Abstract
Objectives
To provide susceptibility data for community-acquired respiratory tract isolates of Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae and Moraxella catarrhalis collected in 2012–14 from four Asian countries.
Methods
MICs were determined using Etest® for all antibiotics except erythromycin, which was evaluated by disc diffusion. Susceptibility was assessed using CLSI, EUCAST and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints. For macrolide/clindamycin interpretation, breakpoints were adjusted for incubation in CO2 where available.
Results
Susceptibility of S. pneumoniae was generally lower in South Korea than in other countries. Penicillin susceptibility assessed using CLSI oral or EUCAST breakpoints ranged from 21.2% in South Korea to 63.8% in Singapore. In contrast, susceptibility using CLSI intravenous breakpoints was much higher, at 79% in South Korea and ∼95% or higher elsewhere. Macrolide susceptibility was ∼20% in South Korea and ∼50%–60% elsewhere. Among S. pyogenes isolates (India only), erythromycin susceptibility (∼20%) was lowest of the antibiotics tested. In H. influenzae antibiotic susceptibility was high except for ampicillin, where susceptibility ranged from 16.7% in South Korea to 91.1% in India. South Korea also had a high percentage (18.1%) of β-lactamase-negative ampicillin-resistant isolates. Amoxicillin/clavulanic acid susceptibility for each pathogen (PK/PD high dose) was between 93% and 100% in all countries except for H. influenzae in South Korea (62.5%).
Conclusions
Use of EUCAST versus CLSI breakpoints had profound differences for cefaclor, cefuroxime and ofloxacin, with EUCAST showing lower susceptibility. There was considerable variability in susceptibility among countries in the same region. Thus, continued surveillance is necessary to track future changes in antibiotic resistance.
Introduction
Community-acquired pneumonia (CAP) remains a significant health problem. Acute respiratory infections are the cause of death in 15% of children under the age of 5 years globally and in 3%–12% in the four countries (India, Singapore, South Korea and Thailand) considered here.1 CAP is also a clinical and economic burden in the expanding ageing population.2 Resistance to antimicrobial agents is a worldwide phenomenon and their use is the main driver of the emergence of resistance.3–5 Although some have not observed a correlation between mortality due to CAP and antimicrobial resistance,6 including patients in Asia,7 others found that penicillin resistance was related to increased mortality in hospitalized patients with pneumococcal pneumonia8 and that macrolide resistance in respiratory pathogens was related to treatment failures in children.9 An Asian Network for Surveillance of Resistant Pathogens (ANSORP) study also found an association between mortality and levofloxacin resistance with invasive pneumococcal disease.10
In Asia antibiotic resistance in CAP is generally high, as demonstrated by data from the ANSORP 2008–09 and the Community-Acquired Respiratory Tract Infection Pathogen Surveillance (CARTIPS) study (2009–10).10,11 Korea also showed high multidrug resistance rates in S. pneumoniae, particularly those strains non-susceptible to levofloxacin.12 Data from India are limited, but another study showed slightly lower non-susceptibility to penicillin in S. pneumoniae than that observed in ANSORP,11 at 14%, but with similar susceptibility to erythromycin.13 In Korea ampicillin resistance was high (59%) in Haemophilus influenzae, as was resistance to clarithromycin (19%) and amoxicillin/clavulanic acid (10%).14
CAP is usually treated empirically, without identification and susceptibility testing of the causative agent, and knowledge of local resistance patterns is therefore especially important when treating this disease. Additionally, surveillance data can provide useful information to assist governments in controlling antimicrobial use and emergence of resistance.
The Survey of Antibiotic Resistance (SOAR) is an ongoing surveillance study of key respiratory pathogens. SOAR has been monitoring antimicrobial resistance in the Middle East, Africa, Latin America, Asia-Pacific and the Commonwealth of Independent States countries since 2002. We present an analysis of recent data from four Asian countries to provide a picture of the current antimicrobial susceptibility situation in four community-acquired respiratory tract infection (CA-RTI) pathogens, Streptococcus pneumoniae, Streptococcus pyogenes, H. influenzae and Moraxella catarrhalis.
Materials and methods
Collaborating centres
Isolates were collected from eight sites in South Korea (Daejoen St Mary's Hospital; Ilsan Hospital; Incheon St Mary's Hospital; Myongji Hospital; Seoul St Mary's Hospital; Severance Hospital; Uijeongbu St Mary's Hospital; and St Vincent's Hospital), four sites in India (Choithram Hospital, Indore; Christian Medical College & Hospital; Vellore, P.D. Hinduja National Hospital & Medical Research Center; and St John's Medical College), four sites in Thailand (Maharaj Nakorn Chiang Mai Hospital; Hat-Yai Hospital; Ramathibodi Hospital; and Srinagarind Hospital) and one site in Singapore (Changi General Hospital).
Clinical isolates
During 2012–14, a total of 1326 clinical respiratory isolates (520 from Thailand, 493 from India, 175 from South Korea and 138 from Singapore), comprising 570 isolates of S. pneumoniae, 78 isolates of S. pyogenes (India only), 148 isolates of M. catarrhalis and 530 isolates of H. influenzae, were analysed.
All patients were from the community (hospitals and university hospitals). Paediatric patients (≤12 years old) accounted for 220 isolates (16.6%), adult patients (13–64 years old) for 696 (52.5%) and the elderly (≥65 years) for 410 isolates (30.9%). All of the S. pyogenes isolates were from throat swabs and the other pathogens were obtained from a variety of specimen types, including blood, bronchoalveolar lavage, middle ear effusion, nasopharyngeal aspirate, pleural fluid, sinus, sputum and tracheal aspirate. Organisms were identified using conventional methods (optochin susceptibility/bile solubility for S. pneumoniae, X and V factor requirement for H. influenzae, bacitracin susceptibility for S. pyogenes and tributyrin test for M. catarrhalis). Duplicate isolates from the same patient were excluded.
