EUCAST criteria, but 71.9% of isolates were intermediate and 28.1% resistant.
Most penicillin-susceptible S. pneumoniae were also susceptible to the β-lactams listed above, except for four isolates that were cefpodoxime intermediate. However, the reverse was not always found. Of the 23 penicillin-non-susceptible isolates, all were amoxicillin, cefepime or ceftriaxone susceptible, 22 (95.7%) were amoxicillin/clavulanic acid susceptible, 18 (78.3%) were cefuroxime susceptible and 15 (65.2%) were cefpodoxime susceptible.
All of the isolates were susceptible to levofloxacin and moxifloxacin by all three criteria; susceptibility to ciprofloxacin was 71.9% and 96.5%, respectively, by EUCAST and PK/PD criteria. Interestingly, the MIC distribution for ciprofloxacin against S. pneumoniae from Lebanon was very low and could be considered ‘sub-wild-type’ as compared with EUCAST data. Susceptibility was 73.7% to both azithromycin and clarithromycin (CLSI in CO2) and also to erythromycin by CLSI and EUCAST disc diffusion criteria.
Oman
Summary MIC and susceptibility data for 60 S. pneumoniae isolates from Oman are shown in Table 6. The complete MIC distribution is given in Table 7. Using the CLSI iv breakpoint, 98.3% of strains were PSSP. With the CLSI oral and EUCAST breakpoints, 41.7% and 41.6% of isolates were PSSP, 51.7% and 56.7% were PISP and 6.7% and 1.7% (one isolate) were PRSP, respectively.
Table 6.
MIC and susceptibility results for all S. pneumoniae isolates from Oman
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| Amoxicillin | 60 | 0.12 | 1 | ≤0.015 | 1 | 100.0 | 0 | 0 | 100.0 | NA | NA | NA |
| AMCa | 60 | 0.12 | 1 | ≤0.015 | 2 | 100.0 | 0 | 0 | 100.0 (100.0) | NA | NA | NA |
| Azithromycin | 60 | 2 | 256 | 0.5 | >256 | 56.7 | 0 | 43.3 | NA | NA | NA | NA |
| Cefaclor | 60 | 2 | 32 | 0.12 | 256 | 46.7 | 8.3 | 45.0 | 40.0 | 0 | 40.0 | 60.0 |
| Cefepime | 60 | 0.12 | 1 | ≤0.015 | 4 | 95.0 | 3.3 | 1.7 | NA | 95.0 | 3.3 | 1.7 |
| Cefixime | 60 | 2 | 8 | 0.12 | 64 | NA | NA | NA | 48.3 | NA | NA | NA |
| Cefpodoxime | 60 | 0.25 | 2 | ≤0.015 | 16 | 61.7 | 18.3 | 20.0 | 61.7 | 60.0 | 1.7 | 38.3 |
| Ceftriaxone | 60 | 0.06 | 0.5 | 0.008 | 2 | 98.3 | 1.7 | 0 | 98.3 | 93.3 | 6.7 | 0 |
| Cefuroxime | 60 | 0.12 | 2 | 0.015 | 8 | 70.0 | 25.0 | 5.0 | 70.0 | 61.7 | 0 | 38.3 |
| Ciprofloxacin | 60 | 1 | 2 | 0.5 | 32 | NA | NA | NA | 75.0 | 0 | 90.0 | 10.0 |
| Clarithromycin | 60 | 0.5 | 32 | 0.06 | >256 | 56.7 | 0 | 43.3 | NA | NA | NA | NA |
| Levofloxacin | 60 | 1 | 1 | 0.25 | 4 | 98.3 | 1.7 | 0 | 98.3 | 98.3 | — | 1.7 |
| Moxifloxacin | 60 | 0.12 | 0.25 | 0.03 | 0.5 | 100.0 | 0 | 0 | 100.0 | 100.0 | — | 0 |
| Penicillin (oral) | 60 | 0.25 | 1 | ≤0.015 | 4 | 41.7 | 51.7 | 6.7 | NA | 41.6 | 56.7 | 1.7 |
| Penicillin (iv) | 60 | 0.25 | 1 | ≤0.015 | 4 | 98.3 | 1.7 | 0 | NA | 68.3 to 98.3 | NA | NA |
| Erythromycinb | 54 | NT | NT | NT | NT | 57.4 | 0 | 42.6 | NA | 57.4 | 0 | 42.6 |
min, minimum; max, maximum; AMC, amoxicillin/clavulanic acid; S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, no breakpoint data available (NA for azithromycin and clarithromycin by EUCAST because Etest breakpoints in CO2 not available).
aPK/PD susceptibility at high dose is shown in parentheses.
bUsing S/I/R zone diameters of CLSI (≤15/16–20/≥21) and EUCAST (≤18/19–21/≥22).
Table 7.
Distribution of MICs for all S. pneumoniae isolates from Oman
| Isolates susceptible at MIC (mg/L) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | n | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | >256 |
| Amoxicillin | 60 | 0 | 23 | 0 | 1 | 5 | 7 | 5 | 11 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AMC | 60 | 0 | 22 | 0 | 1 | 4 | 8 | 5 | 9 | 8 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Azithromycin | 60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 14 | 19 | 0 | 0 | 1 | 2 | 10 | 3 | 8 | 2 |
| Cefaclor | 60 | 0 | 0 | 0 | 0 | 0 | 1 | 14 | 9 | 4 | 5 | 5 | 3 | 5 | 9 | 3 | 1 | 1 | 0 |
| Cefepime | 60 | 0 | 5 | 0 | 15 | 1 | 12 | 3 | 3 | 18 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefixime | 60 | 0 | 0 | 0 | 0 | 0 | 10 | 10 | 4 | 5 | 8 | 3 | 16 | 3 | 0 | 1 | 0 | 0 | 0 |
| Cefpodoxime | 60 | 0 | 5 | 0 | 15 | 2 | 8 | 6 | 1 | 11 | 9 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Ceftriaxone | 60 | 9 | 0 | 11 | 3 | 8 | 3 | 5 | 17 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefuroxime | 60 | 0 | 0 | 19 | 1 | 2 | 10 | 5 | 0 | 5 | 15 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 60 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 40 | 9 | 1 | 3 | 0 | 2 | 0 | 0 | 0 | 0 |
| Clarithromycin | 60 | 0 | 0 | 0 | 0 | 1 | 4 | 20 | 9 | 0 | 0 | 1 | 0 | 10 | 10 | 0 | 0 | 3 | 2 |
| Levofloxacin | 60 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 24 | 29 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moxifloxacin | 60 | 0 | 0 | 0 | 1 | 2 | 46 | 9 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillin | 60 | 0 | 20 | 0 | 1 | 4 | 4 | 7 | 5 | 15 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
All 60 isolates were susceptible to amoxicillin or amoxicillin/clavulanate by both CLSI and PK/PD criteria. Among cephalosporins, cefepime showed 95.0% susceptibility by both CLSI and EUCAST breakpoints; ceftriaxone was also highly active, with 98.3%, 93.3% and 98.3% susceptibility by CLSI, EUCAST and PK/PD criteria, respectively. The isolates were susceptible to cefuroxime with the three breakpoints (70.0%, 61.7% and 70.0%, respectively) and cefpodoxime (61.7%, 60.0% and 61.7%). Cefaclor breakpoints are such that all isolates were non-susceptible by EUCAST criteria (40% intermediate, 60% resistant), as observed in other countries, while 46.7% and 40% were susceptible by CLSI and PK/PD criteria, respectively. By PK/PD criteria, 48.3% of strains were susceptible to cefixime.
All penicillin-susceptible S. pneumoniae were also susceptible to the β-lactams tested. However, the reverse was not always found. Of the 35 penicillin-non-susceptible isolates, all were amoxicillin or amoxicillin/clavulanic acid susceptible, 34 (97.1%) were ceftriaxone susceptible, 28 (80%) were cefepime susceptible and 17 (48.6%) were cefuroxime susceptible. However, only three penicillin non-susceptible S. pneumoniae were cefaclor susceptible (8.6%).
