Abstract
Objectives
To compare antibiotic susceptibility of community-acquired respiratory bacteria in China during 2009–11 and 2013–14.
Methods
Susceptibility was determined by Etest® (bioMérieux) or disc diffusion according to CLSI, EUCAST and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints, except for azithromycin where Etest® breakpoints (in CO2 incubation) were used in place of standard CLSI breakpoints. Statistical significance of differences in susceptibility across time periods was evaluated using Fisher's exact test.
Results
During 2009–11, 434 Streptococcus pneumoniae, 307 Haemophilus influenzae and 140 Moraxella catarrhalis were collected from eight centres and during 2013–14, 208 S. pneumoniae, 185 H. influenzae and 80 M. catarrhalis were collected from five centres. Penicillin-non-susceptible isolates remained stable at ∼66% over both time periods but susceptibility decreased significantly for amoxicillin/clavulanic acid (or amoxicillin) and cefaclor. For H. influenzae, the proportion of β-lactamase-positive isolates and β-lactamase-negative ampicillin-resistant strains (CLSI definition) was higher in 2013–14 (25.4% and 7.0%, respectively) than in 2009–11 (16.3% and 3.6%, respectively), with decreased ampicillin and cephalosporin susceptibility. By 2009–11 and 2013–14, only amoxicillin/clavulanic acid (amoxicillin), levofloxacin, penicillin (intravenously) and chloramphenicol inhibited >70% of S. pneumoniae. During 2013–14, M. catarrhalis showed increasing resistance, with cefaclor and levofloxacin susceptibility decreasing significantly. However, amoxicillin/clavulanic acid, cefuroxime and levofloxacin continued to inhibit >90% of isolates.
Conclusions
On the whole, antimicrobial susceptibility decreased in China between 2009–11 and 2013–14. In 2013–14, amoxicillin/clavulanic acid, levofloxacin and chloramphenicol were the most active antibacterial agents tested against community-acquired respiratory pathogens when assessed by CLSI, EUCAST or PK/PD breakpoints. Resistance to other antibacterials in China was generally high. Our data demonstrate the need to harmonize breakpoints for these pathogens.
Introduction
Community-acquired pneumonia (CAP) and other lower respiratory tract infections are the second most frequent cause of all-age premature deaths.1 Streptococcus pneumoniae and Haemophilus influenzae are the most common bacterial species associated with CAP. A smaller percentage of Moraxella catarrhalis isolates are recovered from patients with this disease. These pathogens are recovered from patients of all age groups but remain a serious threat to young children, the elderly and those with an immunocompromised status. Managing patients with CAP requires risk-scoring systems and reliable and effective empirical therapy due to the rapid progression of this disease.2,3 While advances have been made in determining patients at highest risk of developing progressive disease, antimicrobial resistance among common CAP pathogens has been increasing. Empirical treatment options include β-lactams, macrolides and fluoroquinolones. However, resistance to each of these agents is now common, or increasing, among CAP pathogens collected in most areas of the world and treatment failures have been reported using standard-of-care therapies.3–7
Resistance surveillance programmes are important in that detection of regional variations can be observed, giving care providers knowledge of bacterial resistance rates in their environments. One such study is the ongoing Survey of Antibiotic Resistance (SOAR), which is an antimicrobial resistance surveillance study of key respiratory pathogens conducted in the Middle East, Africa, Latin America, Asia-Pacific and Commonwealth of Independent States countries and has been running since 2002. Here, we report recent data from SOAR for three major respiratory tract pathogens collected from hospital sites in China.
Materials and methods
Collaborating centres
The following eight centres took part in the study in 2009–11: Beijing Hospital, Zhejiang Provincial College of Medicine, Jiangxi Provincial People's Hospital, Peking Union Medical College Hospital, Shanghai Children's Hospital, Shanghai Huashan Hospital, Shanghai Ruijin Hospital and Tongji Hospital. Five centres participated in 2013–14: Beijing Hospital, Jiangxi Provincial People's Hospital, Peking Union Medical College Hospital, Shanghai Children's Hospital and Shanghai Huashan Hospital.
Clinical isolates
During 2009–11, a total of 434 isolates of S. pneumoniae, 307 isolates of H. influenzae and 140 isolates of M. catarrhalis were collected and during 2013–14, a total of 208 isolates of S. pneumoniae, 185 isolates of H. influenzae and 80 isolates of M. catarrhalis were collected (all isolates were from community-acquired infections and provided by a university hospital). In 2009–11, paediatric patients (≤12 years old) accounted for 240 isolates (27.2%), adult patients (13–64 years old) for 405 (46.0%) and the elderly (≥65 years) for 227 isolates (25.8%). A small number (nine isolates, 1.0%) were from patients whose age was not provided. In 2013–14, the collection comprised 248 isolates from paediatric patients (52.4%), 136 from adult patients (28.8%) and 89 isolates from the elderly (18.8%). Pathogens were obtained from a variety of specimen types including sputum, bronchoalveolar lavage, bronchial brush and blood. Organisms were identified using conventional methods (optochin susceptibility/bile solubility for S. pneumoniae and X and V factor requirement for H. influenzae). Duplicate isolates from the same patient were not accepted.
Susceptibility testing
MICs were determined using the Etest® susceptibility method according to the manufacturer's instructions (bioMérieux, Marcy l'Etoile, France). Disc diffusion susceptibility testing was carried out according to CLSI methodology.8 Study drugs for H. influenzae evaluated by Etest® included amoxicillin/clavulanic acid, ampicillin, ampicillin/sulbactam, azithromycin, cefaclor, cefuroxime and levofloxacin. Trimethoprim/sulfamethoxazole, tetracycline and chloramphenicol were evaluated by disc diffusion. Study drugs for S. pneumoniae evaluated by Etest® included penicillin, amoxicillin/clavulanic acid, cefaclor, cefuroxime and levofloxacin. Erythromycin, clindamycin, trimethoprim/sulfamethoxazole, tetracycline and chloramphenicol were evaluated by disc diffusion. For M. catarrhalis, study drugs included amoxicillin/clavulanic acid, azithromycin, cefaclor, cefuroxime and levofloxacin and all were evaluated by Etest®. β-Lactamase production was determined for each H. influenzae and M. catarrhalis isolate using chromogenic cephalosporin (nitrocefin) discs according to the manufacturer's instructions (BD Diagnostics, Sparks, MD, USA) using E. coli ATCC 35218 and H. influenzae ATCC 49247 as the positive and negative controls, respectively. Quality control strains S. pneumoniae ATCC 49619, H. influenzae ATCC 49247, H. influenzae ATCC 49766, Escherichia coli ATCC 25922 and E. coli ATCC 32518 were included on each day of testing. Results of susceptibility testing were accepted if the results of the control strains were within published limits. Susceptibility to the study drugs was calculated based on CLSI breakpoints,9,10 except for azithromycin where bioMérieux Etest® breakpoints for incubation in CO2 were used. In addition, susceptibility based on EUCAST and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints was analysed where applicable11,12 to assess if adoption of these breakpoints would affect susceptibility. EUCAST and PK/PD breakpoints were not evaluated for azithromycin because, unlike CLSI, these are not adjusted for incubation in CO2 by Etest®. The MIC breakpoints used are shown in Table 1 and the disc diffusion zone diameter breakpoints are shown in Table 2.
Table 1.
