Abstract
Objectives
To assess antibiotic susceptibility of community-acquired respiratory tract isolates from Ivory Coast, Kenya, Democratic Republic of Congo (DRC) and Senegal in 2011–14.
Methods
Bacterial isolates were collected and MICs determined using Etest® for all antibiotics except erythromycin, for which testing was by disc diffusion. Susceptibility was assessed using CLSI, EUCAST and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints. For macrolide interpretation, CLSI breakpoints were adjusted for incubation in CO2.
Results
Susceptibility to penicillin (using CLSI oral or EUCAST breakpoints) was low among isolates of Streptococcus pneumoniae from the DRC and Kenya (17.4% and 19%, respectively) but higher among isolates from the Ivory Coast (70%) and Senegal (85.7%). Penicillin susceptibility using CLSI iv breakpoints was higher in all countries, but still only 69.6% in the DRC. Macrolide susceptibility (based on CLSI erythromycin disc diffusion breakpoints) was also low in Kenya (∼65%) but 87%–100% elsewhere. Haemophilus influenzae were only collected in the DRC and Senegal, with β-lactamase prevalence of 39% and 4%, respectively. Furthermore, β-lactamase-negative ampicillin-resistant (BLNAR) isolates were found in DRC (four isolates, 17%), but only two isolates were found in Senegal (by EUCAST definition). Amoxicillin/clavulanic acid in vitro susceptibility was 73.9% in the DRC and 100% in Senegal based on CLSI breakpoints, but this reduced to 65.2% in the DRC when BLNAR rates were considered. Clarithromycin susceptibility was >95% in both countries.
Conclusions
There was considerable variability in antibiotic susceptibility among the African countries participating in the surveillance programme. Thus, continued surveillance is necessary to track future changes in antibiotic resistance. Use of EUCAST versus CLSI breakpoints showed profound differences for cefaclor and ofloxacin against S. pneumoniae, with EUCAST showing lower susceptibility.
Introduction
In Africa, acute respiratory infections are the cause of death in 16% of children under 5 years old.1 Despite this significant health problem, few studies report updates on antimicrobial susceptibility of respiratory pathogens in this continent and the studies to date are often from large-scale global surveillance programmes that present combined data for the entire continent rather than individual countries.2,3 Local antimicrobial surveillance data in individual African countries are very limited,4 with most of the data coming from South Africa.5 However, recent reviews of studies of antimicrobial susceptibility in Africa have shown that resistance patterns vary within Africa5,6 and results from one country should not be assumed to apply to another. This makes surveillance at the country and local level very valuable for guiding empirical therapy, especially in sub-Saharan Africa, where healthcare providers and patients must often rely on affordable first-line antibiotics that may have lost their clinical effectiveness.2 Knowledge of resistance patterns is especially important for community-acquired pneumonia (CAP), since it is usually treated empirically without identification of the causative agent or its antibiogram.
The Survey of Antibiotic Resistance (SOAR) is an ongoing surveillance study of key respiratory pathogens. SOAR has been monitoring antimicrobial resistance in Africa, the Middle East, Latin America, Asia-Pacific and the Commonwealth of Independent States countries since 2002. For this report, recent SOAR data from hospitals in the Democratic Republic of Congo, Ivory Coast, Kenya and the Republic of Senegal were analysed to provide a picture of the current state of antimicrobial susceptibility.
Materials and methods
Collaborating centres
Isolates were collected from five sites in four countries: CUK/RDC, Democratic Republic of Congo; Institut Pasteur de Côte d'Ivoire, Ivory Coast; Université Cheikh Anta Diop, Senegal; Kenyatta National Hospital, Kenya and Aga Khan University Hospital, Kenya.
Clinical isolates (from outpatients who attended the university/national hospitals)
During 2011–14, a total of 277 clinical respiratory isolates, comprising 231 isolates of Streptococcus pneumoniae (23 from the Democratic Republic of Congo, 110 from the Ivory Coast, 84 from Kenya and 14 from Senegal) and 46 isolates of Haemophilus influenzae were analysed (23 from the Democratic Republic of Congo and 23 from Senegal). Paediatric patients (≤12 years old) accounted for 93 (33.6%) isolates, adult patients (13–64 years old) accounted for 170 (61.4%) isolates and the elderly (≥65 years) accounted for 14 (5.1%) isolates. Isolates originated from a variety of infection sources, including blood, tracheal aspirate, bronchoalveolar lavage, middle ear effusion, pleural aspirate and sputum. Organisms were identified using conventional methods (optochin susceptibility/bile solubility for S. pneumoniae and X and V factor requirement for H. influenzae). Duplicate isolates from the same patient were excluded from analysis.
Susceptibility testing
MICs were determined in selected local laboratories using the gradient strip Etest® susceptibility method according to the manufacturer's instructions (bioMérieux, Marcy l'Étoile, France). β-Lactamase production was determined by a chromogenic cephalosporin (nitrocefin) disc method. Disc diffusion susceptibility testing was carried out according to CLSI methodology.7 Study drugs for S. pneumoniae evaluated by Etest® varied by country and included penicillin, amoxicillin, amoxicillin/clavulanic acid, azithromycin, cefaclor, ceftriaxone, cefuroxime, chloramphenicol, clarithromycin, clindamycin, erythromycin (only in Kenya by Etest®), levofloxacin, moxifloxacin, ofloxacin, tetracycline and trimethoprim/sulfamethoxazole. Study drugs for H. influenzae evaluated by Etest® included amoxicillin/clavulanic acid, ampicillin, azithromycin, cefaclor, cefixime, cefuroxime, ciprofloxacin, clarithromycin, levofloxacin, ofloxacin and trimethoprim/sulfamethoxazole. Erythromycin was also evaluated by disc diffusion. Susceptibility to the study drugs was calculated based on CLSI breakpoints,8 except for macrolides and clindamycin, where Etest® breakpoints for incubation in CO2 were used. In addition, susceptibility based on the EUCAST and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints was analysed where applicable to assess whether adoption of these breakpoints would affect rates of susceptibility.9,10 EUCAST and PK/PD breakpoints were not evaluated for macrolides (except erythromycin for EUCAST) or clindamycin because, unlike CLSI, these are not adjusted for incubation in CO2 by bioMérieux. Breakpoints are shown in Table 1.
Table 1.