All of the isolates were susceptible to moxifloxacin by the three criteria and 98.3% were susceptible to levofloxacin. For ciprofloxacin, 75% were susceptible using the PK/PD breakpoint, whereas none was susceptible by EUCAST criteria (90% intermediate, 10% resistant). Susceptibility to azithromycin and clarithromycin was seen in 56.7% of isolates (CLSI in CO2), while susceptibility to erythromycin was seen in 57.4% using both CLSI and EUCAST disc diffusion breakpoints.
UAE
Summary MIC and susceptibility data for 48 S. pneumoniae isolates from the UAE are shown in Table 8. The complete MIC distribution data are shown in Table 9. Only 33.3% of the isolates were PSSP by CLSI oral and EUCAST criteria (41.7% and 54.2% PISP, 25.0% and 12.5% PRSP, respectively). Using the CLSI iv breakpoint, 87.5% were PSSP (12.5% PISP, no PRSP).
Table 8.
MIC and susceptibility results for all S. pneumoniae isolates from the UAE
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| Amoxicillin | 48 | 0.12 | 4 | ≤0.015 | 8 | 89.6 | 8.3 | 2.1 | 89.6 | NA | NA | NA |
| AMCa | 48 | 0.12 | 4 | ≤0.015 | 8 | 87.5 | 8.3 | 4.2 | 87.5 (95.8) | NA | NA | NA |
| Azithromycin | 48 | 16 | >256 | 0.12 | >256 | 47.9 | 0 | 52.1 | NA | NA | NA | NA |
| Cefaclor | 48 | 2 | 128 | 0.12 | >256 | 39.6 | 16.7 | 43.8 | 31.3 | 0 | 31.3 | 68.8 |
| Cefepime | 48 | 0.5 | 2 | ≤0.015 | 2 | 81.3 | 18.8 | 0 | NA | 81.3 | 18.8 | 0 |
| Cefixime | 48 | 4 | 32 | 0.06 | 256 | NA | NA | NA | 31.3 | NA | NA | NA |
| Cefpodoxime | 48 | 0.5 | 4 | ≤0.015 | 16 | 50.0 | 8.3 | 41.7 | 50.0 | 33.3 | 16.7 | 50.0 |
| Ceftriaxone | 48 | 0.25 | 2 | 0.008 | 4 | 89.6 | 8.3 | 2.1 | 89.6 | 77.1 | 20.8 | 2.1 |
| Cefuroxime | 48 | 0.5 | 4 | ≤0.015 | 16 | 54.2 | 20.8 | 25.0 | 54.2 | 47.9 | 6.3 | 45.8 |
| Ciprofloxacin | 48 | 1 | 4 | 0.5 | >32 | NA | NA | NA | 70.8 | 0 | 89.6 | 10.4 |
| Clarithromycin | 48 | 8 | >256 | 0.06 | >256 | 43.7 | 0 | 56.3 | NA | NA | NA | NA |
| Levofloxacin | 48 | 0.5 | 1 | 0.12 | 32 | 97.9 | 0 | 2.1 | 97.9 | 97.9 | 0 | 2.1 |
| Moxifloxacin | 48 | 0.12 | 0.12 | 0.06 | 2 | 97.9 | 2.1 | 0 | 97.9 | 97.9 | 0 | 2.1 |
| Penicillin (oral) | 48 | 0.5 | 4 | ≤0.015 | 4 | 33.3 | 41.7 | 25.0 | NA | 33.3 | 54.2 | 12.5 |
| Penicillin (iv) | 48 | 0.12 | 4 | ≤0.015 | 8 | 87.5 | 12.5 | 0 | NA | 56.3–87.5 | NA | NA |
| Erythromycinb | 48 | NT | NT | NT | NT | 50.0 | 12.5 | 37.5 | NA | 50.0 | 6.3 | 43.7 |
min, minimum; max, maximum; AMC, amoxicillin/clavulanic acid; S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, no breakpoint data available (NA for azithromycin and clarithromycin by EUCAST because Etest® breakpoints in CO2 not available).
aPK/PD susceptibility at high dose is shown in parentheses.
bUsing S/I/R zone diameters of CLSI (≤15/16–20/≥21) and EUCAST (≤18/19–21/≥22).
Table 9.
Distribution of MICs for all S. pneumoniae isolates from the UAE
| Isolates susceptible at MIC (mg/L) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | n | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >32 | 64 | 128 | 256 | >256 |
| Amoxicillin | 48 | 0 | 11 | 0 | 6 | 2 | 5 | 3 | 4 | 6 | 6 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AMC | 48 | 0 | 11 | 0 | 5 | 2 | 7 | 2 | 4 | 4 | 7 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Azithromycin | 48 | 0 | 0 | 0 | 0 | 0 | 3 | 12 | 6 | 0 | 1 | 1 | 0 | 3 | 3 | 0 | 3 | 0 | 1 | 15 |
| Cefaclor | 48 | 0 | 0 | 0 | 0 | 0 | 1 | 7 | 7 | 4 | 8 | 1 | 0 | 2 | 8 | 0 | 5 | 1 | 3 | 1 |
| Cefepime | 48 | 0 | 9 | 0 | 3 | 1 | 1 | 8 | 7 | 10 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefixime | 48 | 0 | 0 | 0 | 0 | 2 | 5 | 3 | 3 | 2 | 8 | 5 | 5 | 6 | 8 | 0 | 0 | 0 | 1 | 0 |
| Cefpodoxime | 48 | 0 | 3 | 0 | 8 | 0 | 3 | 2 | 8 | 4 | 8 | 8 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceftriaxone | 48 | 5 | 0 | 2 | 3 | 4 | 6 | 7 | 10 | 6 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefuroxime | 48 | 0 | 7 | 0 | 4 | 0 | 5 | 7 | 3 | 0 | 10 | 9 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 48 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17 | 17 | 9 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Clarithromycin | 48 | 0 | 0 | 0 | 0 | 7 | 13 | 1 | 0 | 0 | 2 | 0 | 5 | 2 | 2 | 0 | 1 | 0 | 0 | 15 |
| Levofloxacin | 48 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 21 | 22 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Moxifloxacin | 48 | 0 | 0 | 0 | 0 | 18 | 27 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillin | 48 | 0 | 9 | 0 | 5 | 2 | 3 | 4 | 4 | 9 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
Amoxicillin susceptibility was noted for 89.6% of isolates using both CLSI and PK/PD breakpoints, whereas susceptibility to amoxicillin/clavulanic acid was 87.5% (low dose, both criteria). This was an unusual finding, but it was not possible to repeat the test to assess its reproducibility. Using high-dose PK/PD, 95.8% were susceptible to amoxicillin/clavulanic acid. Among the cephalosporins, the best activity was demonstrated by ceftriaxone: 89.6%, 77.1% and 89.6% of strains were susceptible by CLSI, EUCAST and PK/PD criteria, respectively. Susceptibility to cefepime was 81.3% by both CLSI and EUCAST criteria. To cefuroxime, 54.2% of strains were susceptible by CLSI and PK/PD breakpoints and 47.9% by EUCAST. To cefpodoxime, 50% of isolates were susceptible by CLSI and PK/PD criteria and 33.3% by EUCAST criteria. Susceptibility to cefaclor was reported in 39.6%, 0% and 31.3% of isolates, respectively, by CLSI, EUCAST and PK/PD breakpoints; 31.3% were also susceptible to cefixime based on PK/PD.
All penicillin-susceptible S. pneumoniae were also susceptible to the β-lactams tested. However, the reverse was not always found. Of the 32 penicillin-non-susceptible isolates, 27 (84.3%) were amoxicillin or ceftriaxone susceptible, 26 (81.3%) were amoxicillin/clavulanic acid susceptible, 23 (71.9%) were cefepime susceptible and 10 (31.3%) were cefuroxime susceptible. However, only three penicillin-non-susceptible S. pneumoniae were cefaclor susceptible (9.4%).
Both levofloxacin and moxifloxacin scored 97.9% susceptible with all three criteria; ciprofloxacin was less active: 0% and 70.8% susceptible by EUCAST and PK/PD criteria, respectively. Among macrolides, 47.9% and 43.7% of isolates, respectively, were susceptible to azithromycin and clarithromycin using CLSI breakpoints in CO2 (all other isolates were resistant); 50.0% were susceptible to erythromycin by CLSI and EUCAST disc diffusion breakpoints (respectively, 37.5% and 43.7% were resistant).