MIC breakpoints (mg/L) used for S. pneumoniae, H. influenzae and M. catarrhalis isolates
|
S. pneumoniae |
H. influenzae |
M. catarrhalis |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLSI |
EUCAST |
CLSI |
EUCAST |
CLSI |
EUCAST |
PK/PD |
|||||||||||||
| Antimicrobial agent | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | (S only) |
| AMCa | ≤2 | 4 | ≥8 | NA | NA | NA | ≤4 | — | ≥8 | ≤2 | — | ≥4 | ≤4 | — | ≥8 | ≤1 | — | ≥2 | ≤2 (≤4) |
| Ampicillin | NT | NT | NT | NT | NT | NT | ≤1 | 2 | ≥4 | ≤1 | — | ≥2 | NT | NT | NT | NT | NT | NT | NA |
| Ampicillin/sulbactam | NT | NT | NT | NT | NT | NT | ≤2 | — | ≥4 | ≤1 | — | ≥2 | NT | NT | NT | NT | NT | NT | NA |
| Azithromycinb | NT | NT | NT | NT | NT | NT | ≤8 | — | — | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Cefaclor | ≤1 | 2 | ≥4 | ≤0.03 | 0.06–0.5 | ≥1 | ≤8 | 16 | ≥32 | NA | NA | NA | ≤8 | 16 | ≥32 | NA | NA | NA | ≤0.5 |
| Cefuroximec | ≤1 | 2 | ≥4 | ≤0.25 | 0.5 | ≥1 | ≤4 | 8 | ≥16 | ≤0.12 | 0.25–1 | ≥2 | ≤4 | 8 | ≥16 | ≤0.12 | 0.25–4 | ≥8 | ≤1 |
| Levofloxacin | ≤2 | 4 | ≥8 | ≤2 | — | ≥4 | ≤2 | — | — | ≤1 | — | ≥2 | ≤2 | — | — | ≤1 | — | ≥2 | ≤2 |
| Penicillin (oral) | ≤0.06 | 0.12–1 | ≥2 | ≤0.06 | 0.12–2 | ≥4 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NA |
| Penicillin (iv)d | ≤2 | 4 | ≥8 | e | NA | NA | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NA |
AMC, amoxicillin/clavulanic acid; S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, not applicable.
aAmoxicillin/clavulanic acid was tested at a 2 : 1 amoxicillin/clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoint based on high dose (4 g of amoxicillin with 250 mg of clavulanic acid per day for adults) is shown in parentheses.4
bbioMérieux Etest® breakpoints for incubation in CO2.
cBreakpoints used are for cefuroxime axetil.
dParenteral non-meningitis breakpoints.
eEUCAST give iv susceptible breakpoints for pneumonia based on three doses: 1.2 g × 4 (MIC ≤0.5 mg/L = susceptible), 1.2 g × 6 or 2.4 g × 4 (MIC ≤1 mg/L = susceptible) and 2.4 g × 6 (MIC ≤2 mg/L = susceptible).
Table 2.
Zone diameter breakpoints (mm) used for S. pneumoniae and H. influenzae isolates
|
S. pneumoniae |
H. influenzae |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLSI |
EUCAST |
CLSI |
EUCAST |
|||||||||
| Antimicrobial agent | R | I | S | R | I | S | R | I | S | R | I | S |
| Chloramphenicol | ≤20 | — | ≥21 | ≤20 | — | ≥21 | ≤25 | 26–28 | ≥29 | ≤27 | — | ≥28 |
| Clindamycin | ≤15 | 16–18 | ≥19 | ≤18 | — | ≥19 | NT | NT | NT | NT | NT | NT |
| Erythromycin | ≤15 | 16–20 | ≥21 | ≤18 | 19–21 | ≥22 | NT | NT | NT | NT | NT | NT |
| Tetracycline | ≤24 | 25–27 | ≥28 | ≤21 | 22–24 | ≥25 | ≤25 | 26–28 | ≥29 | ≤21 | 22–24 | ≥25 |
| SXT | ≤15 | 16–18 | ≥19 | ≤14 | 15–17 | ≥18 | ≤10 | 11–15 | ≥16 | ≤19 | 20–22 | ≥23 |
SXT, trimethoprim/sulfamethoxazole; NT, not tested.
Statistical analysis
Differences in susceptibility between time periods were assessed with Fisher's exact test, using XLSTAT version 2015.2.02. A P value <0.05 was considered statistically significant. For this analysis only, the five sites that participated in both time periods were included.
Results
S. pneumoniae
A total of 434 S. pneumoniae were collected in China from 2009 to 2011. Nearly all of the pneumococci were collected from sputum cultures (n = 421, 97.0%). Comparing age groups, 139 isolates (32.0%) were collected from paediatric patients (aged ≤12 years old), 107 (24.7%) were from elderly patients (aged ≥65 years old) and 181 (41.7%) were from adults (aged 13–64 years old). Seven isolates were collected from patients whose age was unknown. In 2013–14, 208 S. pneumoniae isolates were collected, with 188 (90.4%) isolates from sputum, 12 (5.8%) isolates from blood and 8 (3.8%) from bronchoalveolar lavage. Paediatric patients accounted for 86 (41.3%) isolates, adult patients for 73 (35.1%) isolates and the elderly for 49 (23.6%) isolates. Summary MIC and susceptibility data for all S. pneumoniae are shown in Table 3. MIC distribution data are given in Table 4.
Table 3.
MIC and susceptibility data for S. pneumoniae isolates collected during 2009–11 and 2013–14
| Years/antimicrobial agent | N | Susceptibility |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| 50% | 90% | min. | max. | %S | %I | %R | %S | %S | %I | %R | ||
| 2009–11 | ||||||||||||
| AMCa,b | 434 | 1 | 4 | ≤0.015 | 128 | 89.2 | 6.0 | 4.8 | 89.2 (95.2) | NA | NA | NA |
| cefaclor | 434 | 16 | >256 | 0.12 | >256 | 40.3 | 2.1 | 57.6 | 35.3 | 0.0 | 35.3 | 64.7 |
| cefuroximec | 434 | 2 | 16 | ≤0.015 | >256 | 44.2 | 9.9 | 45.9 | 44.2 | 41.2 | 3.7 | 58.8 |
| levofloxacin | 434 | 1 | 1 | 0.12 | >32 | 97.5 | 0.2 | 2.3 | 97.5 | 97.5 | — | 2.5 |
| penicillin (oral) | 434 | 1 | 2 | ≤0.015 | 32 | 36.2 | 35.5 | 28.3 | NA | 36.2 | 53.9 | 9.9 |
| penicillin (iv) | 434 | 1 | 2 | ≤0.015 | 32 | 90.1 | 6.5 | 3.4 | NA | 49.1–90.1 | NA | NA |
| chloramphenicold | 434 | NA | NA | NA | NA | 85.9 | 0.0 | 14.1 | NA | 85.9 | 0.0 | 14.1 |
| clindamycind | 434 | NA | NA | NA | NA | 9.2 | 0.5 | 90.3 | NA | 9.2 | 0.0 | 90.8 |
| erythromycind | 434 | NA | NA | NA | NA | 7.8 | 0.7 | 91.5 | NA | 7.8 | 0.2 | 91.9 |
| tetracyclined | 434 | NA | NA | NA | NA | 6.0 | 3.2 | 90.8 | NA | 9.2 | 4.2 | 86.6 |
| SXTd | 434 | NA | NA | NA | NA | 24.2 | 5.3 | 70.5 | NA | 27.0 | 3.0 | 70.1 |
| 2013–14 | ||||||||||||
| AMCa,b | 208 | 1 | 8 | ≤0.015 | 64 | 75.0 | 13.5 | 11.5 | 75.0 (88.5) | NA | NA | NA |
| cefaclor | 208 | >256 | >256 | 0.25 | >256 | 22.6 | 13.0 | 64.4 | 7.7 | 0.0 | 7.7 | 92.3 |
| cefuroximec | 208 | 2 | 32 | ≤0.015 | >256 | 43.3 | 10.5 | 46.2 | 43.3 | 36.5 | 4.8 | 58.7 |
| levofloxacin | 208 | 1 | 2 | 0.25 | >32 | 99.0 | 0.0 | 1.0 | 99.0 | 99.0 | — | 1.0 |
| penicillin (oral) | 208 | 1 | 4 | ≤0.015 | 32 | 32.2 | 33.2 | 34.6 | NA | 32.2 | 51.5 | 16.3 |
| penicillin (iv) | 208 | 1 | 4 | ≤0.015 | 32 | 83.7 | 13.4 | 2.9 | NA | 49.5–83.7 | NA | NA |
| chloramphenicold | 208 | NA | NA | NA | NA | 90.9 | 0.0 | 9.1 | NA | 90.9 | 0.0 | 9.1 |
| clindamycind | 208 | NA | NA | NA | NA | 6.2 | 0.5 | 93.3 | NA | 6.2 | 0.0 | 93.8 |
| erythromycind | 208 | NA | NA | NA | NA | 4.3 | 1.9 | 93.8 | NA | 4.3 | 1.0 | 94.7 |
| tetracyclined | 208 | NA | NA | NA | NA | 0.0 | 0.0 | 100 | NA | 0.0 | 6.2 | 93.8 |
| SXTd | 208 | NA | NA | NA | NA | 26.4 | 4.3 | 69.2 | NA | 28.4 | 2.4 | 69.2 |
AMC, amoxicillin/clavulanic acid; NA, no breakpoint data available; SXT, trimethoprim/sulfamethoxazole.