MIC breakpoints (mg/L) used for S. pneumoniae and H. influenzae isolates
|
S. pneumoniae |
H. influenzae |
All species |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLSI |
EUCAST |
CLSI |
EUCAST |
PK/PD | |||||||||
| Antimicrobial | S | I | R | S | I | R | S | I | R | S | I | R | (S only) |
| Amoxicillin | ≤2 | 4 | ≥8 | NA | NA | NA | NT | NT | NT | NT | NT | NT | ≤2 |
| AMCa | ≤2 | 4 | ≥8 | NA | NA | NA | ≤4 | — | ≥8 | ≤2 | — | ≥4 | ≤2 (≤4) |
| Ampicillin | NT | NT | NT | NT | NT | NT | ≤1 | 2 | ≥4 | ≤1 | — | ≥2 | NA |
| Azithromycinb | ≤4 | 8 | ≥16 | NA | NA | NA | ≤8 | — | —b | NA | NA | NA | NA |
| Cefaclor | ≤1 | 2 | ≥4 | ≤0.03 | 0.06–0.5 | ≥1 | ≤8 | 16 | ≥32 | NA | NA | NA | ≤0.5 |
| Cefixime | NA | NA | NA | NA | NA | NA | ≤1 | — | — | ≤0.12 | — | ≥0.25 | ≤1 |
| Ceftriaxone | ≤1 | 2 | ≥4 | ≤0.5 | 1-2 | ≥4 | NT | NT | NT | NT | NT | NT | ≤1 |
| Cefuroximec | ≤1 | 2 | ≥4 | ≤0.25 | 0.5 | ≥1 | ≤4 | 8 | ≥16 | ≤0.12 | 0.25–1 | ≥2 | ≤1 |
| Ciprofloxacin | NT | NT | NT | NT | NT | NT | ≤1 | — | — | ≤0.5 | — | ≥1 | ≤1 |
| Chloramphenicol | ≤4 | — | ≥8 | ≤8 | — | ≥16 | NT | NT | NT | NT | NT | NT | NA |
| Clarithromycin | ≤0.5 | 1 | ≥2 | NA | NA | NA | ≤16 | 32 | ≥64 | NA | NA | NA | NA |
| Clindamycinb | ≤0.5 | 1 | ≥2 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Erythromycinb | ≤1 | 2 | ≥4 | ≤1 | 2 | ≥4 | NT | NT | NT | NT | NT | NT | NA |
| Levofloxacin | ≤2 | 4 | ≥8 | ≤2 | — | ≥4 | ≤2 | — | — | ≤1 | — | ≥2 | ≤2 |
| Moxifloxacin | ≤1 | 2 | ≥4 | ≤0.5 | — | ≥1 | NT | NT | NT | NT | NT | NT | ≤1 |
| Ofloxacin | ≤2 | 4 | ≥8 | ≤0.12 | 0.25–4 | ≥8 | ≤2 | — | — | ≤0.5 | — | ≥1 | NA |
| Penicillin (oral) | ≤0.06 | 0.12–1 | ≥2 | ≤0.06 | 0.12–2 | ≥4 | NA | NA | NA | NA | NA | NA | NA |
| Penicillin (iv)d | ≤2 | 4 | ≥8 | notee | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Tetracycline | ≤1 | 2 | ≥4 | ≤1 | 2 | ≥4 | NT | NT | NT | NT | NT | NT | NA |
| SXT | ≤0.5 | 1-2 | ≥4 | ≤1 | 2 | ≥4 | ≤0.5 | 1-2 | ≥4 | ≤0.5 | 1 | ≥2 | ≤0.5 |
AMC, amoxicillin/clavulanic acid; SXT, trimethoprim/sulfamethoxazole; S, susceptible; I, intermediate; R, resistant; NA, not applicable; NT, not tested.
aTested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoint based on a high dose (4 g of amoxicillin with 250 mg of clavulanic acid per day for adults) is shown in parentheses.
bbioMérieux Etest® breakpoints for incubation in CO2.
cBreakpoints used are for cefuroxime axetil.
dParenteral non-meningitis breakpoints. EUCAST do not give iv breakpoints.
eEUCAST do not give iv breakpoints but dose-specific susceptible breakpoints are noted for pneumonia: 1.2 g × 4 (MIC ≤0.5 mg/L = susceptible), 1.2 g × 6 or 2.4 g × 4 (MIC ≤1 mg/L = susceptible) and 2.4 g × 6 (MIC ≤2 mg/L = susceptible).
Quality control and data analysis
Quality control strains Staphylococcus aureus ATCC 29213, S. pneumoniae ATCC 49619, H. influenzae ATCC 49247, H. influenzae ATCC 49766, Escherichia coli ATCC 25922 and E. coli ATCC 32518 were included on each day of testing. Results of susceptibility testing were accepted if the results of the control strains were within published limits. Differences in susceptibility between age groups and countries were assessed for statistical significance with Fisher's exact test using XLSTAT version 2011.1.05. A P value <0.05 was considered statistically significant.
Results
S. pneumoniae
Of the 231 S. pneumoniae isolates collected in the four African countries, 85 were from sputum (36.8%), 62 from blood (26.8%), 42 from ear effusion (18.2%), 25 from tracheal aspirate or bronchoalveolar lavage (10.8%) and 17 from pleural aspirate (7.4%). Paediatric patients (≤12 years old) contributed 82 (35.5%) isolates, adults (13–64 years old) 139 isolates (60.2%) and the elderly (≥65 years) 10 isolates (4.3%).
Summary MIC and susceptibility data for S. pneumoniae are shown in Table 2. MIC distribution data are given in Table 3. By CLSI penicillin iv (non-meningitis) breakpoints, >95% of S. pneumoniae were penicillin susceptible in all countries, except Democratic Republic of Congo (69.6%), whereas based on CLSI penicillin oral and EUCAST breakpoints, the proportion of penicillin-susceptible isolates was 85.7% in Senegal (albeit with small sample size of 14 isolates), 70% in Ivory Coast, 19% in Kenya and 17.4% in Democratic Republic of Congo (Table 2).
Table 2.
MIC and susceptibility results for S. pneumoniae isolates
| Country/antimicrobial | n | Susceptibility |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R | ||
| Democratic Republic of Congo | ||||||||||||
| azithromycina | 23 | 0.5 | 2 | 0.03 | 16 | 91.3 | 0 | 8.7 | NA | NA | NA | NA |
| cefixime | 23 | 0.25 | 2 | ≤0.015 | 32 | NA | NA | NA | 87.0 | NA | NA | NA |
| cefuroximeb | 23 | 0.5 | 4 | 0.03 | 32 | 78.3 | 8.7 | 13.0 | 78.3 | 30.4 | 34.8 | 34.8 |
| levofloxacin | 23 | 0.25 | 1 | 0.004 | 2 | 100 | 0 | 0 | 100 | 100 | 0 | 0 |
| ofloxacin | 23 | 0.25 | 1 | 0.004 | >32 | 95.7 | 0 | 4.4 | NA | 43.5 | 52.2 | 4.4 |
| penicillin (oral) | 23 | 0.5 | 8 | 0.06 | 32 | 17.4 | 47.8 | 34.8 | NA | 17.4 | 52.2 | 30.4 |
| penicillin (iv) | 23 | 0.5 | 8 | 0.06 | 32 | 69.6 | 17.4 | 13.0 | NA | 52.2–69.6 | NA | NA |
| SXT | 23 | 0.25 | >32 | 0.004 | >32 | 73.9 | 4.4 | 21.7 | 73.9 | 78.3 | 0 | 21.7 |
| erythromycinc | 23 | NT | NT | NT | NT | 87.0 | 4.3 | 8.7 | NA | 87.0 | 0.0 | 13.0 |
| Ivory Coast | ||||||||||||
| AMCd,e | 110 | 0.03 | 0.12 | ≤0.015 | 2 | 100 | 0 | 0 | 100 (100) | NA | NA | NA |
| cefaclor | 110 | 1 | 4 | 0.25 | 64 | 60.0 | 22.7 | 17.3 | 17.3 | 0 | 17.3 | 82.7 |
| cefuroxime | 110 | 0.06 | 0.25 | ≤0.015 | 1 | 100 | 0 | 0 | 100 | 97.3 | 1.8 | 0.9 |
| clarithromycina | 110 | 0.03 | 0.06 | ≤0.015 | 256 | 95.5 | 0 | 4.5 | NA | NA | NA | NA |
| levofloxacin | 110 | 1 | 1 | 0.25 | 8 | 98.2 | 0.9 | 0.9 | 98.2 | 98.2 | 0 | 1.8 |
| ofloxacin | 110 | 2 | 4 | 0.015 | 32 | 86.4 | 9.1 | 4.5 | NA | 1.8 | 93.6 | 4.6 |
| penicillin (oral) | 110 | 0.03 | 0.5 | ≤0.002 | 4 | 70.0 | 25.5 | 4.5 | NA | 70.0 | 28.2 | 1.8 |
| penicillin (iv) | 110 | 0.03 | 0.5 | ≤0.002 | 4 | 98.2 | 1.8 | 0 | NA | 93.7–98.2 | NA | NA |
| SXT | 110 | 0.5 | 4 | 0.015 | 32 | 50.0 | 26.4 | 23.6 | 50.0 | 54.6 | 21.8 | 23.6 |
| erythromycinc | 110 | NT | NT | NT | NT | 96.4 | 1.8 | 1.8 | NA | 93.6 | 4.6 | 1.8 |
| Kenya | ||||||||||||
| amoxicillin | 64 | 0.5 | 2 | ≤0.015 | 16 | 92.2 | 1.6 | 6.2 | 92.2 | NA | NA | NA |
| AMCd,e | 84 | 0.25 | 2 | ≤0.015 | >256 | 95.2 | 1.2 | 3.6 | 95.2 (96.4) | NA | NA | NA |
| azithromycin | 64 | 2 | >256 | 0.25 | >256 | 65.6 | 6.3 | 28.1 | NA | NA | NA | NA |
| ceftriaxone | 84 | 0.25 | 1 | 0.008 | 8 | 96.4 | 1.2 | 2.4 | 96.4 | 82.1 | 15.5 | 2.4 |
| cefuroxime | 84 | 0.25 | 4 | ≤0.015 | 32 | 66.7 | 17.9 | 15.5 | 66.7 | 54.8 | 4.8 | 40.5 |
| chloramphenicol | 64 | 0.25 | >256 | 0.03 | >256 | 68.7 | 0.0 | 31.3 | NA | 73.4 | 0 | 26.6 |