Comparative susceptibility of S. pneumoniae isolates by country
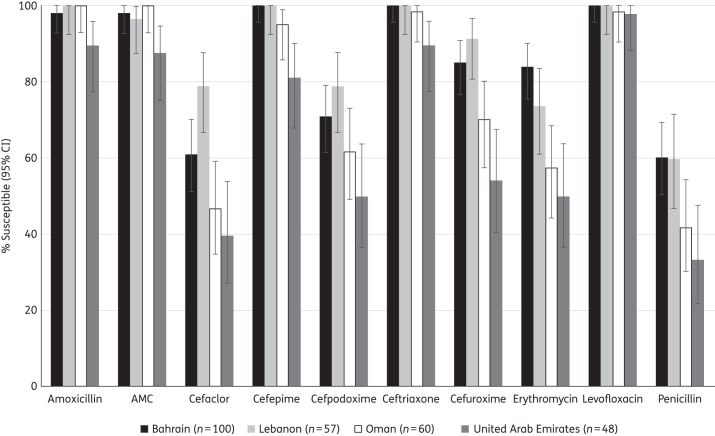
Comparative susceptibility (CLSI breakpoints) of S. pneumoniae by country to 10 antimicrobial agents is depicted in Figure 1. In general, susceptibility was lowest among isolates from the UAE, followed by Oman then Lebanon and Bahrain. Lower susceptibility in the UAE compared with all the other Gulf and Near East countries was statistically significant for amoxicillin, amoxicillin/clavulanic acid and cefepime (P < 0.05). UAE isolates also had significantly lower susceptibility than isolates from Bahrain and Lebanon for cefaclor, ceftriaxone, cefuroxime, cefpodoxime and the macrolides, but this difference was not significant between the UAE and Oman. For several of the agents tested, including penicillin, cephalosporins and macrolides, susceptibilities among strains from Oman and the UAE were statistically significantly lower than for the strains from both Bahrain and Lebanon. Susceptibility in Bahrain and Lebanon was in general not significantly different, although susceptibility to cefaclor in Bahrain was significantly lower than in Lebanon (P < 0.05). Susceptibility to quinolones, on the other hand, was similar in all four countries.
Figure 1.
Susceptibility of S. pneumoniae isolates to 10 antimicrobial agents compared by country (CLSI breakpoints). For penicillin, the CLSI oral breakpoint was utilized. AMC, amoxicillin/clavulanic acid.
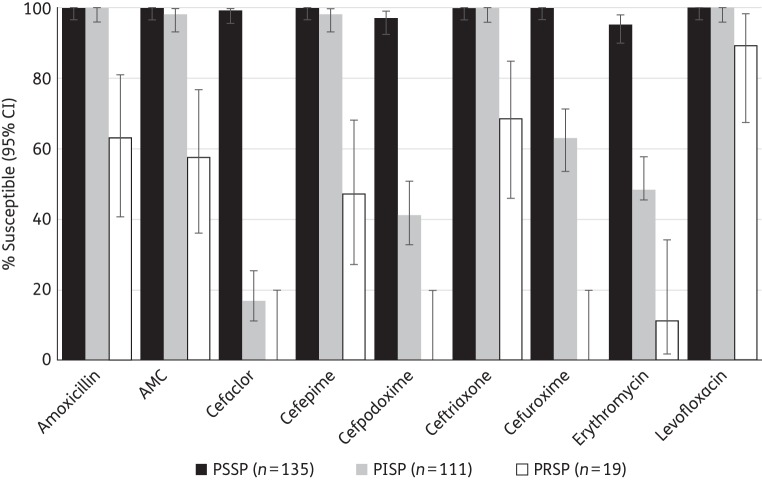
Antimicrobial susceptibility of S. pneumoniae by penicillin susceptibility
Utilizing the CLSI (oral) breakpoints, the relationship between susceptibility to penicillin and to other antimicrobial agents was assessed for the pooled set of 265 S. pneumoniae strains from the four countries (Figure 2). Among 135 PSSP, 100% were susceptible to amoxicillin, amoxicillin/clavulanic acid, cefepime, ceftriaxone and cefuroxime; 99.3% were susceptible to cefaclor and 97.0% to cefpodoxime. Of the 111 PISP, 100% were susceptible to amoxicillin and ceftriaxone, 98.3% susceptible to amoxicillin/clavulanic acid and 98.2% were susceptible to cefepime. Susceptibility to the other cephalosporins was much lower, ranging from 63.1% (cefuroxime) to 17.1% (cefaclor). Among 19 PRSP, 63.2% were susceptible to amoxicillin and 68.4%, 57.9% and 47.4% were susceptible to ceftriaxone, amoxicillin/clavulanic acid and cefepime, respectively. All PRSP were resistant to cefaclor, cefpodoxime and cefuroxime. Levofloxacin susceptibility was seen in 100% of PSSP and PISP, and in 89.5% of PRSP. Erythromycin susceptibility reduced with susceptibility to penicillin, with 95.5%, 48.6% and 11.1% susceptibility among PSSP, PISP and PRSP, respectively. With the exception of cefaclor, activity of the agents versus PRSP isolates was significantly lower than their activity against both PSSP and PISP isolates (P < 0.05). Cefaclor activity was not statistically significantly different between PISP and PRSP. The difference in activity against PISP versus PSSP was statistically significant only for cefaclor, cefpodoxime, cefuroxime and erythromycin.
Figure 2.
Susceptibility of pooled S. pneumoniae isolates from Bahrain, Lebanon, Oman and the UAE to other antimicrobial agents (using CLSI oral breakpoints) by susceptibility to penicillin. AMC, amoxicillin/clavulanic acid.
S. pyogenes susceptibility
As expected, all of the isolates were susceptible to penicillin; MICs were mainly ≤0.015 mg/L, well below the CLSI and EUCAST breakpoints. Similarly, all isolates were susceptible to amoxicillin/clavulanic acid (PK/PD). This full susceptibility was also observed for all cephalosporins against isolates from Bahrain, Lebanon and Oman. Surprisingly, only 96.8% of strains were susceptible to cefepime (based on CLSI breakpoints); 93.6% were susceptible to cefpodoxime (PK/PD) and susceptibility to both cefaclor and cefixime was 90.3% (PK/PD) in the UAE. This reduced cephalosporin activity against isolates fully susceptible to penicillin is unusual, but it was not possible to repeat the test to assess its reproducibility.
Generally, the isolates were highly susceptible to moxifloxacin and levofloxacin (>94%) by EUCAST and PK/PD criteria, but susceptibility to levofloxacin was only 80.0% by CLSI and PK/PD criteria and 78.0% by EUCAST criteria in isolates from Bahrain. Ciprofloxacin susceptibility was also 80.0% against isolates from Bahrain using PK/PD criteria, with similar susceptibility observed in Oman.
Erythromycin susceptibility was the lowest for all agents tested that have available breakpoints. This ranged from 68.4% in Oman, 80% in the UAE, 88% in Lebanon and 94% in Bahrain (CLSI or EUCAST breakpoints).
To erythromycin, Oman isolates had the lowest susceptibility rate, significantly lower than for isolates from Bahrain (P < 0.05) but not significantly lower than isolates from Lebanon or the UAE. Levofloxacin susceptibility was lowest among isolates from Bahrain (but this was not statistically significant).
Overall, there was consistently high susceptibility of S. pyogenes to all β-lactam antibiotics (100% of all strains from three of the countries) and to moxifloxacin, with somewhat more variability in susceptibility to levofloxacin and ciprofloxacin. Non-susceptibility was more often seen to erythromycin.