aAMC, amoxicillin/clavulanic acid PK/PD susceptibility at high dose is shown in parentheses.
bSusceptibility for amoxicillin alone can be inferred from amoxicillin/clavulanic acid.
cBreakpoints used are for cefuroxime axetil.
dCLSI disc diffusion testing method used.
Table 4.
MIC distribution data for S. pneumoniae isolates collected during 2009–11 and 2013–14
| Years/antimicrobial agent | N | Number of isolates at MIC (mg/L) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ≥256 | ||
| 2009–11 | ||||||||||||||||
| AMC | 434 | 111 | 37 | 13 | 8 | 15 | 22 | 92 | 89 | 26 | 12 | 7 | 0 | 0 | 2 | 0 |
| cefaclor | 434 | 0 | 0 | 0 | 1 | 15 | 137 | 22 | 9 | 11 | 8 | 18 | 62 | 66 | 24 | 61 |
| cefuroxime | 434 | 21 | 36 | 37 | 32 | 37 | 16 | 13 | 43 | 78 | 57 | 32 | 16 | 11 | 0 | 5 |
| levofloxacin | 434 | 0 | 0 | 0 | 1 | 4 | 97 | 297 | 24 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| penicillin | 434 | 87 | 50 | 20 | 17 | 12 | 27 | 98 | 80 | 28 | 10 | 4 | 1 | 0 | 0 | 0 |
| 2013–14 | ||||||||||||||||
| AMC | 208 | 31 | 30 | 12 | 3 | 11 | 9 | 33 | 27 | 28 | 16 | 6 | 1 | 1 | 0 | 0 |
| cefaclor | 208 | 0 | 0 | 0 | 0 | 1 | 15 | 31 | 27 | 5 | 3 | 3 | 4 | 2 | 3 | 114 |
| cefuroxime | 208 | 11 | 30 | 11 | 8 | 16 | 10 | 4 | 22 | 30 | 22 | 15 | 15 | 12 | 0 | 2 |
| levofloxacin | 208 | 0 | 0 | 0 | 0 | 3 | 62 | 121 | 20 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| penicillin | 208 | 31 | 22 | 14 | 9 | 8 | 19 | 33 | 38 | 28 | 4 | 1 | 1 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
In 2009–11, using CLSI oral or EUCAST breakpoints (these being identical), only 36.2% of the pneumococci were penicillin susceptible. However, due to differing intermediate and resistant breakpoints, 35.5% were penicillin intermediate and 28.3% penicillin resistant by CLSI oral breakpoints and 53.9% penicillin intermediate and 9.9% penicillin resistant by EUCAST. Using the CLSI intravenous (iv) (non-meningitis) breakpoints, 90.1% of isolates were susceptible to penicillin. These susceptibility level results decreased slightly in 2013–14, with 32.2% of isolates susceptible using the CLSI oral/EUCAST breakpoints and 83.7% susceptible at the CLSI iv breakpoint (Table 3).
Using CLSI or low-dose PK/PD breakpoints, susceptibility to amoxicillin/clavulanic acid (and by inference amoxicillin) was 89.2% but using the high-dose PK/PD breakpoint, 95.2% of pneumococci were susceptible in 2009–11. Apart from high levofloxacin susceptibility (97.5% by all breakpoints) and high chloramphenicol susceptibility (85.9% by CLSI/EUCAST criteria), the other tested antimicrobials exhibited low activity (≤44%) against pneumococci. In 2013–14, amoxicillin/clavulanic acid (amoxicillin) susceptibility decreased to 75.0% by CLSI and low-dose PK/PD breakpoints and to 88.5% using the high-dose PK/PD breakpoint. Levofloxacin and chloramphenicol susceptibility was more stable and actually increased slightly to 99.0% and 90.9% susceptible, respectively. As in 2009–11, susceptibility levels for all other antimicrobials were low (<44%) in 2013–14 (Table 3).
CLSI guidelines indicate that isolates susceptible to penicillin G (MIC ≤0.06 mg/L) can be reported as susceptible to amoxicillin/clavulanic acid, ceftriaxone, cefaclor, cefepime, cefpodoxime and cefuroxime. Data from this study (both periods combined) confirmed this, as in most cases penicillin-susceptible S. pneumoniae (n = 224) were also susceptible to the β-lactams listed above. The exception was with cefaclor, where 20 penicillin-susceptible isolates were intermediate to cefaclor and 4 were cefaclor resistant. However, the reverse was not always found. Of the 418 penicillin-non-susceptible isolates, 319 (76.3%) were amoxicillin/clavulanic acid susceptible and 58 (13.9%) were cefuroxime susceptible. Only a small number of penicillin-non-susceptible isolates (n = 22, 5.3%) were cefaclor susceptible. A similar ‘expert rule’ is provided by EUCAST but for penicillins only, i.e. amoxicillin/clavulanic acid (amoxicillin) in this study. However, unlike by CLSI, individual breakpoints are not provided by EUCAST for amoxicillin/clavulanic acid to make this comparison.
H. influenzae
A total of 307 H. influenzae were collected in China from 2009 to 2011. These isolates were obtained mostly from patient sputum cultures (n = 294, 95.8%). Isolates of H. influenzae came from paediatric patients (n = 72, 23.5%), the elderly (n = 84, 27.4%) and adults (n = 151, 49.2%). In 2013–14, 185 H. influenzae isolates were collected; all but 3 isolates were from sputum (98.4%). The majority of isolates (n = 113, 61.1%) were from paediatric patients, 47 (25.4%) came from adults and 25 (13.5%) from the elderly.