| erythromycina | 64 | 0.25 | >256 | 0.06 | >256 | 64.1 | 3.1 | 32.8 | NA | 64.1 | 3.1 | 32.8 |
| levofloxacin | 20 | 0.5 | 1 | 0.25 | 1 | 100 | 0 | 0 | 100 | 100 | 0 | 0 |
| moxifloxacin | 84 | 0.12 | 0.25 | 0.03 | 0.5 | 100 | 0 | 0 | 100 | 100 | 0 | 0 |
| penicillin (oral) | 84 | 1 | 2 | ≤0.015 | >256 | 19.0 | 60.7 | 20.2 | NA | 19.0 | 69.0 | 4.8 |
| penicillin (iv) | 84 | 1 | 2 | ≤0.015 | >256 | 95.2 | 1.2 | 3.6 | NA | 46.4–95.2 | NA | NA |
| tetracycline | 64 | 8 | 32 | 0.06 | >256 | 46.8 | 1.6 | 51.6 | NA | 46.9 | 1.6 | 51.6 |
| Senegal | ||||||||||||
| AMCd,e | 14 | ≤0.015 | 0.03 | ≤0.015 | 0.03 | 100 | 0 | 0 | 100 (100) | NA | NA | NA |
| cefaclor | 14 | 1 | 2 | 0.12 | 2 | 78.6 | 21.4 | 0 | 42.9 | 0 | 42.9 | 57.1 |
| cefixime | 14 | 0.5 | 2 | 0.12 | 2 | NA | NA | NA | 85.7 | 0 | 0 | 0 |
| cefuroxime | 14 | 0.06 | 0.25 | ≤0.015 | 0.5 | 100 | 0 | 0 | 100 | 92.9 | 7.1 | 0 |
| clarithromycina | 14 | 0.06 | 0.12 | ≤0.015 | 0.12 | 100 | 0 | 0 | NA | NA | NA | NA |
| clindamycin | 14 | 0.12 | 0.25 | 0.06 | 0.25 | 100 | 0 | 0 | NA | NA | NA | NA |
| levofloxacin | 14 | 0.5 | 1 | 0.5 | 1 | 100 | 0 | 0 | 100 | 100 | 0 | 0 |
| ofloxacin | 14 | 2 | 2 | 1 | 2 | 100 | 0 | 0 | NA | 0 | 100 | 0 |
| penicillin (oral) | 14 | 0.03 | 0.12 | ≤0.015 | 0.12 | 85.7 | 14.3 | 0 | NA | 85.7 | 14.3 | 0 |
| penicillin (iv) | 14 | 0.03 | 0.12 | ≤0.015 | 0.12 | 100 | 0 | 0 | NA | 100 | NA | NA |
| SXT | 14 | 4 | 32 | 0.06 | 32 | 14.3 | 28.6 | 57.1 | 14.3 | 21.4 | 21.4 | 57.1 |
| erythromycinc | 14 | NT | NT | NT | NT | 100 | 0 | 0 | NA | 100 | 0 | 0 |
S, susceptible; I, intermediate; R, resistant; NA, no breakpoint data available (NA for azithromycin, clarithromycin and clindamycin by PK/PD and EUCAST and for erythromycin by EUCAST because Etest® breakpoints in CO2 not available); NT, not tested for MIC; SXT, trimethoprim/sulfamethoxazole; AMC, amoxicillin/clavulanic acid.
abioMérieux Etest® breakpoints for incubation in CO2.
bBreakpoints used are for cefuroxime axetil.
cUsing S/I/R zone diameters (mm) of CLSI (≤15/16–20/≥21) and EUCAST (≤18/19–21/≥22).
dAmoxicillin/clavulanic acid PK/PD susceptibility at high dose shown in parentheses.
eFor S. pneumoniae susceptibility to amoxicillin alone can be inferred from amoxicillin/clavulanic acid data.
Table 3.
Distribution of MICs for S. pneumoniae isolates
| Number of isolates at MIC (mg/L) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country/antimicrobial | n | ≤0.002 | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >32 | 128 | >256 |
| Democratic Republic of Congo | ||||||||||||||||||||
| azithromycin | 23 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 2 | 5 | 5 | 2 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| cefixime | 23 | 0 | 0 | 0 | 1 | 0 | 3 | 3 | 1 | 7 | 3 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| cefuroxime | 23 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 4 | 8 | 3 | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| levofloxacin | 23 | 0 | 3 | 1 | 0 | 6 | 1 | 0 | 0 | 4 | 3 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ofloxacin | 23 | 0 | 4 | 2 | 0 | 3 | 0 | 0 | 1 | 6 | 3 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| penicillin | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 2 | 2 | 3 | 1 | 4 | 1 | 0 | 2 | 0 | 0 | 0 |
| SXT | 23 | 0 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 6 | 5 | 1 | 0 | 0 | 1 | 0 | 1 | 3 | 0 | 0 |
| ≤0.002 | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | ||
| Ivory Coast | ||||||||||||||||||||
| AMC | 110 | 0 | 0 | 0 | 35 | 0 | 37 | 17 | 11 | 8 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefaclor | 110 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 18 | 47 | 25 | 14 | 3 | 0 | 1 | 1 | 0 | 0 |
| cefuroxime | 110 | 0 | 0 | 0 | 44 | 0 | 8 | 22 | 22 | 11 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 110 | 0 | 0 | 0 | 35 | 0 | 51 | 17 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 3 |
| levofloxacin | 110 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 36 | 62 | 8 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| ofloxacin | 110 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 4 | 14 | 75 | 10 | 3 | 1 | 1 | 0 | 0 | 0 |
| penicillin | 110 | 2 | 5 | 5 | 0 | 24 | 21 | 20 | 9 | 12 | 5 | 2 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| SXT | 110 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 16 | 21 | 14 | 5 | 24 | 19 | 2 | 1 | 4 | 0 | 0 | 0 |
| ≤0.002 | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | >256 | ||
| Kenya | ||||||||||||||||||||
| amoxicillina | 64 | 0 | 0 | 0 | 9 | 0 | 4 | 3 | 3 | 9 | 4 | 14 | 13 | 1 | 1 | 3 | 0 | 0 | 0 | 0 |
| AMC | 84 | 0 | 0 | 0 | 17 | 0 | 3 | 3 | 15 | 7 | 4 | 21 | 10 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| azithromycina | 64 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 18 | 17 | 4 | 4 | 1 | 2 | 0 | 0 | 15 |
| ceftriaxone | 84 | 0 | 0 | 4 | 0 | 10 | 0 | 7 | 11 | 11 | 26 | 12 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| cefuroxime | 84 | 0 | 0 | 0 | 17 | 0 | 0 | 5 | 10 | 14 | 4 | 6 | 15 | 10 | 0 | 1 | 2 | 0 | 0 | 0 |
| chloramphenicola | 64 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 17 | 18 | 0 | 0 | 2 | 1 | 3 | 2 | 1 | 0 | 0 | 14 |
| erythromycina | 64 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 17 | 14 | 1 | 1 | 2 | 2 | 2 | 3 | 0 | 0 | 0 | 14 |
| levofloxacina | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 11 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| moxifloxacin | 84 | 0 | 0 | 0 | 0 | 0 | 1 | 9 | 60 | 13 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| penicillin | 84 | 0 | 0 | 0 | 12 | 0 | 2 | 2 | 6 | 9 | 8 | 28 | 13 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| tetracyclinea | 64 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 18 | 8 | 2 | 1 | 1 | 0 | 12 | 8 | 7 | 2 | 1 | 3 |
| ≤0.002 | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | ||
| Senegal | ||||||||||||||||||||
| AMC | 14 | 0 | 0 | 0 | 8 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefaclor | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 5 | 5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefixime | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 5 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefuroxime | 14 | 0 | 0 | 0 | 1 | 0 | 5 | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 14 | 0 | 0 | 0 | 1 | 0 | 3 | 4 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| clindamycin | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| levofloxacin | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ofloxacin | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| penicillin | 14 | 0 | 0 | 0 | 4 | 0 | 7 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SXT | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 3 | 1 | 1 | 1 | 5 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid; SXT, trimethoprim/sulfamethoxazole.