H. influenzae susceptibility in individual countries
Bahrain
Summary MIC and susceptibility data for 100 H. influenzae isolates from Bahrain are shown in Table 10 and the complete MIC distribution data are shown in Table 11. Susceptibility to amoxicillin/clavulanic acid by CLSI, EUCAST and PK/PD criteria was 99.0%. Susceptibility to ampicillin was 85.0% by CLSI and EUCAST criteria, which reflects the prevalence of β-lactamase-positive isolates (n = 14, 14%). None of the isolates was β-lactamase negative and ampicillin resistant (BLNAR). All isolates were susceptible to ceftriaxone and 99.0% to cefixime by all three criteria. For cefepime, 100% and 99.0% were susceptible by CLSI and EUCAST criteria, respectively. Cefpodoxime susceptibility was 100%, 79% and 100%, respectively, by CLSI, EUCAST and PK/PD criteria. Susceptibility was lower to cefuroxime (83%, 0% and 63.0%) by CLSI, EUCAST and PK/PD criteria and to cefaclor (31% and 0%) with CLSI and PK/PD breakpoints, respectively.
Table 10.
MIC and susceptibility results for all H. influenzae isolates from Bahrain
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| AMCa | 100 | 1 | 2 | 0.5 | >256 | 99.0 | 0 | 1.0 | 99.0 (99.0) | 99.0 | 0 | 1.0 |
| Ampicillin | 100 | 0.25 | 8 | 0.12 | >256 | 85.0 | 1.0 | 14.0 | NA | 85.0 | 0 | 15.0 |
| Azithromycin | 100 | 2 | 4 | 1 | 8 | 100 | 0 | 0 | NA | NA | NA | NA |
| Cefaclor | 100 | 32 | >256 | 4 | >256 | 31.0 | 10.0 | 59.0 | 0 | NA | NA | NA |
| Cefepime | 100 | 0.12 | 0.25 | 0.03 | 0.5 | 100 | 0 | 0.0 | NA | 99.0 | 0 | 1.0 |
| Cefixime | 100 | 0.06 | 0.12 | ≤0.015 | 2 | 99.0 | 0 | 1.0 | 99.0 | 99.0 | 0 | 1.0 |
| Cefpodoxime | 100 | 0.12 | 0.5 | 0.06 | 0.5 | 100 | 0 | 0.0 | 100 | 79.0 | 21.0 | 0 |
| Ceftriaxone | 100 | 0.008 | 0.015 | ≤0.002 | 0.03 | 100 | 0 | 0.0 | 100 | 100 | 0 | 0 |
| Cefuroxime | 100 | 1 | 8 | 0.5 | 16 | 83.0 | 9.0 | 8.0 | 63.0 | 0 | 63.0 | 37.0 |
| Ciprofloxacin | 100 | 0.015 | 0.5 | 0.008 | >32 | 96.0 | 0 | 4.0 | 96.0 | 90.0 | 0 | 10.0 |
| Clarithromycin | 100 | 16 | 32 | 4 | 64 | 69.0 | 27.0 | 4.0 | NA | NA | NA | NA |
| Levofloxacin | 100 | 0.03 | 0.5 | 0.008 | >32 | 97.0 | 0 | 3.0 | 97.0 | 97.0 | 0 | 3.0 |
| Moxifloxacin | 100 | 0.03 | 0.25 | 0.015 | >32 | 97.0 | 0 | 3.0 | 97.0 | 92.0 | 0 | 8.0 |
min, minimum; max, maximum; AMC, amoxicillin/clavulanic acid; S, susceptible; I, intermediate; R, resistant; NA, no breakpoint data available (NA for azithromycin and clarithromycin by EUCAST because Etest® breakpoints in CO2 not available).
aPK/PD susceptibility at high dose is shown in parentheses.
Table 11.
Distribution of MICs for all H. influenzae isolates from Bahrain
| Isolates susceptible at MIC (mg/L) |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | n | 0.002 | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >32 | 64 | 128 | 256 | >256 |
| AMC | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 28 | 43 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ampicillin | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 49 | 13 | 21 | 1 | 1 | 3 | 4 | 0 | 0 | 0 | 0 | 2 | 4 |
| Azithromycin | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 49 | 39 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefaclor | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 9 | 10 | 15 | 0 | 2 | 3 | 1 | 38 |
| Cefepime | 100 | 0 | 0 | 0 | 0 | 0 | 1 | 22 | 62 | 14 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefixime | 100 | 0 | 0 | 0 | 1 | 0 | 15 | 66 | 17 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefpodoxime | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 63 | 6 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceftriaxone | 100 | 1 | 26 | 46 | 0 | 20 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefuroxime | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 32 | 31 | 14 | 6 | 9 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 100 | 0 | 0 | 35 | 0 | 49 | 5 | 0 | 0 | 0 | 1 | 6 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| Clarithromycin | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 11 | 57 | 27 | 0 | 4 | 0 | 0 | 0 |
| Levofloxacin | 100 | 0 | 0 | 1 | 0 | 44 | 43 | 1 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| Moxifloxacin | 100 | 0 | 0 | 0 | 0 | 18 | 55 | 13 | 2 | 2 | 2 | 5 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
Susceptibility to quinolones was high: 97% of isolates were susceptible to levofloxacin by all three criteria; 97%, 92% and 97% were susceptible to moxifloxacin using the CLSI, EUCAST and PK/PD breakpoints, respectively; and 96%, 90% and 96% were susceptible to ciprofloxacin. By CLSI criteria, all strains were susceptible to azithromycin, but only 69.0% were susceptible to clarithromycin.
Lebanon
Summary MIC and susceptibility data for 47 H. influenzae isolates from Lebanon are shown in Table 12 and the complete MIC distribution data are shown in Table 13. All 47 isolates were in vitro susceptible to amoxicillin/clavulanic acid using CLSI, EUCAST and PK/PD breakpoints. However, four isolates (8.5%) were BLNAR by CLSI definition (ampicillin MIC ≥4 mg/L) and 7 (14.9%) were BLNAR by EUCAST definition (ampicillin MIC ≥2 mg/L). According to expert rules, BLNAR should be considered non-susceptible to amoxicillin/clavulanic acid, so susceptibility should be 91.5% by CLSI and 85.1% by EUCAST. Susceptibility to ampicillin was 70.2% by CLSI and EUCAST criteria. Seven isolates out of 47 (14.9%) were β-lactamase positive. Susceptibility to cefixime, cefpodoxime and ceftriaxone was 100% by all three criteria, and susceptibility to cefepime was 100% using CLSI and EUCAST breakpoints. Susceptibility was lower to the other two cephalosporins tested: 95.7% (87.2% accounting for BLNAR), 6.4% and 91.5%, respectively, to cefuroxime by CLSI, EUCAST and PK/PD criteria; and 95.7% (89.4% accounting for BLNAR) and 8.5% to cefaclor by CLSI and PK/PD criteria, respectively. All isolates were susceptible to ciprofloxacin and levofloxacin by all three breakpoints; 100% were also susceptible to moxifloxacin by CLSI and PK/PD criteria and 97.8% were susceptible by EUCAST criteria.
Table 12.
MIC and susceptibility results for all H. influenzae isolates from Lebanon
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| AMCa,b | 47 | 0.5 | 2 | ≤0.015 | 2 | 100.0 (91.5)c | 0 | 0 (8.5)c | 100.0 (100.0) | 100.0 (85.1)c | 0 (0)c | 0 (14.9)c |
| Ampicillin | 47 | 0.5 | 32 | 0.06 | >256 | 70.2 | 6.4 | 23.4 | NA | 70.2 | 0 | 29.8 |
| Cefaclorb | 47 | 1 | 4 | 0.03 | 32 | 95.7 (87.2)c | 2.1 (2.2)c | 2.1 (10.6)c | 8.5 | NA | NA | NA |
| Cefepime | 47 | 0.03 | 0.06 | 0.03 | 0.06 | 100.0 | 0 | 0 | NA | 100.0 | 0 | 0 |
| Cefixime | 47 | 0.03 | 0.06 | 0.03 | 0.06 | 100.0 | 0 | 0 | 100.0 | 100.0 | 0 | 0 |
| Cefpodoxime | 47 | 0.06 | 0.12 | 0.03 | 0.25 | 100.0 | 0 | 0 | 100.0 | 100.0 | 0.0 | 0 |
| Ceftriaxone | 47 | 0.004 | 0.008 | 0.004 | 0.008 | 100.0 | 0 | 0 | 100.0 | 100.0 | 0 | 0 |
| Cefuroximeb | 47 | 0.5 | 1 | 0.015 | 8 | 95.7 (89.4)c | 4.3 (2.1)c | 0 (8.5)c | 91.5 | 6.4 (6.4)c | 85.1 (72.3)c | 8.5 (21.3)c |
| Ciprofloxacin | 47 | 0.015 | 0.03 | 0.002 | 0.12 | 100.0 | 0 | 0 | 100.0 | 100.0 | 0 | 0 |
| Levofloxacin | 46 | 0.06 | 0.12 | 0.015 | 0.5 | 100.0 | 0 | 0 | 100.0 | 100.0 | 0 | 0 |
| Moxifloxacin | 46 | 0.06 | 0.12 | 0.03 | 1 | 100.0 | 0 | 0 | 100.0 | 97.8 | 0 | 2.2 |
min, minimum; max, maximum; AMC, amoxicillin/clavulanic acid; S, susceptible; I, intermediate; R, resistant; NA, no breakpoint data available (NA for azithromycin and clarithromycin by EUCAST because Etest® breakpoints in CO2 not available).