In 2009–11, 16.3% (50/307) were β-lactamase positive and 83.7% (257/307) were β-lactamase negative, of which 11 (3.6% of all H. influenzae) were β-lactamase negative, ampicillin resistant (BLNAR) by the CLSI definition (ampicillin MIC ≥4 mg/L) and 40 (13.0% of all H. influenzae) by the EUCAST definition (ampicillin MIC ≥2 mg/L). In 2013–14, the percentage of β-lactamase-positive isolates was higher at 25.4% (47/185) and by CLSI and EUCAST definitions 13 (7.0%) and 31 (16.8%) BLNAR isolates were found, respectively. For analysis, the BLNAR were included within the β-lactamase-negative population.
Summary MIC and susceptibility data and MIC distributions for all H. influenzae isolates are shown in Tables 5 and 6, respectively.
Table 5.
MIC and susceptibility data for H. influenzae isolates collected during 2009–11 and 2013–14
| Years/antimicrobial agent | Isolate group | N | Susceptibility |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
||||||||||
| 50% | 90% | min. | max. | %S | %I | %R | %S | %S | %I | %R | |||
| 2009–11 | |||||||||||||
| AMCa,b | all | 307 | 1 | 2 | 0.06 | 512 | 98.4 (97.4)c | — | 1.6 (2.6)c | 93.5 (98.4) | 93.5 (85.0)c | — | 6.5 (15.0)c |
| BL− | 257 | 0.5 | 2 | 0.06 | 8 | 98.8 | — | 1.2 | 94.2 (98.8) | 94.2 | — | 5.8 | |
| BL+ | 50 | 1 | 2 | 0.12 | 512 | 96.0 | — | 4.0 | 90.0 (96.0) | 90.0 | — | 10.0 | |
| ampicillind | all | 307 | 0.5 | 64 | 0.06 | >256 | 71.3 | 9.5 | 19.2 | NA | 71.3 | — | 28.7 |
| BL− | 257 | 0.5 | 2 | 0.06 | >256 | 84.4 | 11.3 | 4.3 | NA | 84.4 | — | 15.6 | |
| BL+ | 50 | 64 | >256 | 0.5 | >256 | 4.0 | 0.0 | 96.0 | NA | 4.0 | — | 96.0 | |
| azithromycine | all | 104 | 2 | 4 | ≤0.015 | >256 | 91.4 | — | — | NA | NA | NA | NA |
| BL− | 85 | 4 | 4 | ≤0.015 | 8 | 92.9 | — | — | NA | NA | NA | NA | |
| BL+ | 19 | 2 | >256 | ≤0.015 | >256 | 84.2 | — | — | NA | NA | NA | NA | |
| cefaclorb | all | 307 | 2 | 32 | 0.25 | >256 | 78.5 (77.5)c | 7.8 (7.2)c | 13.7 (15.3)c | 2.0 | NA | NA | NA |
| BL− | 257 | 2 | 32 | 0.25 | >256 | 79.4 | 7.8 | 12.8 | 2.3 | NA | NA | NA | |
| BL+ | 50 | 4 | 32 | 1 | >256 | 74.0 | 8.0 | 18.0 | 0.0 | NA | NA | NA | |
| cefuroximeb,f | all | 307 | 1 | 4 | 0.06 | 64 | 94.8 (92.8)c | 2.9 (2.0)c | 2.3 (5.2)c | 65.5 | 1.0 (1.0)c | 64.5 (62.9)c | 34.5 (36.2)c |
| BL− | 257 | 1 | 4 | 0.06 | 64 | 94.6 | 3.1 | 2.3 | 66.9 | 1.2 | 65.8 | 33.1 | |
| BL+ | 50 | 1 | 4 | 0.25 | 32 | 96.0 | 2.0 | 2.0 | 58.0 | 0.0 | 58.0 | 42.0 | |
| levofloxacin | all | 307 | 0.06 | 4 | 0.008 | >32 | 78.2 | 78.2 | 71.7 | — | 28.3 | ||
| BL− | 257 | 0.06 | 4 | 0.008 | >32 | 79.8 | — | 20.2 | 79.8 | 72.4 | — | 27.6 | |
| BL+ | 50 | 0.03 | 8 | 0.008 | >32 | 70.0 | 0 | 30 | 70.0 | 68.0 | — | 32.0 | |
| chloramphenicolg | all | 307 | NA | NA | NA | NA | 90.6 | 1.3 | 8.1 | NA | 91.5 | — | 8.5 |
| BL− | 257 | NA | NA | NA | NA | 96.1 | 1.2 | 2.7 | NA | 96.9 | — | 3.1 | |
| BL+ | 50 | NA | NA | NA | NA | 62.0 | 2.0 | 36.0 | NA | 64.0 | — | 36.0 | |
| tetracyclineg | all | 292 | NA | NA | NA | NA | 74.3 | 9.9 | 15.8 | NA | 85.3 | 2.7 | 12.0 |
| BL− | 242 | NA | NA | NA | NA | 79.8 | 10.3 | 9.9 | NA | 90.9 | 2.5 | 6.6 | |
| BL+ | 50 | NA | NA | NA | NA | 48.0 | 8.0 | 44.0 | NA | 58.0 | 4.0 | 38.0 | |
| SXTg | all | 307 | NA | NA | NA | NA | 41.0 | 2.6 | 56.4 | NA | 34.2 | 4.6 | 61.2 |
| BL− | 257 | NA | NA | NA | NA | 46.3 | 3.1 | 50.6 | NA | 38.5 | 5.1 | 56.4 | |
| BL+ | 50 | NA | NA | NA | NA | 14.0 | 0.0 | 86.0 | NA | 12.0 | 2.0 | 86.0 | |
| 2013–14 | |||||||||||||
| AMCa,b | all | 185 | 1 | 4 | 0.12 | 16 | 95.7 (89.1)c | — | 4.3 (10.9)c | 78.4 (95.7) | 78.4 (73.0)c | — | 21.6 (27.0)c |
| BL− | 138 | 1 | 2 | 0.12 | 8 | 97.8 | — | 2.2 | 81.9 (97.8) | 81.9 | — | 18.1 | |
| BL+ | 47 | 2 | 8 | 0.5 | 16 | 89.4 | — | 10.6 | 68.1 (89.4) | 68.1 | — | 31.9 | |
| ampicillind | all | 185 | 1 | >256 | 0.12 | >256 | 58.4 | 9.7 | 31.9 | NA | 58.4 | — | 41.6 |
| BL− | 138 | 1 | 2 | 0.12 | >256 | 77.5 | 13.1 | 9.4 | NA | 77.5 | — | 22.5 | |
| BL+ | 47 | 256 | >256 | 1 | >256 | 2.1 | 0.0 | 97.9 | NA | 2.1 | — | 97.9 | |
| ampicillin/sulbactamb | all | 97 | 1 | 4 | 0.12 | 8 | 86.6 (80.4)c | — | 13.4 (19.6)c | NA | 63.9 (58.9)c | — | 36.1 (41.1)c |
| BL− | 73 | 1 | 2 | 0.12 | 8 | 93.2 | — | 6.8 | NA | 82.2 | — | 17.8 | |
| BL+ | 24 | 2 | 8 | 0.25 | 8 | 66.7 | — | 33.3 | NA | 8.3 | — | 91.7 | |
| azithromycine | all | 185 | 2 | 16 | ≤0.015 | >256 | 89.7 | — | — | NA | NA | NA | NA |
| BL− | 138 | 2 | 4 | ≤0.015 | >256 | 97.8 | — | — | NA | NA | NA | NA | |
| BL+ | 47 | 4 | >256 | ≤0.015 | >256 | 66.0 | — | — | NA | NA | NA | NA | |
| cefaclorb | all | 185 | 16 | >256 | 1 | >256 | 49.2 (47.6)c | 13.0 (13.0)c | 37.8 (39.5)c | 0.0 | NA | NA | NA |
| BL− | 138 | 8 | >256 | 1 | >256 | 52.9 | 10.9 | 36.2 | 0.0 | NA | NA | NA | |
| BL+ | 47 | 16 | >256 | 1 | >256 | 38.3 | 19.2 | 42.5 | 0.0 | NA | NA | NA | |
| cefuroximeb,f | all | 185 | 2 | 32 | 0.25 | >256 | 75.1 (73.0)c | 7.1 (7.0)c | 17.8 (20.0)c | 43.8 | 0.0 (0.0)c | 43.8 (42.2)c | 56.2 (57.8)c |
| BL− | 138 | 2 | 32 | 0.25 | >256 | 76.8 | 7.3 | 15.9 | 46.4 | 0.0 | 46.4 | 53.6 | |
| BL+ | 47 | 2 | 64 | 0.25 | >256 | 70.2 | 6.4 | 23.4 | 36.2 | 0.0 | 36.2 | 63.8 | |
| levofloxacin | all | 185 | 0.015 | 4 | 0.008 | >32 | 89.2 | — | — | 89.2 | 82.7 | — | 17.3 |