Susceptibility testing
MICs were determined by gradient strip (Etest®) according to the manufacturer's instructions (bioMérieux, Marcy l'Étoile, France), including: amoxicillin/clavulanic acid, azithromycin, cefaclor, cefixime, cefotaxime, cefpodoxime, cefuroxime, clarithromycin, clindamycin, erythromycin, levofloxacin, moxifloxacin, ofloxacin, penicillin and trimethoprim/sulfamethoxazole against S. pneumoniae and amoxicillin/clavulanic acid, azithromycin, cefixime, cefpodoxime, cefuroxime, clarithromycin, erythromycin, levofloxacin, ofloxacin, penicillin and trimethoprim/sulfamethoxazole against S. pyogenes (India only). In addition, for these two organisms erythromycin was tested by CLSI disc diffusion methodology.15 For H. influenzae and M. catarrhalis, amoxicillin/clavulanic acid, ampicillin (H. influenzae only), azithromycin, cefaclor, cefixime, cefpodoxime, cefuroxime, ciprofloxacin, clarithromycin, levofloxacin, moxifloxacin, ofloxacin (H. influenzae only) and trimethoprim/sulfamethoxazole (H. influenzae only) were tested. Although not all antimicrobial agents were tested in each country, there was substantial overlap among countries in the panel of antimicrobial agents tested. The particular agents tested in each country can be found in the respective summary tables of MIC data (Tables 1–9). Quality control strains for MIC determination included S. pneumoniae ATCC 49619, H. influenzae ATCC 49247, H. influenzae ATCC 49766 and Escherichia coli ATCC 32518, which were tested concurrently with the clinical isolates. Acceptable MIC ranges were those of CLSI16 or those provided by the Etest® manufacturer (bioMérieux, Marcy l'Étoile, France) for adjustment in CO2. In H. influenzae and M. catarrhalis, production of β-lactamase was assessed using a nitrocefin disc according to the manufacturer's instructions (BD Diagnostics, Sparks, MD, USA), with E. coli ATCC 35218 and H. influenzae ATCC 49247 as the positive and negative controls, respectively.
Table 1.
MIC breakpoints (mg/L) used for S. pneumoniae, S. pyogenes, H. influenzae and M. catarrhalis isolates
|
S. pneumoniae |
S. pyogenes |
H. influenzae |
M. catarrhalis |
All species |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLSI |
EUCAST |
CLSI |
EUCAST |
CLSI |
EUCAST |
CLSI |
EUCAST |
PK/PD | |||||||||||||||||
| Antimicrobial | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | (S only) |
| AMCa | ≤2 | 4 | ≥8 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ≤4 | — | ≥8 | ≤2 | — | ≥4 | ≤4 | — | ≥8 | ≤1 | — | ≥2 | ≤2 (≤4) |
| Ampicillin | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | ≤1 | 2 | ≥4 | ≤1 | — | ≥2 | NT | NT | NT | NT | NT | NT | NA |
| Azithromycinb | ≤4 | 8 | ≥16 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ≤8 | — | —b | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Cefaclor | ≤1 | 2 | ≥4 | ≤0.03 | 0.06–0.5 | ≥1 | NT | NT | NT | NT | NT | NT | ≤8 | 16 | ≥32 | NA | NA | NA | ≤8 | 16 | ≥32 | NA | NA | NA | ≤0.5 |
| Cefixime | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ≤1 | — | — | ≤0.12 | — | ≥0.25 | NA | NA | NA | ≤0.5 | 1 | ≥2 | ≤1 |
| Cefotaxime | ≤1 | 2 | ≥4 | ≤0.5 | 1–2 | ≥4 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NA |
| Cefpodoxime | ≤0.5 | 1 | ≥2 | ≤0.25 | 0.5 | ≥1 | NA | NA | NA | NA | NA | NA | ≤2 | — | — | ≤0.25 | 0.5 | ≥0.1 | NA | NA | NA | NA | NA | NA | ≤0.5 |
| Cefuroximec | ≤1 | 2 | ≥4 | ≤0.25 | 0.5 | ≥1 | NA | NA | NA | NA | NA | NA | ≤4 | 8 | ≥16 | ≤0.12 | 0.25–1 | ≥2 | ≤4 | 8 | ≥16 | ≤0.12 | 0.25–4 | ≥8 | ≤1 |
| Ciprofloxacin | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | ≤1 | — | — | ≤0.5 | — | ≥1 | ≤1 | — | — | ≤0.5 | — | ≥1 | ≤1 |
| Clarithromycinb | ≤0.5 | 1 | ≥2 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ≤16 | 32 | ≥64 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Clindamycinb | ≤0.5 | 1 | ≥2 | NA | NA | NA | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NA |
| Erythromycinb | ≤1 | 2 | ≥4 | ≤1 | 2 | ≥4 | ≤1 | 2 | ≥4 | NA | NA | NA | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NA |
| Levofloxacin | ≤2 | 4 | ≥8 | ≤2 | — | ≥4 | ≤2 | 4 | ≥8 | ≤1 | 2 | ≥4 | ≤2 | — | — | ≤1 | — | ≥2 | ≤2 | — | — | ≤1 | — | ≥2 | ≤2 |
| Moxifloxacin | ≤1 | 2 | ≥4 | ≤0.5 | — | ≥1 | NT | NT | NT | NT | NT | NT | ≤1 | — | — | ≤0.5 | — | ≥1 | NA | NA | NA | ≤0.5 | — | ≥1 | ≤1 |
| Ofloxacin | ≤2 | 4 | ≥8 | ≤0.12 | 0.25–4 | ≥8 | ≤2 | 4 | ≥8 | NA | NA | NA | ≤2 | — | — | ≤0.5 | — | ≥1 | NT | NT | NT | NT | NT | NT | NA |
| Penicillin (oral) | ≤0.06 | 0.12–1 | ≥2 | ≤0.06 | 0.12–2 | ≥4 | ≤0.12 | — | — | ≤0.25 | — | ≥0.5 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NA |
| Penicillin (iv)d | ≤2 | 4 | ≥8 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NA |
| SXTe | ≤0.5 | 1–2 | ≥4 | 2 | ≥4 | NA | NA | ≤1 | 2 | ≥4 | ≤0.5 | 1–2 | ≥4 | ≤0.5 | 1 | ≥2 | NT | NT | NT | NT | NT | NT | ≤0.5 | ||
AMC, amoxicillin/clavulanic acid; S, susceptible; I, intermediate; R, resistant; NA, not applicable; NT, not tested; SXT, trimethoprim/sulfamethoxazole.