aNot all isolates were tested with this antibiotic.
The most consistent in vitro activity in the region was shown by levofloxacin, with >98% of pneumococcal isolates showing susceptibility by all three breakpoints in all four countries. Similarly, 100% of isolates were susceptible to amoxicillin/clavulanic acid (and by inference of amoxicillin alone) by both CLSI and PK/PD breakpoints in Ivory Coast and Senegal, and >95% susceptible by these breakpoints in Kenya. Cefuroxime was very active against isolates from the Ivory Coast and Senegal with >90% susceptibility using all three breakpoints. However, isolates from the Democratic Republic of Congo and Kenya showed <80% susceptibility by CLSI and PK/PD breakpoints and 30.4% and 54.8%, respectively, using the EUCAST breakpoint. Macrolides were active against >95% of S. pneumoniae in Ivory Coast and Senegal, 87% in Democratic Republic of Congo, but only 64.1% in Kenya, based on erythromycin susceptibility using CLSI breakpoints. Using all three breakpoints, trimethoprim/sulfamethoxazole activity was low in Democratic Republic of Congo (73.9%–78.3%), Ivory Coast (50%–54.6%) and Senegal (14.3%–21.4%), as was tetracycline activity in Kenya (<50%).
Cefaclor and ofloxacin demonstrated significant variation in percentage susceptibility when different breakpoints were applied. Cefaclor was active against 60% of isolates from the Ivory Coast and 78.6% from Senegal using CLSI breakpoints, whereas the PK/PD breakpoint resulted in 17.3% and 42.9% susceptibility, respectively, and EUCAST breakpoints showed non-susceptibility in both countries. Percentage susceptibility values for ofloxacin using CLSI breakpoints were >85% in Democratic Republic of Congo, Ivory Coast and Senegal, but only 43.5%, 1.8% and 0%, respectively, using EUCAST breakpoints.
Figure 1 shows the direct comparison between countries of percentage antimicrobial susceptibility (based on CLSI breakpoints) tested by at least two countries collecting a minimum of 20 isolates. Isolates recovered from patients in the Ivory Coast showed significantly higher susceptibility to cefuroxime and penicillin than both Kenya and Democratic Republic of Congo. Susceptibility to trimethoprim/sulfamethoxazole was lowest (14.3%) in Senegal but was 50% in Ivory Coast and 73.9% in Democratic Republic of Congo. Antimicrobial susceptibility was generally lower in Kenya than in the two other countries, with significantly lower susceptibility to azithromycin (65.6%) compared with Democratic Republic of Congo and to cefuroxime and erythromycin compared with Ivory Coast.
Figure 1.
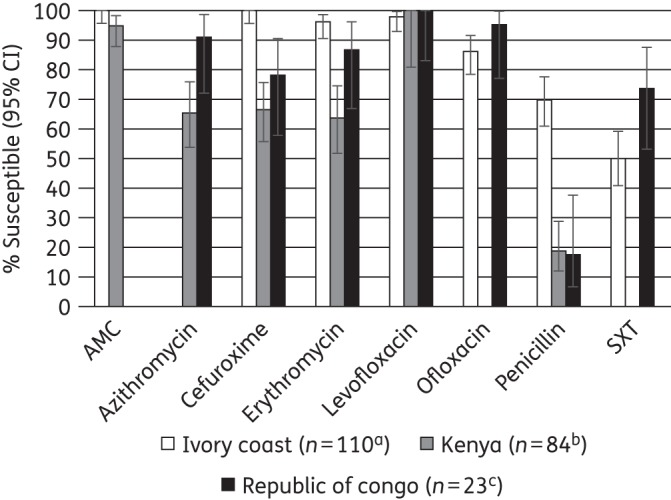
Percentage susceptibility rates (with 95% confidence intervals) for antimicrobials against S. pneumoniae by country according to CLSI breakpoints. aAzithromycin was not tested in Ivory Coast. bSample sizes in Kenya were: azithromycin and erythromycin, n = 64; levofloxacin, n = 20. cAmoxicillin/clavulanic acid was not tested in Democratic Republic of Congo. In Senegal only 14 S. pneumoniae isolates were collected. Therefore Senegal data have not been included in this figure. AMC, amoxicillin/clavulanic acid; SXT, trimethoprim/sulfamethoxazole.
CLSI guidelines indicate that isolates susceptible to penicillin G (MIC ≤0.06 mg/L) can be reported as susceptible to amoxicillin/clavulanic acid, ceftriaxone, cefaclor and cefuroxime. Data from this study (all isolates combined) confirmed this, in that all penicillin-susceptible S. pneumoniae were also susceptible to the β-lactam antibiotics listed above apart from 24 out of 114 penicillin-susceptible isolates (21.1%) that were non-susceptible to cefaclor. However, the reverse was not always found. All 206 penicillin-non-susceptible isolates were amoxicillin/clavulanic acid susceptible and all but one was ceftriaxone susceptible. For cefuroxime, 74.2% of penicillin-non-susceptible isolates were susceptible. However, only 14.5% of penicillin-non-susceptible S. pneumoniae were cefaclor susceptible (27.7%). A similar ‘expert rule’ is provided by EUCAST but for penicillins only, i.e. amoxicillin/clavulanic acid (amoxicillin). However, unlike CLSI, individual breakpoints are not provided by EUCAST for amoxicillin/clavulanate to make this comparison.