| BL− | 138 | 0.015 | 2 | 0.008 | 8 | 90.6 | — | — | 90.6 | 83.3 | — | 16.7 | |
| BL+ | 47 | 0.03 | 4 | 0.008 | >32 | 85.1 | — | — | 85.1 | 80.9 | — | 19.2 | |
| chloramphenicolg | all | 185 | NA | NA | NA | NA | 91.4 | 2.1 | 6.5 | NA | 91.9 | — | 8.1 |
| BL− | 138 | NA | NA | NA | NA | 96.4 | 1.4 | 2.2 | NA | 97.1 | — | 2.9 | |
| BL+ | 47 | NA | NA | NA | NA | 76.6 | 4.3 | 19.1 | NA | 76.6 | — | 23.4 | |
| tetracyclineg | all | 185 | NA | NA | NA | NA | 86.5 | 3.8 | 9.7 | NA | 90.3 | 1.6 | 8.1 |
| BL− | 138 | NA | NA | NA | NA | 92.0 | 2.9 | 5.1 | NA | 94.9 | 0.0 | 5.1 | |
| BL+ | 47 | NA | NA | NA | NA | 70.2 | 6.4 | 23.4 | NA | 76.6 | 6.4 | 17.0 | |
| SXTg | all | 185 | NA | NA | NA | NA | 41.1 | 3.2 | 55.7 | NA | 38.9 | 1.6 | 59.5 |
| BL− | 138 | NA | NA | NA | NA | 45.7 | 2.9 | 51.4 | NA | 42.8 | 2.1 | 55.1 | |
| BL+ | 47 | NA | NA | NA | NA | 27.7 | 4.2 | 68.1 | NA | 27.7 | 0.0 | 72.3 | |
NA, no breakpoint data available; BL−, β-lactamase negative; BL+, β-lactamase positive; AMC, amoxicillin/clavulanic acid; SXT, trimethoprim/sulfamethoxazole.
aAmoxicillin/clavulanic acid PK/PD susceptibility at high dose is shown in parentheses.
bIn the clinical setting, isolates of BLNAR are considered resistant to amoxicillin/clavulanic acid, ampicillin/sulbactam, cefaclor and cefuroxime (see main text).
cClinical susceptibility to amoxicillin/clavulanic acid ampicillin/sulbactam, cefaclor and cefuroxime reduced (data in parentheses) due to corrections according to BLNAR (see main text).
dIn clinical setting, all β-lactamase-positive H. influenzae should be considered resistant.
ebioMérieux Etest® breakpoints for incubation in CO2.
fBreakpoints used are for cefuroxime axetil.
gCLSI disc diffusion testing method used.
Table 6.
MIC distribution data for H. influenzae isolates collected during 2009–11 and 2013–14
| Years/antimicrobial agent | N | Number of isolates at MIC (mg/L) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ≥256 | ||
| 2009–11 | ||||||||||||||||
| AMC | 307 | 0 | 0 | 2 | 5 | 30 | 108 | 101 | 41 | 15 | 3 | 1 | 0 | 0 | 0 | 1 |
| ampicillin | 307 | 0 | 0 | 2 | 8 | 48 | 98 | 63 | 29 | 7 | 4 | 7 | 8 | 7 | 1 | 25 |
| azithromycin | 104 | 7 | 0 | 0 | 0 | 0 | 6 | 8 | 32 | 42 | 6 | 0 | 0 | 0 | 0 | 3 |
| cefaclor | 307 | 0 | 0 | 0 | 0 | 2 | 4 | 29 | 125 | 54 | 27 | 24 | 20 | 4 | 8 | 10 |
| cefuroxime | 307 | 0 | 0 | 1 | 2 | 19 | 59 | 120 | 57 | 33 | 9 | 2 | 3 | 2 | 0 | 0 |
| levofloxacin | 307 | 100 | 46 | 15 | 23 | 10 | 9 | 17 | 20 | 49 | 10 | 2 | 6 | 0 | 0 | 0 |
| 2013–14 | ||||||||||||||||
| AMC | 185 | 0 | 0 | 0 | 4 | 4 | 40 | 49 | 48 | 32 | 5 | 3 | 0 | 0 | 0 | 0 |
| ampicillin | 185 | 0 | 0 | 0 | 4 | 27 | 34 | 43 | 18 | 10 | 8 | 8 | 4 | 1 | 3 | 25 |
| ampicillin/sulbactam | 97 | 0 | 0 | 0 | 5 | 12 | 20 | 25 | 22 | 9 | 4 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 185 | 20 | 1 | 0 | 6 | 1 | 3 | 10 | 65 | 55 | 5 | 1 | 0 | 4 | 4 | 10 |
| cefaclor | 185 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 32 | 31 | 24 | 24 | 16 | 18 | 9 | 27 |
| cefuroxime | 185 | 0 | 0 | 0 | 0 | 8 | 31 | 42 | 32 | 26 | 13 | 11 | 7 | 6 | 1 | 8 |
| levofloxacin | 185 | 105 | 11 | 2 | 20 | 1 | 7 | 7 | 12 | 16 | 1 | 0 | 3 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
In 2009–11, in vitro susceptibility to amoxicillin/clavulanic acid was high at 98.4% for all H. influenzae by CLSI breakpoints and by high-dose PK/PD breakpoints (Table 5). However, CLSI guidelines state that all BLNAR should be considered non-susceptible to amoxicillin/clavulanic acid, thereby reducing clinical susceptibility to 97.4%. Similarly, BLNAR corrections have been made for susceptibility to cefaclor and cefuroxime (Table 5). Using EUCAST and low-dose PK/PD breakpoints, in vitro susceptibility to amoxicillin/clavulanic acid was reduced slightly to 93.5%. With a similar BLNAR correction, susceptibility is reduced to 85%. In 2013–14, in vitro susceptibility to amoxicillin/clavulanic acid remained high (95.7%) using CLSI/high-dose PK/PD breakpoints (89.1% with BLNAR correction), but decreased to 78.4% (73.0% with BLNAR correction) by EUCAST/low-dose PK/PD criteria. Interestingly, susceptibility to ampicillin/sulbactam (2013–14 only) was lower than that seen with amoxicillin/clavulanic acid. High in vitro susceptibility (94.8%) was observed in 2009–11 for cefuroxime by CLSI breakpoints (92.8% with BLNAR correction). However, with PK/PD and EUCAST breakpoints, susceptibility to cefuroxime was reduced considerably to 65.5% and 1.0%, respectively. By 2013–14, susceptibility to cefuroxime had been reduced to 75.1% (73.0% corrected) using CLSI breakpoints and was even lower by PK/PD (43.8%) and EUCAST (0%) breakpoints. Similarly, cefaclor showed wide variation in susceptibility if different breakpoints are used, with 78.5% (77.5% corrected) of H. influenzae susceptible in 2009–11 by CLSI breakpoints, but virtually zero susceptibility using PK/PD breakpoints. EUCAST does not provide breakpoints for cefaclor against H. influenzae, but using BLNAR prevalence as a marker, 87.0% appeared susceptible. Again, susceptibility decreased in 2013–14 with only 49.2% (47.6% corrected) of isolates susceptible by CLSI criteria (Table 5).