aThis agent was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoint based on high-dose (4 g of amoxicillin with 250 mg of clavulanic acid per day for adults) is shown in parentheses.
bbioMérieux Etest® breakpoints for incubation in CO2.
cBreakpoints used are for cefuroxime axetil.
dParenteral non-meningitis breakpoints. EUCAST does not indicate iv breakpoints but dose-specific susceptible breakpoints are noted for pneumonia: 1.2 g × 4 (MIC ≤0.5 mg/L = susceptible), 1.2 g × 6 or 2.4 g × 4 (MIC ≤1 mg/L = susceptible) and 2.4 g × 6 (MIC ≤2 mg/L = susceptible).
eTrimethoprim/sulfamethoxazole was tested at a 1:19 ratio.
Table 9.
Distribution of M. catarrhalis MICs by country
| Number of isolates at MIC (mg/L) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | na | ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >32 | 64 | >256 |
| India | ||||||||||||||||
| AMC | 61 | 0 | 4 | 5 | 18 | 7 | 7 | 8 | 8 | 3 | 0 | 1 | 0 | 0 | 0 | 0 |
| azithromycin | 61 | 4 | 3 | 0 | 9 | 11 | 9 | 7 | 7 | 4 | 3 | 1 | 0 | 0 | 0 | 3 |
| cefixime | 37 | 0 | 0 | 5 | 6 | 7 | 18 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefpodoxime | 61 | 0 | 0 | 0 | 4 | 8 | 16 | 15 | 14 | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| cefuroxime | 59 | 0 | 0 | 0 | 0 | 1 | 2 | 11 | 24 | 10 | 4 | 5 | 1 | 0 | 1 | 0 |
| ciprofloxacin | 61 | 1 | 0 | 9 | 1 | 1 | 9 | 20 | 9 | 0 | 7 | 1 | 0 | 3 | 0 | 0 |
| clarithromycin | 49 | 2 | 1 | 1 | 0 | 7 | 21 | 4 | 0 | 5 | 4 | 3 | 0 | 0 | 1 | 0 |
| levofloxacin | 61 | 0 | 2 | 8 | 2 | 1 | 5 | 17 | 14 | 1 | 7 | 1 | 0 | 3 | 0 | 0 |
| South Korea | ||||||||||||||||
| AMC | 18 | 2 | 3 | 1 | 3 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 4 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| cefaclor | 18 | 1 | 0 | 1 | 0 | 2 | 5 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefuroxime | 18 | 0 | 1 | 0 | 0 | 3 | 1 | 4 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ciprofloxacin | 18 | 0 | 4 | 12 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 18 | 0 | 0 | 0 | 1 | 5 | 9 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| levofloxacin | 18 | 0 | 15 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Singapore | ||||||||||||||||
| AMC | 15 | 1 | 3 | 4 | 1 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 20 | 0 | 0 | 0 | 13 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefaclor | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 14 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefuroxime | 14 | 0 | 0 | 0 | 1 | 0 | 1 | 6 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| ciprofloxacin | 20 | 0 | 4 | 15 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 20 | 0 | 1 | 0 | 6 | 11 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| levofloxacin | 20 | 0 | 2 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| moxifloxacin | 20 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thailand | ||||||||||||||||
| AMC | 49 | 0 | 2 | 3 | 10 | 28 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 49 | 4 | 7 | 4 | 5 | 9 | 15 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| cefuroxime | 49 | 0 | 0 | 1 | 0 | 2 | 6 | 22 | 17 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 49 | 0 | 5 | 4 | 13 | 16 | 6 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| levofloxacin | 49 | 0 | 3 | 40 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
AMC, amoxicillin/clavulanic acid.
aNot all isolates were tested.
The principal MIC susceptibility criteria utilized were those of CLSI,16 with the exception of the macrolides and clindamycin, for which bioMérieux Etest® breakpoints for incubation at elevated CO2 tension were used. In addition, susceptibility based on the EUCAST criteria17 and on pharmacokinetic/pharmacodynamic (PK/PD) breakpoints18 was assessed. These additional breakpoints were not evaluated for macrolides (except erythromycin MIC by EUCAST) because susceptibility criteria for Etest® incubation in elevated CO2 are available only relative to the CLSI breakpoints. Both CLSI and EUCAST disc diffusion criteria are available for erythromycin. The breakpoints for all three methods of evaluation are shown in Table 1.