aPK/PD susceptibility at high dose is shown in parentheses.
bIn clinical setting, isolates of BLNAR are considered resistant to amoxicillin/clavulanic acid, cefaclor and cefuroxime (see main text).
cSusceptibility to amoxicillin/clavulanic acid, cefaclor and cefuroxime (data in parenthesis) due to corrections according to BLNAR (see main text).
Table 13.
Distribution of MICs for all H. influenzae isolates from Lebanon
| Isolates susceptible at MIC (mg/L) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | n | 0.002 | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 256 | >256 |
| AMC | 47 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 7 | 21 | 8 | 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ampicillin | 47 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 19 | 7 | 3 | 3 | 2 | 2 | 2 | 2 | 1 | 2 |
| Cefaclor | 47 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 21 | 14 | 6 | 0 | 1 | 1 | 0 | 0 |
| Cefepime | 47 | 0 | 0 | 0 | 0 | 0 | 32 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefixime | 47 | 0 | 0 | 0 | 0 | 0 | 26 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefpodoxime | 47 | 0 | 0 | 0 | 0 | 0 | 11 | 28 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceftriaxone | 47 | 0 | 36 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefuroxime | 47 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 3 | 25 | 12 | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 47 | 1 | 7 | 13 | 0 | 20 | 2 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Levofloxacin | 46 | 0 | 0 | 0 | 0 | 5 | 15 | 20 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moxifloxacin | 46 | 0 | 0 | 0 | 0 | 0 | 18 | 22 | 4 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
Oman
Summary MIC and susceptibility data for 108 H. influenzae isolates from Oman are shown in Table 14, and the complete MIC distribution data are in Table 15. Amoxicillin susceptibility was 90.7% by both EUCAST and PK/PD criteria; reflecting the prevalence of β-lactamase (11/108, 10.2%). As ampicillin was not tested, BLNAR cannot be clearly defined; however, the amoxicillin MIC for β-lactamase-negative isolates was ≤2 mg/L, which suggests that none was BLNAR. Susceptibility to amoxicillin/clavulanic acid was 95.4%, 92.6% and 92.6% by CLSI, EUCAST and PK/PD criteria, respectively, and 95.4% were susceptible by the high-dose PK/PD criteria. Based on CLSI, EUCAST and PK/PD breakpoints, respectively, the isolates were susceptible to ceftriaxone at 100%, 99.1% and 100%, to cefixime at 100%, 98.2% and 100%, to cefpodoxime at 99.1%, 94.4% and 99.1%, and to cefuroxime at 98.2%, 16.7% and 86.1%. Susceptibility to cefepime was 99.1% and 96.3% by CLSI and EUCAST criteria, respectively; to cefaclor, 88.9% and 12% of the isolates, respectively, were susceptible by CLSI and PK/PD criteria. Among the quinolones, 100% of isolates were susceptible to levofloxacin by all three criteria, and 98.1% were susceptible to both moxifloxacin and ciprofloxacin by all three criteria. By CLSI breakpoints, 100% of isolates were susceptible to azithromycin and 85.2% to clarithromycin.
Table 14.
MIC and susceptibility results for all H. influenzae isolates from Oman
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| Amoxicillin | 108 | 0.12 | 2 | 0.03 | 256 | NA | NA | NA | 90.7 | 90.7 | 9.3 | |
| AMCa | 108 | 0.25 | 2 | ≤0.015 | 16 | 95.4 | 0 | 4.6 | 92.6 (95.4) | 92.6 | — | 7.4 |
| Azithromycin | 105 | 2 | 4 | 0.06 | 4 | 100.0 | 0 | 0.0 | NA | NA | NA | NA |
| Cefaclor | 108 | 2 | 16 | 0.25 | >256 | 88.9 | 2.8 | 8.3 | 12.0 | NA | NA | NA |
| Cefepime | 108 | 0.06 | 0.25 | ≤0.015 | 4 | 99.1 | 0 | 0.9 | NA | 96.3 | 0 | 3.7 |
| Cefixime | 108 | 0.03 | 0.06 | ≤0.015 | 0.5 | 100.0 | 0 | 0.0 | 100.0 | 98.2 | 0 | 1.9 |
| Cefpodoxime | 108 | 0.06 | 0.25 | ≤0.015 | 8 | 99.1 | 0 | 0.9 | 99.1 | 94.4 | 4.6 | 0.9 |
| Ceftriaxone | 108 | 0.004 | 0.015 | ≤0.002 | 0.5 | 100.0 | 0 | 0.0 | 100.0 | 99.1 | 0 | 0.9 |
| Cefuroxime | 108 | 0.5 | 2 | 0.03 | 64 | 98.2 | 0.9 | 0.9 | 86.1 | 16.7 | 69.4 | 13.9 |
| Ciprofloxacin | 108 | 0.008 | 0.015 | ≤0.002 | 4 | 98.1 | 0 | 1.9 | 98.2 | 98.2 | 0 | 1.9 |
| Clarithromycin | 108 | 8 | 32 | 0.5 | 32 | 85.2 | 14.8 | 0.0 | NA | NA | NA | NA |
| Levofloxacin | 108 | 0.015 | 0.015 | 0.004 | 1 | 100.0 | 0 | 0.0 | 100.0 | 100.0 | 0 | 0.0 |
| Moxifloxacin | 108 | 0.015 | 0.03 | ≤0.002 | 4 | 98.1 | 0 | 1.9 | 98.1 | 98.1 | 0 | 1.9 |
min, minimum; max, maximum; AMC, amoxicillin/clavulanic acid; S, susceptible; I, intermediate; R, resistant; NA, no breakpoint data available (NA for azithromycin and clarithromycin by EUCAST because Etest® breakpoints in CO2 not available).
aAmoxicillin/clavulanic acid PK/PD susceptibility at high dose is shown in parentheses.
Table 15.
Distribution of MICs for all H. influenzae isolates from Oman
| Isolates susceptible at MIC (mg/L) |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | n | ≤0.002 | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | >256 |
| Amoxicillin | 108 | 0 | 0 | 0 | 0 | 0 | 7 | 18 | 33 | 22 | 10 | 3 | 5 | 0 | 4 | 3 | 1 | 0 | 1 | 1 | 0 |
| AMC | 108 | 0 | 0 | 0 | 2 | 0 | 3 | 7 | 10 | 32 | 26 | 17 | 3 | 3 | 4 | 1 | 0 | 0 | 0 | 0 | 0 |
| Azithromycina | 105 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 4 | 10 | 29 | 38 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefaclor | 108 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 11 | 25 | 37 | 15 | 6 | 3 | 6 | 2 | 0 | 0 | 1 |
| Cefepime | 108 | 0 | 0 | 0 | 9 | 0 | 13 | 44 | 31 | 7 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefixime | 108 | 0 | 0 | 0 | 47 | 0 | 48 | 9 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefpodoxime | 108 | 0 | 0 | 0 | 12 | 0 | 12 | 48 | 19 | 11 | 5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceftriaxone | 108 | 22 | 49 | 21 | 0 | 11 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefuroxime | 108 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 13 | 22 | 42 | 11 | 9 | 4 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Ciprofloxacin | 108 | 2 | 19 | 50 | 0 | 33 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Clarithromycin | 108 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 7 | 10 | 31 | 38 | 16 | 0 | 0 | 0 | 0 |
| Erythromycin | 108 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 5 | 10 | 38 | 37 | 14 | 0 | 0 | 0 | 0 | 0 | 0 |
| Levofloxacin | 108 | 0 | 8 | 45 | 0 | 50 | 1 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moxifloxacin | 108 | 1 | 4 | 19 | 0 | 51 | 25 | 3 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
aQuality control data were out of range for some MIC batches so these isolates are not included.