Using CLSI breakpoints, susceptibility was seen in 91.4% and 90.6% of H. influenzae isolates, respectively, in 2009–11. This proved to be relatively stable across time periods with 89.7% and 91.4% susceptible in 2013–14 (Table 5).
By CLSI or EUCAST breakpoints, 71.3% of H. influenzae isolates were susceptible to ampicillin in 2009–11 and 58.4% in 2013–14. As would be expected, ampicillin was inactive against the β-lactamase-positive strains. Levofloxacin and tetracycline were active against 78.2% and 74.3% (by CLSI criteria) of H. influenzae isolates in 2009–11, but susceptibility levels were higher in 2013–14 (89.2% and 86.5%, respectively). Trimethoprim/sulfamethoxazole was poorly active (41% susceptible) against H. influenzae in both time periods using CLSI breakpoints (Table 5).
M. catarrhalis
A total of 140 M. catarrhalis were collected in China from 2009 to 2011. These isolates were obtained mostly from patient sputum cultures (n = 138, 98.6%). Isolates of M. catarrhalis came from paediatric patients (n = 29, 20.7%), the elderly (n = 36, 25.7%) and adults (n = 73, 52.1%). Two isolates were collected from patients where age is unknown. In 2013–14, 80 M. catarrhalis isolates were collected, all of them from sputum. Paediatric patients accounted for 49 isolates (61.3%), adults for 16 (20.0%) and the elderly for 15 (18.8%). All M. catarrhalis, except for three isolates collected in 2009–11, were β-lactamase positive.
Summary MIC and susceptibility data and MIC distributions for M. catarrhalis are shown in Tables 7 and 8.
Table 7.
MIC and susceptibility data for M. catarrhalis isolates collected during 2009–11 and 2013–14
| Years/antimicrobial agent | N | Susceptibility |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| 50% | 90% | min. | max. | %S | %I | %R | %S | %S | %I | %R | ||
| 2009–11 | ||||||||||||
| AMC | 140 | 0.25 | 0.25 | 0.03 | 1 | 100 | — | 0.0 | 100 (100) | 100 | — | 0.0 |
| azithromycin | 140 | 0.25 | >256 | 0.03 | >256 | NA | NA | NA | NA | NA | NA | NA |
| cefaclor | 140 | 2 | 8 | 0.5 | 32 | 91.4 | 4.3 | 4.3 | 1.4 | NA | NA | NA |
| cefuroximea | 140 | 2 | 8 | 0.5 | 8 | 90.0 | 10.0 | 0.0 | 27.1 | 0.0 | 90.0 | 10.0 |
| levofloxacin | 140 | 0.06 | 0.5 | 0.03 | 2 | 100 | — | — | 100 | 96.4 | — | 3.6 |
| 2013–14 | ||||||||||||
| AMC | 80 | 0.25 | 0.25 | 0.03 | 0.5 | 100 | — | 0.0 | 100 (100) | 100 | — | 0.0 |
| azithromycin | 80 | 0.03 | 0.5 | ≤0.015 | >256 | NA | NA | NA | NA | NA | NA | NA |
| cefaclor | 80 | 4 | 16 | 0.25 | 32 | 63.8 | 26.3 | 10.0 | 2.5 | NA | NA | NA |
| cefuroximea | 80 | 2 | 4 | 0.06 | 8 | 93.8 | 6.3 | 0.0 | 20.0 | 1.3 | 92.5 | 6.3 |
| levofloxacin | 80 | 0.06 | 1 | 0.015 | >32 | 91.3 | — | — | 91.3 | 91.3 | — | 8.8 |
AMC, amoxicillin/clavulanic acid; NA, no breakpoint data available.
aBreakpoints used are for cefuroxime axetil.
Table 8.
MIC distribution data for M. catarrhalis isolates collected during 2009–11 and 2013–14
| Years/antimicrobial agent | N | Number of isolates at MIC (mg/L) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ≥256 | ||
| 2009–11 | ||||||||||||||||
| AMC | 140 | 0 | 19 | 15 | 25 | 69 | 11 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 140 | 0 | 2 | 4 | 31 | 36 | 23 | 7 | 6 | 2 | 1 | 0 | 0 | 0 | 0 | 28 |
| cefaclor | 140 | 0 | 0 | 0 | 0 | 0 | 2 | 42 | 66 | 8 | 10 | 6 | 6 | 0 | 0 | 0 |
| cefuroxime | 140 | 0 | 0 | 0 | 0 | 0 | 12 | 26 | 48 | 40 | 14 | 0 | 0 | 0 | 0 | 0 |
| levofloxacin | 140 | 0 | 27 | 93 | 3 | 1 | 4 | 7 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2013–14 | ||||||||||||||||
| AMC | 80 | 0 | 3 | 9 | 20 | 41 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 80 | 9 | 36 | 18 | 6 | 1 | 5 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| cefaclor | 80 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 30 | 14 | 3 | 21 | 8 | 0 | 0 | 0 |
| cefuroxime | 80 | 0 | 0 | 1 | 0 | 5 | 5 | 5 | 26 | 33 | 5 | 0 | 0 | 0 | 0 | 0 |
| levofloxacin | 80 | 3 | 20 | 36 | 9 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
In 2009–11, all isolates were susceptible to amoxicillin/clavulanic acid and levofloxacin using CLSI and PK/PD breakpoint criteria. Levofloxacin susceptibility was reduced to 96.4% when EUCAST breakpoints were applied. In 2013–14, amoxicillin/clavulanic acid susceptibility remained at 100%, but levofloxacin activity had decreased to 91.3% by all three breakpoints. Dramatic differences in susceptibility were observed across breakpoints (but not across time periods) for cefuroxime. Using CLSI breakpoints, susceptibility was 90.0% and 93.8% in 2009–11 and 2013–14, respectively, whereas it was 27.1% and 20.0%, respectively, using PK/PD breakpoints and 0% and 1.3%, respectively, using EUCAST breakpoint criteria. Cefaclor showed similar differences between CLSI and PK/PD breakpoints, but also a decrease in activity over time. In 2009–11, susceptibility was 91.4% by CLSI criteria compared with 1.4% by PK/PD breakpoints, with respective values of 63.8% and 2.5% in 2013–14 (Table 7).