Statistical analysis
Differences in susceptibility between countries and age groups were assessed with Fisher's exact test using XLSTAT version 2011.1.05. A P value <0.05 was considered statistically significant. Between-country comparisons were made for species where antibiotics were tested by at least three countries against at least 20 isolates per country.
Results
Sources of isolates from all sites combined
Of the 570 S. pneumoniae isolates, 345 were from sputum (60.5%), 125 from blood (21.9%), 57 from tracheal aspirate (10%) and the remainder from bronchoalveolar lavage, bronchial aspirate, sinus, middle ear effusion, nasopharyngeal aspirate or pleural fluid. Most isolates were from adults (n = 303, 53.2%) with 191 isolates from elderly (33.5%) and 76 from paediatric patients (13.3%).
All 78 S. pyogenes isolates were from throat swabs and all were collected in India. The majority were from paediatric patients (n = 46, 59.0%); 32 isolates (41.0%) were from adult patients and there were none from elderly patients.
The majority of the 530 H. influenzae isolates came from sputum (n = 386, 72.8%); 38 isolates were from sinus or tracheal aspirates (7.2% each), 37 (7.0%) were from bronchoalveolar lavage and 21 (4.0%) were from blood. The remainder were from middle ear effusions, nasopharyngeal aspirate or bronchial aspirate. Adults accounted for 290 isolates (54.7%), elderly patients for 166 isolates (31.3%) and paediatric patients for 74 isolates (14.0%).
For M. catarrhalis, the vast majority of the 148 isolates were from sputum (n = 111, 75.0%), with 17 isolates from tracheal aspirate (11.5%) and the remainder from sinus, bronchoalveolar lavage, nasopharyngeal aspirate and blood. Adults accounted for 71 isolates (48.0%), elderly patients for 53 isolates (35.8%) and paediatric patients for 24 isolates (16.2%). All isolates from Singapore and Thailand were β-lactamase positive, as were 83.3% and 72.1% from South Korea and India, respectively.
S. pneumoniae susceptibility in individual countries
Summary MIC and susceptibility data for S. pneumoniae isolates for each individual country are shown in Table 2 and MIC distribution data are shown in Table 3.
Table 2.
MIC and susceptibility results for S. pneumoniae isolates by country
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | na | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| India | ||||||||||||
| AMCb,c | 219 | 0.06 | 2 | ≤0.015 | >256 | 91.8 | 2.7 | 5.5 | 91.8 (94.5) | NA | NA | NA |
| azithromycind | 199 | 1 | >256 | ≤0.015 | >256 | 66.3 | 13.6 | 20.1 | NA | NA | NA | NA |
| cefixime | 219 | 2 | 32 | ≤0.015 | >256 | NA | NA | NA | 49.8 | NA | NA | NA |
| cefpodoxime | 219 | 0.25 | 4 | ≤0.015 | >256 | 67.1 | 9.1 | 23.7 | 67.1 | 55.3 | 11.9 | 32.9 |
| cefuroximee | 218 | 0.25 | 4 | ≤0.015 | >256 | 75.2 | 8.7 | 16.1 | 75.2 | 51.4 | 13.8 | 34.9 |
| clarithromycind | 199 | 0.25 | >256 | ≤0.015 | >256 | 54.8 | 2.0 | 43.2 | NA | NA | NA | NA |
| erythromycind | 183 | 0.25 | >256 | ≤0.015 | >256 | 57.4 | 8.2 | 34.4 | NA | 57.4 | 8.2 | 34.4 |
| levofloxacin | 219 | 1 | >32 | 0.12 | >32 | 85.8 | 3.2 | 11.0 | 85.8 | 85.8 | 0.0 | 14.2 |
| ofloxacin | 219 | 2 | >32 | 0.5 | >32 | 77.2 | 9.1 | 13.7 | NA | 0.0 | 86.3 | 13.7 |
| penicillin (oral) | 219 | 0.12 | 2 | ≤0.015 | >32 | 49.3 | 33.8 | 16.9 | NA | 49.3 | 46.1 | 4.6 |
| penicillin (iv) | 219 | 0.12 | 2 | ≤0.015 | >32 | 95.4 | 2.7 | 1.8 | NA | 73.5–95.4 | NA | NA |
| SXT | 219 | 1 | 16 | ≤0.015 | >32 | 32.9 | 39.3 | 27.9 | 32.9 | 55.3 | 16.9 | 27.8 |
| erythromycinf | 218 | — | — | — | — | 49.1 | 12.8 | 38.1 | — | 49.1 | 4.6 | 46.3 |
| South Korea | ||||||||||||
| AMCb,c | 85 | 1 | 4 | ≤0.015 | 8 | 81.2 | 16.5 | 2.4 | 81.2 (97.6) | NA | NA | NA |