UAE
Summary MIC and susceptibility data for 81 H. influenzae isolates from the UAE are shown in Table 16; the complete MIC distribution data are in Table 17. Eleven isolates (13.6%) were β-lactamase positive; there was a single (1.2%) BLNAR isolate by CLSI criteria (ampicillin MIC ≥4 mg/L) and 2 (2.5%) were BLNAR by EUCAST criteria (ampicillin MIC ≥2 mg/L). All 81 (100%) isolates were susceptible in vitro to amoxicillin/clavulanic acid by CLSI, EUCAST and PK/PD criteria; however, BLNAR are considered clinically non-susceptible to amoxicillin/clavulanic acid so susceptibility should be corrected to 98.8% by CLSI and 97.5% by EUCAST. Susceptibility to ampicillin was 87.7% by CLSI and EUCAST criteria. All isolates were susceptible to ceftriaxone by all three criteria. To cefixime, 100%, 98.8% and 100% of isolates were susceptible by CLSI, EUCAST and PK/PD criteria, respectively; to cefpodoxime, 100%, 92.6% and 100%, respectively, were susceptible. To cefepime, susceptibility was 100% and 93.8%, respectively, by CLSI and EUCAST criteria. Cefuroxime susceptibility was 100%, 17.3% and 86.4%, respectively, by CLSI, EUCAST and PK/PD breakpoints, although CLSI susceptibility should be corrected to 98.8% on account of BLNAR (EUCAST susceptibility remains unchanged). Cefaclor susceptibility was 98.8% and 21% by CLSI and PK/PD criteria. Among the quinolones tested, all isolates were susceptible to levofloxacin and moxifloxacin by CLSI and PK/PD breakpoints and 98.8% susceptible by EUCAST breakpoints; ciprofloxacin susceptibility was 93.8% by CLSI and PK/PD criteria and 92.6% by EUCAST criteria. By CLSI criteria, 98.8% of the isolates were susceptible to azithromycin and 90.1% were susceptible to clarithromycin.
Table 16.
MIC and susceptibility results for all H. influenzae isolates from the UAE
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| AMCa,b | 81 | 0.25 | 1 | ≤0.015 | 2 | 100.0 (98.8)c | 0 | 0 (1.2)c | 100.0 (100.0) | 100.0 (97.5)c | 0 | 0 (2.5)c |
| Ampicillin | 81 | 0.12 | 2 | ≤0.015 | 32 | 87.7 | 4.9 | 7.4 | NA | 87.7 | 0 | 12.3 |
| Azithromycin | 81 | 1 | 2 | 0.12 | 16 | 98.8 | 0 | 1.2 | NA | NA | NA | NA |
| Cefaclorb | 81 | 2 | 4 | ≤0.015 | 16 | 98.8 (97.5)c | 1.2 (1.2)c | 0 (1.2)c | 21.0 | NA | NA | NA |
| Cefepime | 81 | 0.12 | 0.25 | ≤0.015 | 0.5 | 100.0 | 0 | 0 | NA | 93.8 | 0 | 6.2 |
| Cefixime | 81 | 0.03 | 0.03 | ≤0.015 | 0.25 | 100.0 | 0 | 0 | 100.0 | 98.8 | 0 | 1.2 |
| Cefpodoxime | 81 | 0.06 | 0.25 | ≤0.015 | 0.5 | 100.0 | 0 | 0 | 100.0 | 92.6 | 7.4 | 0 |
| Ceftriaxone | 81 | 0.004 | 0.015 | ≤0.002 | 0.03 | 100.0 | 0 | 0 | 100.0 | 100.0 | 0 | 0 |
| Cefuroximeb | 81 | 0.5 | 2 | ≤0.015 | 4 | 100.0 (98.8)c | 0 | 0 (1.2)c | 86.4 | 17.3 (17.3)c | 69.1 (66.7)c | 13.6 (16.0)c |
| Ciprofloxacin | 81 | 0.008 | 0.5 | ≤0.002 | 4 | 93.8 | 0 | 6.2 | 93.8 | 92.6 | 0 | 7.4 |
| Clarithromycin | 81 | 8 | 16 | 0.5 | >256 | 90.1 | 8.7 | 1.2 | NA | NA | NA | NA |
| Levofloxacin | 81 | 0.008 | 0.25 | ≤0.002 | 2 | 100.0 | 0 | 0 | 100.0 | 98.8 | 0 | 1.2 |
| Moxifloxacin | 81 | 0.015 | 0.25 | ≤0.002 | 1 | 100.0 | 0 | 0 | 100.0 | 98.8 | 0 | 1.2 |
min, minimum; max, maximum; AMC, amoxicillin/clavulanic acid; S, susceptible; I, intermediate; R, resistant; NA, no breakpoint data available (NA for azithromycin and clarithromycin by EUCAST because Etest® breakpoints in CO2 not available).
aAmoxicillin/clavulanic acid PK/PD susceptibility at high dose is shown in parentheses.
bIn clinical settings, isolates of BLNAR are considered resistant to amoxicillin/clavulanic acid, cefaclor and cefuroxime (see main text).
cSusceptibility to amoxicillin/clavulanic acid, cefaclor and cefuroxime (data in parentheses) due to corrections according to BLNAR (see main text).
Table 17.
Distribution of MICs for all H. influenzae isolates from the UAE
| Isolates susceptible at MIC (mg/L) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | n | 0.002 | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >256 |
| AMC | 81 | 0 | 0 | 0 | 4 | 0 | 3 | 2 | 8 | 25 | 23 | 12 | 4 | 0 | 0 | 0 | 0 | 0 |
| Ampicillin | 81 | 0 | 0 | 0 | 3 | 0 | 2 | 13 | 25 | 17 | 10 | 1 | 4 | 2 | 2 | 1 | 1 | 0 |
| Azithromycin | 81 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 14 | 36 | 19 | 5 | 1 | 1 | 0 | 0 |
| Cefaclor | 81 | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 0 | 1 | 11 | 18 | 28 | 10 | 7 | 1 | 0 | 0 |
| Cefepime | 81 | 0 | 0 | 0 | 3 | 0 | 10 | 27 | 31 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefixime | 81 | 0 | 0 | 0 | 33 | 0 | 40 | 6 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefpodoxime | 81 | 0 | 0 | 0 | 20 | 0 | 9 | 22 | 17 | 7 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceftriaxone | 81 | 16 | 36 | 15 | 0 | 9 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cefuroxime | 81 | 0 | 0 | 0 | 3 | 0 | 5 | 0 | 6 | 19 | 21 | 16 | 10 | 1 | 0 | 0 | 0 | 0 |
| Ciprofloxacin | 81 | 4 | 18 | 37 | 0 | 6 | 3 | 2 | 0 | 0 | 5 | 1 | 4 | 1 | 0 | 0 | 0 | 0 |
| Clarithromycin | 81 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 4 | 15 | 32 | 19 | 7 | 1 |
| Levofloxacin | 81 | 3 | 21 | 29 | 0 | 11 | 4 | 3 | 1 | 2 | 3 | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| Moxifloxacin | 81 | 2 | 14 | 24 | 0 | 17 | 5 | 3 | 7 | 4 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
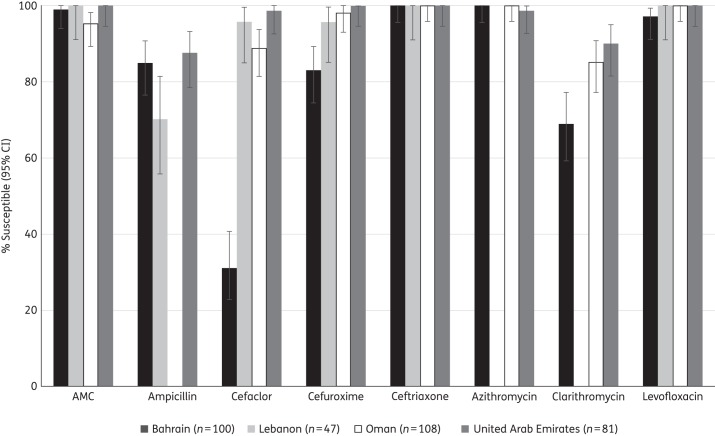
Comparative susceptibility of H. influenzae isolates by country
Figure 3 shows a comparison, by country, of the susceptibility of H. influenzae to eight antimicrobial agents, utilizing CLSI breakpoints. Ampicillin susceptibility was significantly lower (70.2%) in Lebanon than in Bahrain and the UAE (it was not tested in Oman). Isolates from Bahrain had significantly lower susceptibility rates (P < 0.05) than all the other three countries for cefaclor and cefuroxime, and susceptibility was significantly lower in Bahrain than Oman and the UAE for clarithromycin (which was not tested in Lebanon).