Prevalence of antibiotic susceptibility among PSSP, PISP and PRSP isolates (based on CLSI penicillin oral breakpoints)
Combining all isolates from the four African countries, susceptibility was compared among penicillin-susceptible S. pneumoniae (PSSP), penicillin-intermediate S. pneumoniae (PISP) and penicillin-resistant S. pneumoniae (PRSP) isolates for those agents where at least 20 isolates were tested in at least two penicillin susceptibility subsets (Figure 2). PSSP isolates were 100% susceptible to amoxicillin/clavulanic acid, cefuroxime and levofloxacin and >90% were susceptible to clarithromycin, ofloxacin and erythromycin. The activities of cefaclor and trimethoprim/sulfamethoxazole were lower, at 72% and 51%, respectively. Among the PISP isolates, >95% were susceptible to amoxicillin/clavulanic acid, clarithromycin, levofloxacin and erythromycin. Susceptibility to all other agents was <80%, except to ofloxacin (85% susceptible). Sample sizes for PRSP isolates were small for most agents, resulting in susceptibility estimates with wide confidence intervals. Levofloxacin (95% susceptible) and amoxicillin/clavulanic acid (86%) were the two most active agents against these isolates, with all other agents showing susceptibility rates of <70%.
Figure 2.
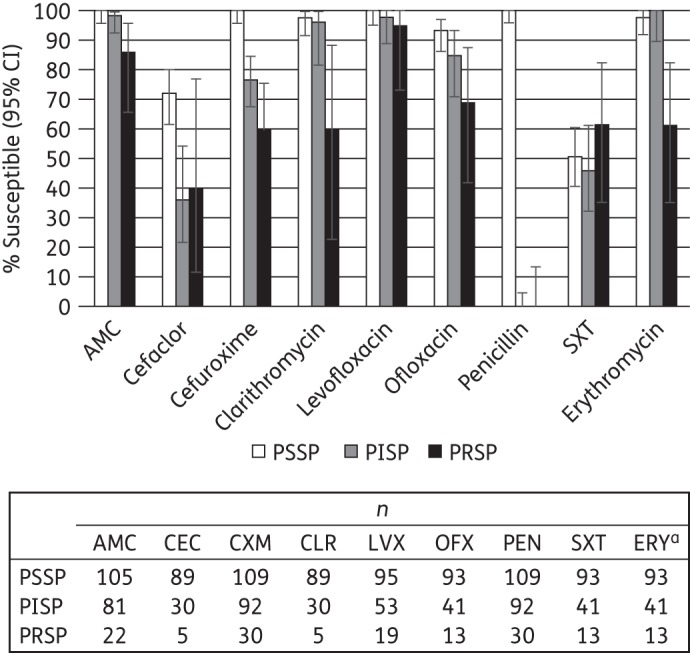
Percentage susceptibility rates (with 95% CIs) for antimicrobials according to CLSI breakpoints against penicillin-susceptible (PSSP), penicillin-intermediate (PISP) and penicillin-resistant S. pneumoniae (PRSP), combining results from Democratic Republic of Congo, Ivory Coast, Kenya and Senegal. Penicillin susceptibility categories are based on oral penicillin CLSI breakpoints. Sample sizes varied by antimicrobial agents and are shown below the bar chart. aOnly isolates with erythromycin testing by disc diffusion were included. AMC, amoxicillin/clavulanic acid; CEC, cefaclor; CXM, cefuroxime; CLR, clarithromycin; LVX, levofloxacin; OFX, ofloxacin; PEN, penicillin; SXT, trimethoprim/sulfamethoxazole; ERY, erythromycin.
Age group analysis
Differences in the susceptibility of S. pneumoniae from adult and paediatric patients were investigated for isolates from the Ivory Coast and Kenya (sample sizes ≥20). For Ivory Coast isolates, a statistically significant difference was found only for ofloxacin, with paediatric isolates almost 20 percentage points less susceptible than isolates from adults (Figure 3). In Kenya no significant differences between adult and paediatric patients were found for any of the tested antimicrobials (data not shown).
Figure 3.
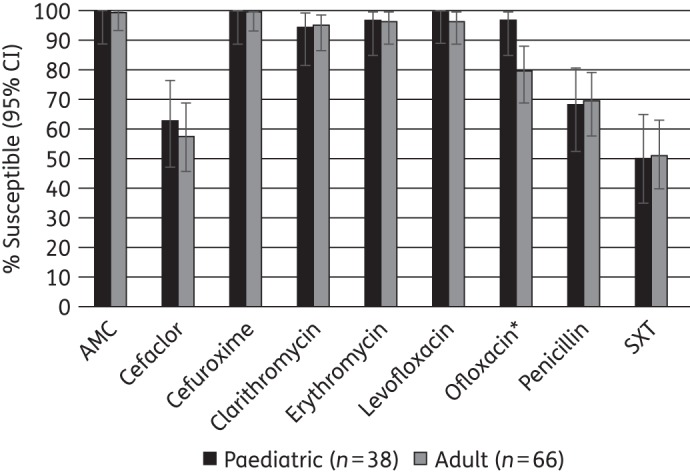
Percentage susceptibility rates (with 95% CIs) for antimicrobials against S. pneumoniae from Ivory Coast by age group according to CLSI breakpoints. *Susceptibility significantly lower in paediatric than adult patients (P < 0.05). Elderly patients contributed only six isolates and were not included in this analysis. AMC, amoxicillin/clavulanic acid; SXT, trimethoprim/sulfamethoxazole.
H. influenzae
Isolates of H. influenzae were only collected from the Democratic Republic of Congo and Senegal. The isolates from these countries comprised 11 from paediatric patients (24%), 31 from adult patients (67%) and 4 from elderly patients (9%).
Of the 23 H. influenzae isolates from Democratic Republic of Congo, 22 (96%) came from middle ear effusion and one from pleural fluid. The Senegal isolates came from sputum (14/23, 61%), tracheal aspirate (7/23, 30%) and middle ear effusion (2/23, 9%).
In Democratic Republic of Congo, 9 of 23 isolates were β-lactamase-positive (39.1%), whereas in Senegal it was only 1 of 23 (4.3%). Of the 14 β-lactamase-negative isolates in Democratic Republic of Congo, 4 isolates (17.4% of all H. influenzae) were β-lactamase negative, ampicillin resistant by CLSI definition (BLNAR, ampicillin MIC ≥4 mg/L), whereas in Senegal no such isolates were found. Using the EUCAST BLNAR definition (MIC ≥2 mg/L), the number of isolates from the Democratic Republic of Congo remained at four (17.4%) and two isolates (10.0%) were found in Senegal. For analysis, the BLNAR strains from Democratic Republic of Congo were included with the other β-lactamase-negative isolates.
Summary MIC and susceptibility data for H. influenzae are shown in Table 4. MIC distribution data are given in Table 5. In the Democratic Republic of Congo, amoxicillin/clavulanic acid in vitro susceptibility was 73.9% for H. influenzae by CLSI and high-dose PK/PD breakpoints, but slightly lower, at 69.6%, using low-dose PK/PD and EUCAST criteria. However, all BLNAR strains (four by CLSI or EUCAST) under clinical conditions should be considered non-susceptible to amoxicillin/clavulanic acid, cefaclor and cefuroxime, irrespective of the MIC. With this correction, clinical susceptibility to amoxicillin/clavulanic acid was reduced to 65.2% by CLSI and to 60.9% by EUCAST. In Senegal, >90% of isolates were susceptible to amoxicillin/clavulanic acid irrespective of the breakpoint used. Ciprofloxacin, levofloxacin, ofloxacin and clarithromycin also showed high activity in this country, with susceptibility rates of >90% by all breakpoints. In contrast, in Democratic Republic of Congo, cefuroxime, cefaclor and clarithromycin showed >95% susceptibility, whereas ampicillin and trimethoprim/sulfamethoxazole showed susceptibility of <70% based on CLSI breakpoints. In both countries, the proportion of isolates susceptible to cefuroxime and cefixime varied markedly depending on the breakpoints used, due to the differences between CLSI, EUCAST and PK/PD for these antibiotics. Cefuroxime in vitro activity was >95% in both countries by the CLSI breakpoint but dropped to 78.3% and 26.1% by PK/PD and EUCAST breakpoints, respectively, in Democratic Republic of Congo and to 82.6% and 0%, respectively, in Senegal. Cefixime was active against 82.6% of isolates based on CLSI and PK/PD breakpoints in Democratic Republic of Congo but susceptibility dropped to 30.4% based on EUCAST breakpoints. In Senegal, 100% of isolates were susceptible to cefixime and 87% to cefaclor based on CLSI breakpoints. Based on EUCAST breakpoints, percentage susceptibility to cefixime was 91.3% and to cefaclor by PK/PD was 0%. Ampicillin activity was 69.6% by CLSI and EUCAST breakpoints in Democratic Republic of Congo and 91.3% in Senegal. Trimethoprim/sulfamethoxazole showed the lowest activity, with 60.9% susceptible across all breakpoints in Democratic Republic of Congo and only 8.7% in Senegal.