| azithromycind | 85 | >256 | >256 | 0.12 | >256 | 20.0 | 1.2 | 78.8 | NA | NA | NA | NA |
| cefaclor | 85 | 32 | >256 | 0.12 | >256 | 24.7 | 3.5 | 71.8 | 23.5 | 0.0 | 23.5 | 76.5 |
| cefotaxime | 85 | 0.5 | 2 | ≤0.015 | 32 | 85.9 | 5.9 | 8.2 | NA | 50.6 | 41.2 | 8.2 |
| cefuroximee | 85 | 2 | 8 | ≤0.015 | >256 | 36.5 | 18.8 | 44.7 | 36.5 | 29.4 | 0.0 | 70.6 |
| clarithromycind | 85 | >256 | >256 | 0.06 | >256 | 18.8 | 0.0 | 81.2 | NA | NA | NA | NA |
| clindamycind | 85 | >256 | >256 | ≤0.015 | >256 | 31.8 | 1.2 | 67.1 | NA | NA | NA | NA |
| levofloxacin | 85 | 1 | 2 | 0.25 | >32 | 91.8 | 0.0 | 8.2 | 91.8 | 91.8 | 0.0 | 8.2 |
| ofloxacin | 85 | 2 | 4 | 1 | >32 | 75.3 | 16.5 | 8.2 | NA | 0.0 | 91.8 | 8.2 |
| penicillin (oral) | 85 | 1 | 4 | ≤0.015 | 16 | 21.2 | 35.3 | 43.5 | NA | 21.2 | 57.6 | 21.2 |
| penicillin (iv) | 85 | 1 | 4 | ≤0.015 | 16 | 78.8 | 17.7 | 3.5 | NA | 37.6–78.8 | NA | NA |
| SXT | 85 | 1 | >32 | 0.12 | >32 | 43.5 | 11.8 | 44.7 | 43.5 | 54.1 | 1.2 | 44.7 |
| erythromycinf | 85 | — | — | — | — | 18.8 | 0.0 | 81.2 | — | 17.6 | 1.2 | 81.2 |
| Singapore | ||||||||||||
| AMCb,c | 58 | 0.03 | 2 | ≤0.015 | 4 | 94.8 | 5.2 | 0.0 | 94.8 (100) | NA | NA | NA |
| azithromycind | 58 | 2 | >256 | 0.25 | >256 | 56.9 | 5.2 | 37.9 | NA | NA | NA | NA |
| cefaclor | 58 | 1 | >256 | 0.5 | >256 | 67.2 | 5.2 | 27.6 | 19.0 | 0.0 | 19.0 | 81.0 |
| cefuroximee | 58 | 0.06 | 4 | ≤0.015 | 32 | 79.3 | 3.5 | 17.2 | 79.3 | 72.4 | 3.5 | 24.1 |
| clarithromycind | 58 | 0.25 | >256 | 0.06 | >256 | 56.9 | 0.0 | 43.1 | NA | NA | NA | NA |
| levofloxacin | 58 | 1 | 2 | 0.5 | 4 | 98.3 | 1.7 | 0.0 | 98.3 | 98.3 | 0.0 | 1.7 |
| moxifloxacin | 58 | 0.12 | 0.25 | 0.06 | 0.25 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 |
| ofloxacin | 58 | 4 | 4 | 0.06 | 8 | 43.1 | 53.5 | 3.5 | NA | 1.7 | 94.8 | 3.5 |
| penicillin (oral) | 58 | 0.03 | 2 | ≤0.015 | 4 | 63.8 | 25.9 | 10.3 | NA | 63.8 | 31.0 | 5.2 |
| penicillin (iv) | 58 | 0.03 | 2 | ≤0.015 | 4 | 94.8 | 5.2 | 0.0 | NA | 81.0–94.8 | NA | NA |
| SXT | 58 | 1 | >32 | 0.12 | >32 | 43.1 | 10.3 | 46.6 | 43.1 | 50.0 | 3.5 | 46.6 |
| erythromycinf | 58 | — | — | — | — | 55.2 | 1.7 | 43.1 | — | 55.2 | 0.0 | 44.8 |
| Thailand | ||||||||||||
| AMCb,c | 208 | 0.06 | 1 | ≤0.015 | 32 | 97.1 | 1.9 | 1.0 | 97.1 (99.0) | NA | NA | NA |
| azithromycind | 208 | 2 | >256 | 0.03 | >256 | 53.4 | 1.0 | 45.7 | NA | NA | NA | NA |
| cefuroximee | 208 | 0.12 | 2 | ≤0.015 | 32 | 77.9 | 16.3 | 5.8 | 77.9 | 59.1 | 7.2 | 33.7 |
| clarithromycind | 208 | 0.25 | >256 | 0.03 | >256 | 52.4 | 0.0 | 47.6 | NA | NA | NA | NA |
| levofloxacin | 208 | 1 | 2 | 0.5 | 16 | 98.1 | 1.4 | 0.5 | 98.1 | 98.1 | 0.0 | 1.9 |
| penicillin (oral) | 208 | 0.12 | 1 | ≤0.015 | 8 | 49.0 | 46.2 | 4.8 | NA | 49.0 | 49.0 | 1.9 |
| penicillin (iv) | 208 | 0.12 | 1 | ≤0.015 | 8 | 98.1 | 1.0 | 1.0 | NA | 84.1–98.1 | NA | NA |
| erythromycinf | 208 | — | — | — | — | 51.9 | 1.9 | 46.2 | NA | 51.9 | 0.0 | 48.1 |
min, minimum; max, maximum; S, susceptible; I, intermediate; R, resistant; AMC, amoxicillin/clavulanic acid; NA, no breakpoint data available (NA for azithromycin, clindamycin and clarithromycin by EUCAST or PK/PD because Etest® breakpoints in CO2 not available); SXT, trimethoprim/sulfamethoxazole.
aNot all isolates were tested. Although amoxicillin has not been tested against S. pneumoniae, percentage susceptibility to amoxicillin and amoxicillin/clavulanic acid is expected to be the same.
bPK/PD susceptibility at high dose is shown in parentheses.
cFor S. pneumoniae susceptibility to amoxicillin alone can be inferred from amoxicillin/clavulanic acid data.
dbioMérieux Etest® breakpoints for incubation in CO2.
eBreakpoints used are for cefuroxime axetil.
fUsing S/I/R zone diameters (mm) of CLSI (≤15/16–20/≥21) and EUCAST (≤18/19–21/≥22).