Figure 3.
Susceptibility of H. influenzae isolates to eight antimicrobial agents compared by country (CLSI breakpoints). Ampicillin was not tested against isolates from Oman; azithromycin and clarithromycin were not tested in Lebanon. AMC, amoxicillin/clavulanic acid.
Age group analysis
Using CLSI breakpoints, susceptibility to tested antimicrobial agents was compared across age groups in each country except for Lebanon, which submitted isolates only from paediatric patients. For isolates from Bahrain, there were no differences among age groups in the susceptibilities of any of the three pathogens.
For isolates from Oman, the susceptibility differences between isolates from paediatric and adult patients were analysed for S. pneumoniae and H. influenzae. S. pneumoniae isolates from adult patients had lower susceptibility to a number of antimicrobial agents. However, susceptibility was significantly lower than for paediatric isolates for cefpodoxime, cefuroxime and erythromycin, as well as for the other macrolides (data not shown). There were no age group differences in susceptibilities of H. influenzae isolates.
For isolates from the UAE, there were no differences among age groups in the susceptibilities of S. pneumoniae isolates. Among S. pyogenes isolates, those from paediatric patients were significantly less susceptible to erythromycin than those from adults. Among H. influenzae isolates, those from paediatric and adult patients generally had similar susceptibilities; however, ampicillin susceptibility in isolates from adults was significantly lower than in isolates from paediatric patients.
Discussion
The SOAR is an ongoing surveillance study (which began in 2002) of key community-acquired respiratory pathogens in various parts of the world. The data presented here concern the susceptibility of S. pneumoniae, S. pyogenes and H. influenzae isolated during 2011–13 in Bahrain, Lebanon, Oman and the UAE. Resistance of respiratory pathogens to antimicrobial agents is considered a worldwide problem, although susceptibility can differ widely among countries. There is relatively little surveillance and epidemiological information from Middle East countries in the published literature. However, previous SOAR susceptibility data for H. influenzae and S. pneumoniae have been presented for the UAE and Lebanon for 2004–0616,17 and further data were presented for both pathogens from the UAE in 2007–09.18
For H. influenzae, the prevalence of β-lactamase in the UAE reduced from 17.6% in 2004–0616 to 14.5% in 2007–0918 and 13.6% in the current study (2011–13). However, the apparent change in β-lactamase prevalence over this period is not statistically significant. Generally, antibiotic resistance has remained stable in this pathogen in the UAE since 2004. On the other hand antibiotic susceptibility in S. pneumoniae from the UAE has reduced significantly in most cases. In 2004–06, penicillin susceptibility was already relatively low, at 57.0% (CLSI oral breakpoint),17 and dropped to 40% in 2007–0918 and 33.3% in 2011–13 (P < 0.05). Interestingly, azithromycin susceptibility in S. pneumoniae from the UAE was also low in 2004–06, at 67%,17 but there was a significant (P < 0.05) increase to 82.3% in 2007–09.18 Azithromycin susceptibility in the UAE was also found to be low in this current study, at 47.9%, which is significantly lower not only than in 2007–09 and but also than in 2004–06 (P < 0.05). Therefore, the overall trend is for macrolide resistance, like penicillin resistance, to also be increasing in the UAE. Similarly, susceptibility to amoxicillin (with or without clavulanate) was high (>98%) in both 2004–0617 and 2007–0918 but significantly lower in 2011–2013, at 89.6% (P < 0.05). Nevertheless, amoxicillin/clavulanic acid remains one of the more potent agents against S. pneumoniae from the UAE, especially at the high PK/PD breakpoint (95.8% susceptible). The only agents that did not show reduced susceptibility in 2011–13 in the UAE were the fluoroquinolones. Using ofloxacin as a marker, fluoroquinolone susceptibility was 64% in 2004–0617 and reduced considerably to 28.6% in 2007–09,18 but levofloxacin susceptibility was 97.9% in 2011–13. This may reflect different fluoroquinolone usage in recent years. The marked difference in susceptibility in isolates from the UAE collected in 2007–09 (very high macrolide susceptibility and very low fluoroquinolone susceptibility) may be due to different circulating clones of pneumococci over that period. These data emphasize the importance of regular surveillance to track such effects. Temporal changes in antibiotic susceptibility between 2004–06 and the current study can also be compared in the Lebanon. For S. pneumoniae, penicillin susceptibility was very low (34.4%) compared with other countries in the region17 in 2004–06, but in the current study it was found to be higher, at 59.6% (a statistically significant increase). Azithromycin susceptibility was also lower in 2004–06 (64.4%) compared with 2011–13 (73.7%), but this difference is not statistically significant. As with the UAE, these differences may be due to variability in dominant clones over the two time periods. For H. influenzae, β-lactamase prevalence in 2004–06 in Lebanon was 20.4% as opposed to 14.9% in 2011–13. This difference is not statistically significant.
Overall, antibiotic resistance was a greater problem in S. pneumoniae than S. pyogenes or H. influenzae. Antibiotic susceptibility was generally lower in S. pneumoniae from the UAE than other countries, followed by Oman then Lebanon and Bahrain. All countries had low oral penicillin susceptibility in S. pneumoniae (33.3%–60%) and those pneumococci resistant to penicillin were also less susceptible to other antibiotics, apart from fluoroquinolones. However, using CLSI iv breakpoints, penicillin susceptibility was around 90% or higher in all countries, which was also the case for amoxicillin/clavulanic acid, amoxicillin and ceftriaxone. Almost full susceptibility was observed for fluoroquinolones in all countries.
Despite the small number of isolates collected from children, an analysis of susceptibility by age group for each country was also made (except Lebanon, where all isolates were from children). These results were mixed and varied by country. For example, in Bahrain there was no age group difference, but in Oman isolates from adults tended to have lower antibiotic susceptibility than isolates from children. A global demographic study found significantly higher penicillin and erythromycin resistance in infants compared with both adults and the elderly19 and SOAR data from Turkey also found this to be true for penicillin but not macrolides.20 SOAR data from Vietnam demonstrated that paediatric isolates were less susceptible to most antibiotics than isolates from the elderly, but there was no difference compared with adults (except for azithromycin).21 It would appear that these age differences, like susceptibility in general, are very country specific and may relate to other factors, such as specimen source.
Antibiotic susceptibility in H. influenzae was generally high, although ampicillin susceptibility was seen in only 70.2% of isolates from Lebanon but >85% elsewhere. This was due to β-lactamase production. The lower susceptibility in Lebanon also reflected a high proportion of BLNAR strains (8.5% by CLSI and 14.9% by EUCAST). BLNAR were rare elsewhere in the region. Susceptibility to amoxicillin/clavulanic acid was >95% in Bahrain, Oman and UAE and >90% in Lebanon in H. influenzae using CLSI breakpoints. Azithromycin susceptibility was also high, being virtually 100% in all countries tested, with clarithromycin susceptibility low in the UAE (90%) and even lower in Bahrain (69%). There was little difference in susceptibility of H. influenzae associated with age group in these four Gulf and Near East countries, as has also been found in Turkey and Vietnam,20,21 which is probably due to low overall resistance.