Comparison of susceptibility in 2009–11 versus 2013–14
An analysis was performed comparing antimicrobial susceptibility between 2009–11 and 2013–14, using only the five sites that participated in both time periods. Susceptibility according to CLSI breakpoints by time period is given in Figures 1–3 for S. pneumoniae, H. influenzae and M. catarrhalis, respectively. For S. pneumoniae, from 2009–11 to 2013–14, susceptibility decreased significantly to amoxicillin/clavulanic acid (from 88.6% to 75.0%), cefaclor (from 41.3% to 22.6%) and tetracycline (from 6.4% to 0%; all P < 0.05). Statistically significant changes were more numerous for H. influenzae, where susceptibility to ampicillin (from 71.3% to 58.4%), cefaclor (from 78.5% to 49.2%) and cefuroxime (from 94.8% to 75.1%) decreased significantly, whereas levofloxacin (from 78.2% to 89.2%) and tetracycline (from 74.3% to 86.5%) showed significant increases in activity. For M. catarrhalis, cefaclor and levofloxacin showed significant changes, decreasing from 91.4% to 63.8% and from 100% to 91.3%, respectively.
Figure 2.
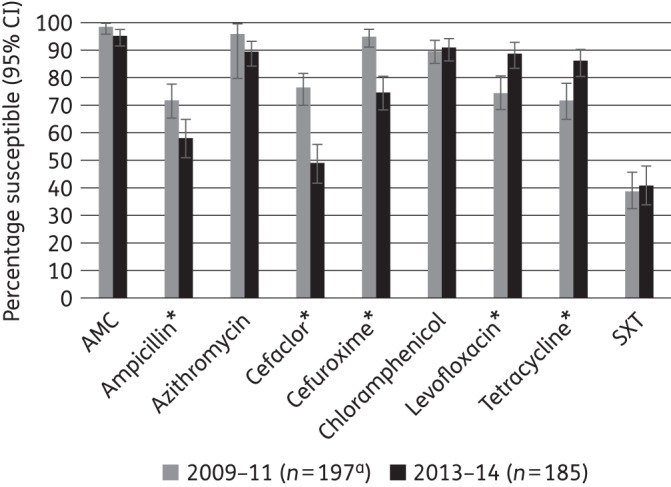
Percentage susceptibility rates (with 95% CI) for antimicrobials against H. influenzae according to CLSI breakpoints, comparing 2009–11 and 2013–14 using only sites that participated in both time periods. AMC, amoxicillin/clavulanic acid; SXT, trimethoprim/sulfamethoxazole. An asterisk indicates a statistically significant difference between time periods (P < 0.05). aSample sizes in 2009–11 were: azithromycin, n = 27; tetracycline, n = 183.
Figure 1.
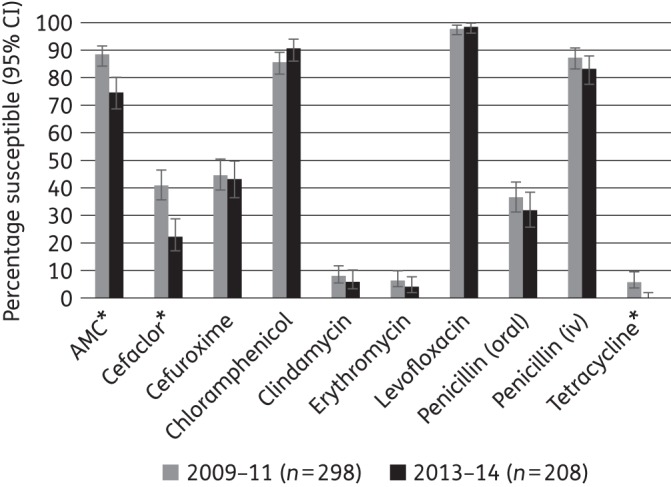
Percentage susceptibility rates (with 95% CI) for antimicrobials against S. pneumoniae according to CLSI breakpoints, comparing 2009–11 and 2013–14 using only sites that participated in both time periods. AMC, amoxicillin/clavulanic acid (it can be assumed that amoxicillin activity alone is the same). An asterisk indicates a statistically significant difference between time periods (P < 0.05).
Figure 3.
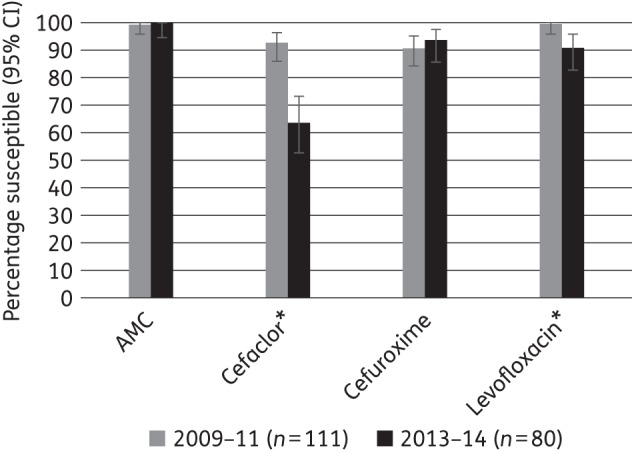
Percentage susceptibility rates (with 95% CI) for antimicrobials against M. catarrhalis according to CLSI breakpoints, comparing 2009–11 and 2013–14 using only sites that participated in both time periods. AMC, amoxicillin/clavulanic acid. An asterisk indicates a statistically significant difference between time periods (P < 0.05).
Discussion
The increasing prevalence of antimicrobial resistance among common pulmonary pathogens including S. pneumoniae and H. influenzae is worrisome. In S. pneumoniae, resistance to antibiotics has been a growing problem that has been monitored since penicillin resistance was observed in this species several decades ago. H. influenzae and M. catarrhalis have been associated with β-lactam resistance due to production of β-lactamase enzymes. β-Lactamase production can be quite variable among H. influenzae, but is usually ≥90% among M. catarrhalis isolates. Unfortunately, contemporary isolates of both of these species are now becoming resistant to agents other than β-lactams. Macrolide and fluoroquinolone resistance is becoming a growing concern.
Data from this current China SOAR study report highlight numerous important features with respect to a countrywide contemporary surveillance over two distinct time periods. For S. pneumoniae, based on CLSI oral and standard EUCAST penicillin breakpoints, activity of penicillin was quite stable but about two-thirds of pneumococci were penicillin non-susceptible during both time periods. However, using the CLSI iv penicillin breakpoints, about 84% may be considered to be susceptible to penicillin. Higher-dose amoxicillin/clavulanic acid (amoxicillin) increased susceptibility when the PK/PD breakpoint was applied. Therefore, the use of relevant breakpoints and dosing of penicillin and amoxicillin/clavulanic acid (amoxicillin alone as would be expected to have identical activity) are important factors to consider when assessing appropriate antimicrobial use. Other β-lactams (cefaclor and cefuroxime) were less active (<44% susceptible in 2013–14) than amoxicillin/clavulanic acid (amoxicillin) or penicillin iv as determined by any breakpoint method. Other classes of antimicrobial, including macrolides, were also poorly active. The exceptions to this were levofloxacin and chloramphenicol. By 2013–14, only amoxicillin/clavulanic acid (by PK/PD high-dose breakpoint amoxicillin alone as would be expected to have identical activity), levofloxacin and chloramphenicol showed ∼90% or higher susceptibility rates in S. pneumoniae from China.
The data from this study confirm that isolates of S. pneumoniae susceptible to penicillin G are also susceptible to other penicillins as inferred by CLSI and EUCAST guidelines and to cephalosporins as inferred by CLSI guidelines. Interestingly, the data from this study found the reverse was not always correct using CLSI breakpoints, i.e. most penicillin-non-susceptible S. pneumoniae were susceptible to amoxicillin/clavulanic acid (amoxicillin). This warrants further investigation.