Table 3.
Distribution of S. pneumoniae MICs by country
| Number of isolates at MIC (mg/L) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | na | ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >32 | 64 | 128 | 256 | >256 |
| India | ||||||||||||||||||
| AMC | 219 | 59 | 43 | 12 | 20 | 12 | 13 | 21 | 21 | 6 | 4 | 3 | 2 | 0 | 0 | 1 | 0 | 2 |
| azithromycin | 199 | 2 | 1 | 4 | 10 | 11 | 31 | 46 | 15 | 12 | 27 | 8 | 3 | 0 | 1 | 1 | 0 | 27 |
| cefixime | 219 | 1 | 0 | 3 | 16 | 32 | 37 | 20 | 23 | 24 | 12 | 15 | 22 | 0 | 5 | 1 | 0 | 8 |
| cefpodoxime | 219 | 15 | 44 | 29 | 17 | 16 | 26 | 20 | 22 | 17 | 8 | 2 | 0 | 0 | 1 | 0 | 0 | 2 |
| cefuroxime | 218 | 22 | 53 | 8 | 12 | 17 | 30 | 22 | 19 | 16 | 11 | 4 | 1 | 0 | 1 | 0 | 0 | 2 |
| clarithromycin | 199 | 1 | 8 | 23 | 54 | 22 | 1 | 4 | 19 | 29 | 8 | 3 | 4 | 0 | 0 | 0 | 0 | 23 |
| erythromycin | 183 | 5 | 13 | 21 | 47 | 18 | 0 | 1 | 15 | 21 | 13 | 0 | 4 | 0 | 0 | 0 | 1 | 24 |
| levofloxacin | 219 | 0 | 0 | 0 | 1 | 2 | 27 | 116 | 42 | 7 | 1 | 0 | 0 | 23 | 0 | 0 | 0 | 0 |
| ofloxacin | 219 | 0 | 0 | 0 | 0 | 0 | 4 | 41 | 124 | 20 | 6 | 1 | 0 | 23 | 0 | 0 | 0 | 0 |
| penicillin | 219 | 36 | 56 | 16 | 22 | 16 | 15 | 21 | 27 | 6 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 |
| SXT | 219 | 1 | 5 | 8 | 16 | 18 | 24 | 49 | 37 | 15 | 21 | 9 | 1 | 15 | 0 | 0 | 0 | 0 |
| South Korea | ||||||||||||||||||
| AMC | 85 | 16 | 1 | 3 | 2 | 4 | 11 | 13 | 19 | 14 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 85 | 0 | 0 | 0 | 2 | 5 | 9 | 1 | 0 | 0 | 1 | 4 | 4 | 0 | 1 | 0 | 0 | 58 |
| cefaclor | 85 | 0 | 0 | 0 | 6 | 9 | 5 | 1 | 3 | 1 | 2 | 14 | 12 | 0 | 13 | 6 | 3 | 10 |
| cefotaxime | 85 | 14 | 0 | 1 | 7 | 3 | 18 | 30 | 5 | 1 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| cefuroxime | 85 | 14 | 0 | 0 | 4 | 7 | 0 | 6 | 16 | 25 | 7 | 4 | 0 | 0 | 1 | 0 | 0 | 1 |
| clarithromycin | 85 | 0 | 0 | 2 | 9 | 5 | 0 | 0 | 0 | 1 | 6 | 2 | 1 | 0 | 1 | 0 | 1 | 57 |
| clindamycin | 85 | 1 | 0 | 2 | 10 | 13 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 57 |
| levofloxacin | 85 | 0 | 0 | 0 | 0 | 1 | 9 | 63 | 5 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 |
| ofloxacin | 85 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 63 | 14 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 |
| penicillin | 85 | 11 | 5 | 2 | 2 | 5 | 7 | 16 | 19 | 15 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| SXT | 85 | 0 | 0 | 0 | 18 | 16 | 3 | 9 | 1 | 9 | 7 | 3 | 2 | 17 | 0 | 0 | 0 | 0 |
| Singapore | ||||||||||||||||||
| AMC | 58 | 5 | 32 | 1 | 1 | 5 | 2 | 3 | 6 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 58 | 0 | 0 | 0 | 0 | 2 | 6 | 13 | 12 | 0 | 3 | 2 | 2 | 0 | 1 | 1 | 0 | 16 |
| cefaclor | 58 | 0 | 0 | 0 | 0 | 0 | 11 | 28 | 3 | 2 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 8 |
| cefuroxime | 58 | 0 | 20 | 9 | 3 | 7 | 2 | 2 | 2 | 7 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 58 | 0 | 0 | 6 | 18 | 9 | 0 | 0 | 2 | 3 | 2 | 1 | 0 | 0 | 2 | 0 | 0 | 15 |
| levofloxacin | 58 | 0 | 0 | 0 | 0 | 0 | 2 | 48 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| moxifloxacin | 58 | 0 | 0 | 1 | 43 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ofloxacin | 58 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 24 | 31 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| penicillin | 58 | 15 | 18 | 4 | 2 | 6 | 2 | 5 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SXT | 58 | 0 | 0 | 0 | 2 | 20 | 3 | 4 | 2 | 3 | 10 | 5 | 0 | 9 | 0 | 0 | 0 | 0 |
| Thailand | ||||||||||||||||||
| AMC | 208 | 63 | 31 | 14 | 13 | 14 | 37 | 23 | 7 | 4 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 208 | 0 | 1 | 0 | 4 | 15 | 16 | 63 | 8 | 4 | 2 | 7 | 25 | 0 | 5 | 0 | 1 | 57 |
| cefuroxime | 208 | 63 | 21 | 10 | 12 | 17 | 15 | 24 | 34 | 10 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 208 | 0 | 4 | 17 | 75 | 12 | 1 | 0 | 0 | 18 | 21 | 6 | 3 | 0 | 0 | 0 | 0 | 51 |
| levofloxacin | 208 | 0 | 0 | 0 | 0 | 0 | 29 | 142 | 33 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| penicillin | 208 | 71 | 18 | 13 | 15 | 20 | 38 | 23 | 6 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid (2:1); SXT, trimethoprim/sulfamethoxazole (1:19).