Table 4.
MIC and susceptibility results for H. influenzae isolates
| Susceptibility |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
||||||||||
| Antimicrobial | Isolate group | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| Democratic Republic of Congo | |||||||||||||
| AMCa,b | all | 23 | 1 | 16 | 0.03 | 32 | 73.9 (65.2)e | 0 | 26.1 (34.8)e | 69.6 (73.9) | 69.6 (60.9)e | 0 | 30.4 (39.1)e |
| BL− | 14 | 0.5 | 8 | 0.03 | 16 | 78.6 | 0 | 21.4 | 78.6 (78.6) | 78.6 | 0 | 21.4 | |
| ampicillin | all | 23 | 0.5 | 32 | 0.03 | >256 | 69.6 | 8.7 | 21.7 | NA | 69.6 | 0 | 30.4 |
| BL− | 14 | 0.5 | 32 | 0.06 | >256 | 71.4 | 0 | 28.6 | NA | 71.4 | 0 | 28.6 | |
| azithromycinc | all | 23 | 0.25 | 16 | 0.03 | >256 | 82.6 | 0 | 17.4 | NA | NA | NA | NA |
| BL− | 14 | 0.25 | 16 | 0.03 | 32 | 85.7 | 0 | 14.3 | NA | NA | NA | NA | |
| cefaclorb | all | 23 | 0.25 | 4 | 0.03 | 8 | 100 (82.6)e | 0 | 0 (17.4)e | 73.9 | NA | NA | NA |
| BL− | 14 | 0.25 | 2 | 0.03 | 4 | 100 | 0 | 0 | 71.4 | NA | NA | NA | |
| cefixime | all | 23 | 0.5 | 16 | ≤0.015 | 16 | 82.6 | 0 | 17.4 | 82.6 | 30.4 | 0 | 69.6 |
| BL− | 14 | 0.25 | 16 | ≤0.015 | 16 | 85.7 | 0 | 14.3 | 85.7 | 35.7 | 0 | 64.3 | |
| cefuroximeb,d | all | 23 | 0.5 | 4 | 0.03 | 16 | 95.7 (78.3)e | 0 | 4.4 (21.7)e | 78.3 | 26.1 (21.7)e | 52.2 (47.8)e | 21.7 (30.4)e |
| BL− | 14 | 0.5 | 4 | 0.03 | 16 | 92.9 | 0 | 7.1 | 71.4 | 21.4 | 50.0 | 28.6 | |
| ciprofloxacin | all | 23 | 0.25 | 2 | 0.004 | 4 | 82.6 | 0 | 17.4 | 82.6 | 82.6 | 0 | 17.4 |
| BL− | 14 | 0.25 | 2 | 0.004 | 4 | 85.7 | 0 | 14.3 | 85.7 | 85.7 | 0 | 14.3 | |
| clarithromycinc | all | 23 | 0.25 | 16 | 0.03 | >256 | 95.7 | 0 | 4.4 | NA | NA | NA | NA |
| BL− | 14 | 0.25 | 16 | 0.03 | >256 | 92.9 | 0 | 7.1 | NA | NA | NA | NA | |
| SXT | all | 23 | 0.5 | >32 | 0.004 | >32 | 60.9 | 17.4 | 21.7 | 60.9 | 60.9 | 8.7 | 30.4 |
| BL− | 14 | 0.5 | >32 | 0.004 | >32 | 57.1 | 14.3 | 28.6 | 57.1 | 57.1 | 7.1 | 35.7 | |
| Senegal | |||||||||||||
| AMCa,b | All | 23 | 0.5 | 2 | 0.12 | 4 | 100 | 0 | 0 | 95.7 (100) | 95.7 (91.3)e | 0 | 4.3 (8.7)e |
| BL− | 22 | 0.5 | 1 | 0.12 | 4 | 100 | 0 | 0 | 95.5 (100) | 95.5 | 0 | 4.6 | |
| ampicillin | all | 23 | 0.25 | 1 | 0.06 | 2 | 91.3 | 8.7 | 0 | NA | 91.3 | 0 | 8.7 |
| BL− | 22 | 0.25 | 1 | 0.06 | 2 | 90.9 | 9.1 | 0 | NA | 90.9 | 0 | 9.1 | |
| cefaclorc | all | 23 | 4 | 16 | 1 | 64 | 87.0 | 8.7 | 4.3 | 0 | NA | NA | NA |
| BL− | 22 | 4 | 16 | 1 | 64 | 86.4 | 9.1 | 4.6 | 0 | NA | NA | NA | |
| cefixime | all | 23 | 0.06 | 0.12 | ≤0.015 | 0.5 | 100 | 0 | 0 | 100 | 91.3 | 0 | 8.7 |
| BL− | 22 | 0.06 | 0.12 | ≤0.015 | 0.5 | 100 | 0 | 0 | 100 | 90.9 | 0 | 9.1 | |
| cefuroximeb,d | all | 23 | 1 | 4 | 0.25 | 4 | 100 | 0 | 0 | 82.6 | 0 | 82.6 | 17.4 |
| BL− | 22 | 1 | 2 | 0.25 | 4 | 100 | 0 | 0 | 86.4 | 0 | 86.4 | 13.6 | |
| ciprofloxacin | all | 23 | 0.015 | 0.03 | 0.004 | 32 | 95.7 | 0 | 4.3 | 95.7 | 95.7 | 0 | 4.3 |
| BL− | 22 | 0.015 | 0.03 | 0.004 | 32 | 95.5 | 0 | 4.6 | 95.5 | 95.5 | 0 | 4.6 | |
| clarithromycinc | all | 23 | 8 | 16 | 2 | 32 | 95.7 | 4.3 | 0 | NA | NA | NA | NA |
| BL− | 22 | 8 | 16 | 2 | 32 | 95.5 | 4.6 | 0 | NA | NA | NA | NA | |
| levofloxacin | all | 23 | 0.015 | 0.015 | 0.008 | 32 | 95.7 | 0 | 4.3 | 95.7 | 95.7 | 0 | 4.3 |
| BL− | 22 | 0.015 | 0.015 | 0.008 | 32 | 95.5 | 0 | 4.6 | 95.5 | 95.5 | 0 | 4.6 | |
| ofloxacin | all | 23 | 0.03 | 0.06 | 0.015 | 32 | 95.7 | 0 | 4.3 | NA | 95.7 | 0. | 4.3 |
| BL− | 22 | 0.03 | 0.06 | 0.015 | 32 | 95.5 | 0 | 4.6 | NA | 95.5 | 0 | 4.6 | |
| SXT | all | 23 | 32 | 32 | 0.12 | 32 | 8.7 | 4.3 | 87.0 | 8.7 | 8.7 | 4.3 | 87.0 |
| BL− | 22 | 32 | 32 | 0.12 | 32 | 9.1 | 4.6 | 86.4 | 9.1 | 9.1 | 4.6 | 86.4 | |
S, susceptible; I, intermediate; R, resistant; AMC, amoxicillin/clavulanic acid; BL−, β-lactamase negative; NA, no breakpoint data available (NA for azithromycin and clarithromycin by PK/PD and EUCAST because Etest® breakpoints in CO2 not available); SXT, trimethoprim/sulfamethoxazole.
aAmoxicillin/clavulanic acid PK/PD susceptibility at high dose shown in parentheses.
bIn the clinical setting, BLNAR isolates are considered resistant to amoxicillin/clavulanic acid, cefaclor and cefuroxime (see main text).
cbioMérieux Etest® breakpoints for incubation in CO2.
dBreakpoints used are for cefuroxime axetil.
eClinical susceptibility to amoxicillin/clavulanic acid, cefaclor and cefuroxime reduced (data in parentheses) due to corrections according to BLNAR (see main text).