All S. pyogenes isolates were penicillin susceptible, although there was evidence of reduced erythromycin susceptibility, especially in Oman (68.4%). S. pyogenes were generally highly susceptible to the other antibiotics, except for levofloxacin in Bahrain (80% susceptible), despite the fact that S. pyogenes from other countries were 100% susceptible. No age group analysis was performed for isolates from Lebanon (all paediatric isolates) or Oman (low isolate numbers). No significant difference in susceptibility was found by age group for isolates from Bahrain, but paediatric isolates from the UAE were significantly less susceptible to erythromycin than those from adults. As with S. pneumoniae, age group-related susceptibility is country specific.
In this analysis we measured susceptibility using three breakpoint criteria. In general these produced similar results. However, there were quite notable exceptions. The first of these was considerably lower cefaclor susceptibility by EUCAST compared with CLSI with S. pneumoniae. To a lesser extent this was also the case for cefuroxime. Similarly, in H. influenzae cefuroxime susceptibility was considerably lower with EUCAST than with CLSI. This has been noted elsewhere22 and in other SOAR publications.20,21 As has been stated previously, considerable harmonization of breakpoints is necessary to avoid confusion and potentially poor therapeutic decisions.
The data from this study confirm that isolates of S. pneumoniae susceptible to penicillin G are also susceptible to other penicillins as inferred by CLSI and EUCAST guidelines and cephalosporins as inferred by CLSI guidelines. Interestingly, the data from this study found the reverse was not always correct using CLSI breakpoints; i.e. most penicillin-non-susceptible S. pneumoniae were susceptible to amoxicillin/clavulanic acid, amoxicillin and cephalosporins. This warrants further investigation.
In summary, we have analysed antibiotic susceptibility in community-acquired respiratory pathogens. There are large country-specific differences in antibiotic susceptibility even within the same region, with overall antibiotic resistance being the highest in S. pneumoniae and isolates from the UAE. These data reinforce the need for regular antibiotic resistance surveillance to track changes in susceptibility over time.
Funding
This study was funded by GlaxoSmithKline.
Transparency declarations
This article forms part of a Supplement sponsored by GlaxoSmithKline. D. Torumkuney is an employee of GlaxoSmithKline and also holds shares in GlaxoSmithKline. I. Morrissey is an employee of IHMA, a medical communication and consultancy company, who participated in the exploration, interpretation of the results and preparation of this manuscript on behalf of GSK. IHMA also provided medical writing support in the form of writing assistance, collating author's comments, grammatical editing and referencing that was paid for by GSK. All other authors declare that they have no conflict of interest.
Editorial assistance was provided by Tracey Morris, Livewire Editorial Communications.
Acknowledgements
We would like to thank Dr Keith Barker (GSK) for reviewing the manuscript; Dr Mansour Al-Zarouni (UAE), Dr Dolla Sarkis (Lebanon) and Mrs Shahanaz Al-Khawaja (Bahrain) for collecting isolates; and Runa Mithani (GSK) for helping to manage the study in Gulf and Near East countries.
References
- 1.World Health Organization. World Health Statistics 2014. http://www.who.int/gho/publications/world_health_statistics/EN_WHS2014_Part3.pdf?ua=1.
- 2.Torres A, Blasi F, Peetermans WE et al. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: a literature review. Eur J Clin Microbiol Infect Dis 2014; 33: 1065–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Musawi M. A retrospective epidemiological study of invasive pneumococcal infections in children aged 0–5 years in Bahrain from 1 January 1999 to 31 December 2003. Vaccine 2012; 30 Suppl 6: G2–6. [DOI] [PubMed] [Google Scholar]
- 4.Riedel S, Beekmann SE, Heilmann KP et al. Antimicrobial use in Europe and antimicrobial resistance in Streptococcus pneumoniae. Eur J Clin Microbiol Infect Dis 2007; 26: 485–90. [DOI] [PubMed] [Google Scholar]
- 5.Goossens H, Ferech M, Vander Stichele R et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005; 365: 579–87. [DOI] [PubMed] [Google Scholar]
- 6.Bronzwaer SL, Cars O, Bucholz U et al. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis 2002; 8: 278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67: 71–9. [DOI] [PubMed] [Google Scholar]
- 8.Tleyjeh IM, Tlaygeh HM, Hejal R et al. The impact of penicillin resistance on short-term mortality in hospitalized adults with pneumococcal pneumonia: a systematic review and meta-analysis. Clin Infect Dis 2006; 42: 778–97. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs MR, Johnson CE. Macrolide resistance: an increasing concern for treatment failure in children. Pediatr Infect Dis 2003; 22 Suppl 8: S131–8. [DOI] [PubMed] [Google Scholar]
- 10.Al-Muhairi S, Zoubeidi T, Ellis M et al. Demographics and microbiological profile of pneumonia in United Arab Emirates. Monaldi Arch Chest Dis 2006; 65: 13–8. [DOI] [PubMed] [Google Scholar]
- 11.Hanna-Wakim R, Chehab H, Mahfouz I et al. Epidemiologic characteristics, serotypes, and antimicrobial susceptibilities of invasive Streptococcus pneumoniae isolates in a nationwide surveillance study in Lebanon. Vaccine 2012; 30 Suppl 6: G11–7. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests—Twelfth Edition: Approved Standard M02-A12. CLSI, Wayne, PA, USA, 2015. [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement M100-S25. CLSI, Wayne, PA, USA, 2015. [Google Scholar]
- 14.The European Committee on Antimicrobial Susceptibility testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 5.0, 2014. http://www.eucast.org.
- 15.Anon JB, Jacobs MR, Poole MD et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg 2004; 130 Suppl 1: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sievers J, SOAR Study Group. Antibacterial resistance among Haemophilus influenzae from 8 countries in Africa and the Middle East: results from the Survey of Antibiotic Resistance (SOAR) 2004–2006. In: Abstracts of the Eighth European Congress of Chemotherapy and Infection, Budapest, Hungary, 2006. Abstract 279. [Google Scholar]
- 17.Sievers J, SOAR Study Group. Antibacterial resistance among Streptococcus pneumoniae from 8 countries in Africa and the Middle East: results from the Survey of Antibiotic Resistance (SOAR) 2004–2006. In: Abstracts of the Forty-sixth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, 2006. Abstract C2-0425. American Society for Microbiology, Washington, DC, USA. [Google Scholar]
- 18.Torumkuney D, O'Brien D, SOAR Study group. Antibacterial resistance among Streptococcus pneumoniae, Haemophilus influenzae and Streptococcus pyogenes from 9 countries in Africa and the Middle East: results from the Survey of Antibiotic Resistance (SOAR) 2007–2009. In: Abstracts of the Forty-ninth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, 2009. Abstract C2-1402. American Society for Microbiology, Washington, DC, USA. [Google Scholar]
- 19.Hoban D, Baquero F et al. Demographic analysis of antimicrobial resistance among Streptococcus pneumoniae: worldwide results from PROTEKT 1999-2000. Int J Infect Dis 2005; 9: 262–73. [DOI] [PubMed] [Google Scholar]
- 20.Soyletir G, Altinkanat G, Gur D et al. Results from the Survey of Antibiotic Resistance (SOAR) 2011–13 in Turkey. J Antimicrob Chemother 2016; 71 Suppl 1: i71–i83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van PH, Binh PT, Minh NHL et al. Results from the Survey of Antibiotic Resistance (SOAR) 2009–11 in Vietnam. J Antimicrob Chemother 2016; 71 Suppl 1: i93–i102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchese A, Esposito S, Barbieri R et al. Does the adoption of EUCAST susceptibility breakpoints affect the selection of antimicrobials to treat acute community-acquired respiratory tract infections? BMC Infect Dis 2012; 12: 181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]