aNot all isolates were tested.
India
Using the CLSI iv breakpoint, 95.4% of the isolates from India were susceptible to penicillin (PSSP), whereas only 49.3% were PSSP according to the CLSI oral and the EUCAST criteria. With these two breakpoints, 33.8% and 46.1% were scored as penicillin intermediate (PISP), respectively, and 16.9% and 4.6% of isolates were penicillin resistant (PRSP), respectively (Table 2).
Only amoxicillin/clavulanic acid (or amoxicillin alone as inferred from the amoxicillin/clavulanic acid data) had >90% susceptibility by both CLSI and PK/PD criteria; 94.5% of isolates were susceptible to high-dose amoxicillin/clavulanic acid (PK/PD) (Table 2).
The fluoroquinolones were more active, with 85.8% of isolates susceptible to levofloxacin (by all three breakpoint criteria) and 77.2% susceptible to ofloxacin by CLSI breakpoints. EUCAST breakpoints, on the other hand, considered all S. pneumoniae to be non-susceptible to ofloxacin (Table 2).
Of the three macrolides tested, azithromycin had the highest activity (CLSI breakpoints in CO2), at 66.3% susceptible, and clarithromycin and erythromycin had lower activity, at 54.8% and 57.4%, respectively (CLSI breakpoints in CO2). By disc, erythromycin susceptibility was 49.1% according to both CLSI and EUCAST breakpoints (Table 2).
South Korea
Using the CLSI iv breakpoint, 78.8% of isolates were PSSP, whereas only 21.2% were PSSP according to both the CLSI oral and the EUCAST criteria. With these two breakpoints, 35.3% and 57.6%, respectively, were scored as PISP and 43.5% and 21.2%, respectively, were PRSP (Table 2).
Only for levofloxacin was there >90% susceptibility by CLSI or EUCAST breakpoints (91.8%), although amoxicillin/clavulanic acid had 97.6% susceptibility according to high-dose PK/PD breakpoints and 81.2% susceptibility by CLSI breakpoints. Cefotaxime susceptibility was also high by CLSI breakpoints (85.9%), but this dropped to 50.6% susceptible by EUCAST breakpoints. Other cephalosporins, cefaclor and cefuroxime, were less active, at 24.7% and 36.5% susceptible by CLSI breakpoints, respectively. According to EUCAST breakpoints, susceptibility to the two cephalosporins was 0% and 29.4%, respectively (Table 2).
Susceptibility to macrolides in South Korea was very low, at around 20% (CLSI breakpoints in CO2), which was confirmed by disc diffusion for erythromycin according to both CLSI and EUCAST breakpoints.
Singapore
Using the CLSI iv breakpoint, 94.8% of strains were PSSP. With the CLSI oral and EUCAST breakpoints, 63.8% and 63.8% of isolates were PSSP, respectively; 25.9% and 31.0% were PISP and 10.3% and 5.2% were PRSP (Table 2).
Susceptibility to amoxicillin/clavulanic acid (amoxicillin) was 94.8% according to CLSI breakpoints and low-dose PK/PD breakpoints, but all isolates were susceptible at the high-dose PK/PD breakpoint. Similarly, all isolates were susceptible to moxifloxacin and 98.3% of isolates susceptible to levofloxacin (by all three breakpoints). Ofloxacin susceptibility was much lower, at 43.1% by CLSI and 1.7% by EUCAST.
Among cephalosporins, cefuroxime showed 79.3% susceptibility by both CLSI and PK/PD breakpoints and 72.4% by EUCAST breakpoints. Cefaclor susceptibility was 0% by EUCAST breakpoints (as shown in South Korea), 19.0% by PK/PD criteria and 67.2% by CLSI. Azithromycin susceptibility was 56.9% (CLSI breakpoints in CO2) and erythromycin susceptibility by CLSI or EUCAST disc diffusion was 55.2% (Table 2).
Thailand
Using the CLSI iv breakpoint, 98.1% of isolates were PSSP (1.0% PISP, 1.0% PRSP), but only 49.0% of the isolates were PSSP by CLSI oral and EUCAST criteria (46.2% and 49.0% PISP, respectively; 4.8% and 1.9% PRSP). Susceptibility to amoxicillin/clavulanic acid (amoxicillin) was 97.1% by CLSI or low-dose PK/PD breakpoints and 99.0% by high-dose PK/PD. Levofloxacin susceptibility was also high, at 98.1% (by all three breakpoint criteria). Cefuroxime was the only cephalosporin tested; 77.9% of strains were susceptible by CLSI and PK/PD breakpoints and 59.1% by EUCAST. Among macrolides, around 50% of isolates were susceptible to azithromycin and clarithromycin using CLSI breakpoints in CO2 or to erythromycin by CLSI and EUCAST disc diffusion breakpoints (Table 2).