Table 5.
Distribution of H. influenzae MICs
| Number of isolates at MIC (mg/L) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country/antimicrobial | n | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >32 | >256 |
| Democratic Republic of Congo | ||||||||||||||||||
| AMC | 23 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 4 | 4 | 3 | 1 | 2 | 2 | 2 | 0 | 0 |
| ampicillin | 23 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 4 | 6 | 4 | 2 | 1 | 0 | 1 | 2 | 0 | 1 |
| azithromycin | 23 | 0 | 0 | 0 | 0 | 3 | 4 | 4 | 2 | 2 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 1 |
| cefaclor | 23 | 0 | 0 | 0 | 0 | 4 | 2 | 2 | 6 | 3 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 0 |
| cefixime | 23 | 0 | 0 | 1 | 0 | 6 | 0 | 0 | 4 | 6 | 2 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| cefuroxime | 23 | 0 | 0 | 0 | 0 | 3 | 2 | 1 | 5 | 3 | 4 | 2 | 2 | 0 | 1 | 0 | 0 | 0 |
| ciprofloxacin | 23 | 1 | 2 | 0 | 4 | 1 | 0 | 2 | 6 | 3 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 23 | 0 | 0 | 0 | 0 | 4 | 3 | 1 | 5 | 4 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 1 |
| SXT | 23 | 2 | 1 | 0 | 0 | 0 | 3 | 0 | 1 | 7 | 2 | 2 | 1 | 1 | 0 | 0 | 3 | 0 |
| n | 0.004 | 0.008 | ≤0.015 | 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 256 | |
| Senegal | ||||||||||||||||||
| AMC | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 10 | 6 | 2 | 1 | 0 | 0 | 0 | 0 | 0 |
| ampicillin | 23 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 8 | 6 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefaclor | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 9 | 8 | 2 | 2 | 0 | 1 | 0 |
| cefixime | 23 | 0 | 0 | 1 | 0 | 7 | 8 | 5 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefuroxime | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 14 | 1 | 3 | 0 | 0 | 0 | 0 | 0 |
| ciprofloxacin | 23 | 2 | 4 | 0 | 11 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| clarithromycin | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 7 | 9 | 2 | 1 | 0 | 0 |
| levofloxacin | 23 | 0 | 7 | 0 | 14 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| ofloxacin | 23 | 0 | 0 | 0 | 4 | 10 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| SXT | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 19 | 0 | 0 |
AMC, amoxicillin/clavulanic acid; SXT, trimethoprim/sulfamethoxazole.
Discussion
There is a paucity of antimicrobial surveillance data for Africa in peer-reviewed journals, especially in the central region, with the limited available data showing marked variation between different geographical regions within Africa as well as an increasing trend in resistance over time.5,6 These findings underscore the importance of monitoring susceptibility trends at the country or local level, and incorporating the most recent susceptibility patterns into empirical therapy decisions. This study provides country-specific antimicrobial surveillance data for several countries across West, Central and East Africa for the period 2011–14. Sample sizes were generally limited, especially for isolates from Senegal, but it has recently been highlighted that increased surveillance is important in Africa even if only small size samples are available.6
Previous data from SOAR for Africa in 2007–09 showed variable penicillin susceptibility in S. pneumoniae from Nigeria (26.6%), Kenya (33.3%), Senegal (66%) and the Ivory Coast (89%).11,12 Very similar data for penicillin susceptibility were observed in 2004–06 for the Ivory Coast and Senegal (data were not reported for Nigeria and Kenya).13 The current study has seen penicillin susceptibility reduced in the Ivory Coast (to 70%) and reduced even further in Kenya (19%). The current level of penicillin susceptibility in Senegal would appear to have increased in 2011–14 compared with earlier time periods, but this may simply be an artefact due to the small sample size in the current study. In SOAR 2004–06 and 2007–09, macrolide susceptibility was high, at ≥85%, in all African countries.11–13 This was also true for the current time period, 2011–14, with the exception of Kenya, where 64.1% erythromycin and 65.6% azithromycin susceptibility was observed. In the current study β-lactamase prevalence in H. influenzae from Senegal was low (4%) but the previous SOAR report in 2007–09 indicated higher levels, at 27%.12 As mentioned above, this may relate to the small sample size.
Several antimicrobial agents were found to have strong activity against respiratory pathogens across most of the African countries included in this study. Using CLSI breakpoints, both organisms in this study showed >90% susceptibility to amoxicillin/clavulanic acid, except for H. influenzae in Democratic Republic of Congo (73.9% susceptible based on CLSI breakpoints). Cefuroxime demonstrated strong activity against both species, except S. pneumoniae in Democratic Republic of Congo and Kenya. Both species generally demonstrated susceptibility to macrolides (∼83% or higher), except for S. pneumoniae in Kenya, where all tested macrolides showed susceptibility <70%. In contrast to these generally very active antimicrobials, several agents that are widely used in Africa showed high resistance rates. Susceptibility to trimethoprim/sulfamethoxazole did not exceed 75% in any country against either species, with susceptibility as low as 14.3% and 8.7% for S. pneumoniae and H. influenzae, respectively, in Senegal (albeit with small sample sizes). Similarly, chloramphenicol showed low activity in Kenya (the only country where the agent was tested) against S. pneumoniae (68.7% susceptible). S. pneumoniae penicillin susceptibility was only 17.4% in Democratic Republic of Congo, 19% in Kenya, 70% in Ivory Coast and 85.7% in Senegal based on CLSI penicillin oral breakpoints. These results are especially important because PISP and PRSP isolates were usually multiply resistant.
The data from this study confirm that isolates of S. pneumoniae susceptible to penicillin G are also susceptible to other penicillins as inferred by CLSI and EUCAST guidelines and cephalosporins as inferred by CLSI guidelines. Interestingly, the data from this study found the reverse was not always true using CLSI breakpoints; i.e. all penicillin-non-susceptible S. pneumoniae were susceptible to amoxicillin/clavulanic acid (amoxicillin) and most were also susceptible to ceftriaxone. Therefore, either the amoxicillin/clavulanic acid or ceftriaxone breakpoints are not correct or the CLSI cross-resistance statement within the β-lactam class is not correct. This warrants further investigation.