For H. influenzae, both the proportion of β-lactamase-positive isolates and the proportion of BLNAR strains were higher in 2013–14 than in 2009–11. As a result, susceptibility was lower for ampicillin and the cephalosporins, a finding confirmed in the direct comparison of isolates from the five sites that participated during both time periods. Interestingly, susceptibility to levofloxacin and tetracycline appeared to increase over the same time period. However, by 2013–14, only amoxicillin/clavulanic acid, azithromycin, levofloxacin and chloramphenicol inhibited ∼90% or more of H. influenzae isolates using CLSI breakpoints.
M. catarrhalis also showed increasing resistance over time, with cefaclor and levofloxacin susceptibility decreasing significantly. However, three of the four agents with available breakpoints continued to inhibit >90% of isolates: amoxicillin/clavulanic acid, cefuroxime and levofloxacin.
This study demonstrated the differences observed when comparing the susceptibility of respiratory tract pathogens using CLSI, EUCAST and PK/PD breakpoints. For example, cefaclor and cefuroxime breakpoint differences had a dramatic effect on percentage susceptibility. It is important to understand these breakpoint differences as some non-European laboratories may utilize EUCAST breakpoints for determining susceptibility or, alternatively, laboratories currently using CLSI breakpoints may consider changing to EUCAST breakpoints. A concerted effort should be made to better align these different breakpoints to avoid confusion among clinicians and to allow for better comparison of surveillance data across geographical regions and over time.
Several studies have also documented high antibiotic resistance among CAP pathogens in China. In 2011, Jones et al.13 reported pneumococcal resistance to ceftriaxone at an alarming 35.1% in China. Another study group found <50% penicillin susceptibility in six cities located in China during 2009–10.14 In a further study from China over the same time period, significant regional differences in antimicrobial susceptibilities were observed including penicillin-non-susceptibility rates ranging from 46% to 100% and macrolide resistance ranging from 0% to 88%.15 Although the high prevalence of antibiotic resistance could be due to overuse of antibiotics and their availability without prescription in China, the antimicrobial resistance rates in pneumococci have been attributed to the capsular serotypes and international clones that are circulating in China. Close monitoring of pneumococcal resistance will be required as various polyvalent pneumococcal conjugate vaccines are introduced into the Chinese population.7,16
A study conducted during 2000–04, in the outpatient paediatric department of a single Chinese hospital, demonstrated that H. influenzae remained highly susceptible to oral cephalosporins and amoxicillin/clavulanic acid.17 During 1999–2000 in Beijing, high rates of resistance to H. influenzae were observed for tetracycline and trimethoprim/sulfamethoxazole, whereas amoxicillin/clavulanic acid and cefuroxime were uniformly active.18 In agreement with these findings, our more recent study data showed that H. influenzae had reduced susceptibility to tetracycline and trimethoprim/sulfamethoxazole and isolates were highly susceptible to amoxicillin/clavulanic acid. However, cefuroxime activity was much lower at 75% in the current study in 2013–14 than in the older study from Beijing.
The data presented from this SOAR study highlight the importance of antimicrobial resistance surveillance in China. Resistance to common antimicrobial agents among CAP pathogens is relatively high in this country and is increasing (based upon previously published literature in this region and the current study). Future data from China will further assist in understanding the implications of longitudinal trends related to antimicrobial resistance in this country.
Funding
This study was funded by GlaxoSmithKline.
Transparency declarations
This article forms part of a Supplement sponsored by GlaxoSmithKline. D. Torumkuney is an employee of GlaxoSmithKline. D. Torumkuney also holds shares in GlaxoSmithKline. I. Morrissey is an employee of IHMA, a medical communication and consultancy company, who participated in the exploration, interpretation of the results and preparation of this manuscript on behalf of GSK. IHMA also provided medical writing support in the form of writing assistance, collating author's comments, grammatical editing and referencing that was paid for by GSK. All other authors declare that they have no conflict of interest.
Editorial assistance was provided by Tracey Morris, Livewire Editorial Communications.
Acknowledgements
We thank Hong Zhang, Quan Lu, Jing Sun, Hui Chen, Yingchun Xu, Xiuli Xie, Yunjian Hu, Dongke Chen, Yuxing Ni, Jingyong Sun, Ziyong Sun, Zhongju Chen, Yunsong Yu, Qing Yang and Weili Zhang for collecting isolates and Dr Keith Barker (GlaxoSmithKline) for reviewing the manuscript.
References
- 1.Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44 Suppl 2: S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez JA, Anzueto AR. Changing needs of community-acquired pneumonia. J Antimicrob Chemother 2011; 66 Suppl 3: iii3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lonks JR, Garau J, Gomez L et al. Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniae. Clin Infect Dis 2002; 35: 556–64. [DOI] [PubMed] [Google Scholar]
- 4.Song JH, Jung SI, Ko KS et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother 2004; 48: 2101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SH, Song JH, Chung DR et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother 2012; 56: 1418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Zhao C, Zhang F et al. High prevalence and molecular analysis of macrolide-nonsusceptible Moraxella catarrhalis isolated from nasopharynx of healthy children in China. Microb Drug Resist 2012; 18: 417–26. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Gertz RE Jr, Liu Z et al. Characterization of highly antimicrobial-resistant clinical pneumococcal isolates recovered in a Chinese hospital during 2009–2010. J Med Microbiol 2012; 61: 42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests—Eleventh Edition: Approved Standard M02-A11. CLSI, Wayne, PA, USA, 2012. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-fourth Informational Supplement M100-S24. CLSI, Wayne, PA, USA, 2014. [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria—Second Edition: Approved Guideline M45-A2. CLSI, Wayne, PA, USA, 2010. [Google Scholar]
- 11.The European Committee on Antimicrobial Susceptibility testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 4.0, 2014 http://www.eucast.org.
- 12.Anon JB, Jacobs MR, Poole MD et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg 2004; 130 Suppl 1: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RN, Castanheira M, Hu B et al. Update of contemporary antimicrobial resistance rates across China: reference testing results for 12 medical centers (2011). Diagn Microbiol Infect Dis 2013; 77: 258–66. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Zu Y, Chen M et al. In vitro activity of cefditoren and other comparators against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis causing community-acquired respiratory tract infections in China. Diagn Microbiol Infect Dis 2012; 73: 187–91. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Chen M, Zu Y et al. Antimicrobial susceptibility of bacterial pathogens associated with community-acquired respiratory tract infections in Asia: report from the Community-Acquired Respiratory Tract Infection Pathogen Surveillance (CARTIPS) study, 2009–2010. Int J Antimicrob Agents 2011; 38: 376–83. [DOI] [PubMed] [Google Scholar]
- 16.Geng Q, Zhang T, Ding Y et al. Molecular characterization and antimicrobial susceptibility of Streptococcus pneumoniae isolated from children hospitalized with respiratory infections in Suzhou, China. PLoS One 2014; 9: e93752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang A, Yu S, Yao K et al. Antimicrobial susceptibility of Haemophilus influenzae strains and antibiotics usage patterns in pediatric outpatients: results from a children's hospital in China (2000–2004). Pediatr Pulmonol 2008; 43: 457–62. [DOI] [PubMed] [Google Scholar]
- 18.Hu YY, Yu SH, Liu G et al. Antimicrobial susceptibility of Haemophilus influenzae among children in Beijing, China, 1999–2000. Acta Paediatr 2002; 91: 136–40. [DOI] [PubMed] [Google Scholar]