Comparing our results with findings in the literature is limited not only because of paucity of data from Africa, but also because different studies have reported data from different countries. For example, two reports using data from the Tigecycline Evaluation and Surveillance Trial (T.E.S.T) included mainly North African countries and South Africa.3,4 Comparing the current study with studies that focused exclusively or predominantly on sub-Saharan Africa, we saw several similar patterns. S. pneumoniae and H. influenzae have been shown to have low susceptibility to trimethoprim/sulfamethoxazole, either in all African regions or in West Africa, with resistance increasing for both organisms.5,6 Trimethoprim/sulfamethoxazole is commonly used in Africa for a broad range of bacterial infections as well as for routine prophylaxis in HIV patients, yet current susceptibility levels in our study, as well as findings in the literature, make its effectiveness against respiratory pathogens doubtful. Similarly, chloramphenicol, another inexpensive, widely used antibiotic, was shown to have low activity against H. influenzae.5,6 Our data agree with the recommendation to use this agent in sub-Saharan Africa only if appropriate susceptibility results are available.6 Our study showed low tetracycline activity against S. pneumoniae in Kenya (the only country that tested this agent), similar to findings in a meta-analysis of three decades of published studies.5 In contrast, a recent systematic review found tetracycline resistance to be high in West Africa, but less so in East Africa.6 Interestingly, tetracycline resistance may be due in part to environmental contamination with this agent, as it is widely used in agriculture.5 Similar to a review of macrolide susceptibility in sub-Saharan Africa,14 the current study found generally good activity of macrolides against S. pneumoniae, with the notable exception of isolates from Kenya. For H. influenzae, on the other hand, the current study found higher macrolide activity compared with ∼70% susceptibility reported in the macrolide review,14 although sample sizes were small in both studies. Interestingly, in the macrolide review, isolates from younger children tended to be more susceptible,14 while in the current study macrolide susceptibility estimates were very similar in children and adults.
Comparisons between studies are often difficult because different breakpoints are used or the MIC interpretive criteria are not even reported.6 This is especially pertinent for S. pneumoniae. Not only do the penicillin breakpoints for S. pneumoniae differ between CLSI and EUCAST, but the CLSI guidelines themselves include three separate breakpoints for oral, parenteral (non-meningitis) and parenteral (meningitis) penicillin, with studies frequently not specifying which were used. Different breakpoints can result in dramatically diverging susceptibility estimates, a problem clearly illustrated in the current study by widely varying susceptibility results for cefaclor, ofloxacin and cefuroxime based on CLSI, PK/PD and EUCAST breakpoints. For assessing comparability between studies it is crucial that future studies clearly identify which breakpoints were used. Furthermore, MIC interpretive criteria should be better aligned, avoiding different breakpoints altogether and thus avoiding confusion among healthcare providers making therapy decisions and among microbiologists trying to compare results from different geographical regions and time periods.
The main limitations of this study stem from small sample sizes and the attempt to draw conclusions about antimicrobial susceptibility levels in the country using data from mainly one hospital site. However, despite these limitations, patterns emerged that can be useful for clinicians in selecting empirical therapy for CAP. Some of these patterns were not unexpected considering other publications, such as the high resistance levels to trimethoprim/sulfamethoxazole found across all four participating countries. Others were surprising, such as the high macrolide resistance of S. pneumoniae isolates from Kenya. These results highlight the importance of ensuring access to antimicrobial agents beyond the inexpensive first-level drugs that are widely used in Africa, as well as the need for continued monitoring of antimicrobial resistance at the country and even the local level.
Funding
This work was funded by GlaxoSmithKline.
Transparency declarations
This article forms part of a Supplement sponsored by GlaxoSmithKline. D. Torumkuney, W. Mwiti and M. J. Anguibi-Pokou are employees of GlaxoSmithKline. D. Torumkuney and W. Mwiti also hold shares in GlaxoSmithKline. I. Morrissey is an employee of IHMA, a medical communication and consultancy company, who participated in the exploration, interpretation of the results and preparation of this manuscript on behalf of GSK. IHMA also provided medical writing support in the form of writing assistance, collating author's comments, grammatical editing and referencing that was paid for by GSK. All other authors declare that they have no conflict of interest.
Editorial assistance was provided by Tracey Morris, Livewire Editorial Communications.
Acknowledgements
We would like to thank Nathalie Guessennd (Institute Pasteur, Ivory Coast), Mireille Dosso (Institute Pasteur, Ivory Coast), Cheikh Boye (Institute Pasteur, Senegal) and Jean Jacques Muyembe Tamfun (INRB, Democratic Republic of Congo) for their participation in the study. We would also like to thank Dr Keith Barker (GSK) for reviewing the manuscript.
References
- 1.World Health Organization. Word Health 2014. Part III. Global health indicators http://www.who.int/gho/publications/world_health_statistics/EN_WHS2014_Part3.pdf?ua=1.
- 2.Kariuki S, Dougan G. Antibacterial resistance in sub-Saharan Africa: an underestimated emergency. Ann N Y Acad Sci 2014; 1323: 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandon M, Dowzicky MJ. Antimicrobial susceptibility among Gram-positive organisms collected from pediatric patients globally between 2004 and 2011: results from the Tigecycline Evaluation and Surveillance Trial. J Clin Microbiol 2013; 51: 2371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomic V, Dowzicky MJ. Regional and global antimicrobial susceptibility among isolates of Streptococcus pneumoniae and Haemophilus influenzae collected as part of the Tigecycline Evaluation and Surveillance Trial (T.E.S.T.) from 2009 to 2012 and comparison with previous years of T.E.S.T. (2004–2008). Ann Clin Microbiol Antimicrob 2014; 13: 52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginsburg AS, Tinkham L, Riley K et al. Antibiotic non-susceptibility among Streptococcus pneumoniae and Haemophilus influenzae isolates identified in African cohorts: a meta-analysis of three decades of published studies. Int J Antimicrob Agents 2013; 42: 482–91. [DOI] [PubMed] [Google Scholar]
- 6.Leopold SJ, van Leth F, Tarekegn H et al. Antimicrobial drug resistance among clinically relevant bacterial isolates in sub-Saharan Africa: a systematic review. J Antimicrob Chemother 2014; 69: 2337–53. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests—Eleventh Edition: Approved Standard M02-A11. CLSI, Wayne, PA, USA, 2012. [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fourth Informational Supplement M100-S24. CLSI, Wayne, PA, USA, 2014. [Google Scholar]
- 9.The European Committee on Antimicrobial Susceptibility testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 4.0, 2014 http://www.eucast.org.
- 10.Anon JB, Jacobs MR, Poole MD et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg 2004; 130 Suppl 1: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iregbu KC, Sonibare SA, Nwoked E et al. Antibacterial resistance among Streptococcus pneumoniae from three centres in Nigeria: results from the Survey of Antibiotic Resistance (SOAR) 2007–2009. In: Abstracts of the 26th Paediatric Association Congress of Paediatrics, Johannesburg, South Africa, 2010. Abstract 1434. [Google Scholar]
- 12.Torumkuney O'Brien D, SOAR Study group. Antibacterial resistance among Streptococcus pneumoniae, Haemophilus influenzae and Streptococcus pyogenes from 9 countries in Africa and the Middle East: results from the Survey of Antibiotic Resistance (SOAR) 2007–2009. In: Abstracts of the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, USA, 2009. Abstract C2–1402, p. 118. American Society for Microbiology, Washington, DC, USA. (Poster/Data on file GSK-2014N216846_00, 2014). [Google Scholar]
- 13.Sievers J, SOAR Study Group. Antibacterial resistance among Streptococcus pneumoniae from 8 countries in Africa and the Middle East: results from the Survey of Antibiotic Resistance (SOAR) 2004–2006. In: Abstracts of the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, USA, 2006. Abstract C2-0425. American Society for Microbiology, Washington, DC, USA. [Google Scholar]
- 14.Lubell Y, Turner P, Ashley EA et al. Susceptibility of bacterial isolates from community-acquired infections in sub-Saharan Africa and Asia to macrolide antibiotics. Trop Med Int Health 2011; 16: 1192–205. [DOI] [PubMed] [Google Scholar]