Comparative susceptibility of S. pneumoniae isolates by country
Comparative susceptibility (CLSI breakpoints) of S. pneumoniae by country to nine antimicrobial agents is depicted in Figure 1. Susceptibility to amoxicillin/clavulanic acid (amoxicillin) or levofloxacin was high in all countries tested. Nevertheless, susceptibility to amoxicillin/clavulanic acid (amoxicillin) was significantly lower in South Korea than the other Asian countries (P < 0.05). Furthermore, susceptibility to cefuroxime, clarithromycin, erythromycin and penicillin was also significantly lower in South Korea. Susceptibility to ofloxacin in Singapore was significantly lower than in South Korea or India.
Figure 1.
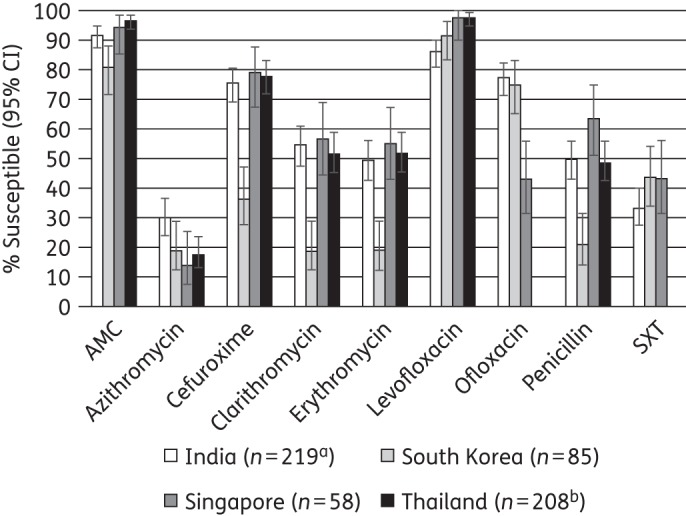
Percentage susceptibility rates (with 95% confidence intervals) for antimicrobials against S. pneumoniae by country according to CLSI breakpoints. Note that only disc diffusion test results are shown for erythromycin. aSample sizes in India were: azithromycin and clarithromycin, n = 199; cefuroxime and erythromycin, n = 218. bOfloxacin and trimethoprim/sulfamethoxazole were not tested in Thailand. AMC, amoxicillin/clavulanic acid; SXT, trimethoprim/sulfamethoxazole.
Prevalence of antibiotic susceptibility among PSSP, PISP and PRSP isolates (based on CLSI penicillin oral breakpoints)
Utilizing the CLSI (oral) breakpoints, the relationship between susceptibility to penicillin and susceptibility to other antimicrobial agents was assessed for the pooled set of 570 S. pneumoniae strains from the four countries (Figure 2).
Figure 2.
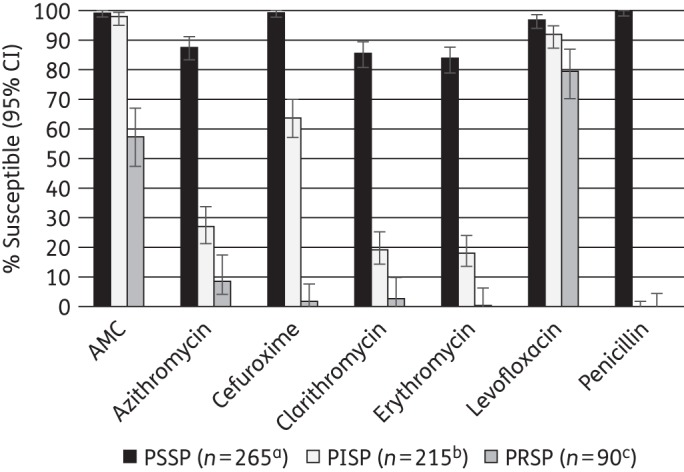
Percentage susceptibility rates (with 95% confidence intervals) for antimicrobials according to CLSI breakpoints against PSSP, PISP and PRSP, combining India, South Korea, Singapore and Thailand. Penicillin susceptibility category is based on oral penicillin CLSI breakpoints. Sample sizes varied by antimicrobial agents and are shown at the bottom. Only antimicrobials that were tested in each country are included. Only results for erythromycin by disc diffusion are included. aSample sizes for PSSP were: azithromycin and clarithromycin, n = 261; erythromycin, n = 264. bSample sizes for PISP were: azithromycin and clarithromycin, n = 204; cefuroxime, n = 214. cSample sizes for PRSP were: azithromycin and clarithromycin, n = 85. AMC, amoxicillin/clavulanic acid.
Among 265 PSSP, 99.6% were susceptible to amoxicillin/clavulanic acid (amoxicillin) or cefuroxime, 84%–88% were susceptible to macrolides and 97.0% to levofloxacin.
The 215 PISP were 98.1% susceptible to amoxicillin/clavulanic acid (amoxicillin) and 92.1% were susceptible to levofloxacin. Susceptibility to cefuroxime was 64.0% and macrolide susceptibility ranged from 19% to 27%.
For the 90 PRSP, 57.8% were susceptible to amoxicillin/clavulanic acid (amoxicillin) and 80% susceptible to levofloxacin. Macrolide susceptibility ranged from 1% to 9% and cefuroxime susceptibility was 2.2%.
Susceptibility to all agents tested was significantly lower in PRSP compared with PISP and PISP compared with PSSP (P < 0.05) with the exception of amoxicillin/clavulanic acid (amoxicillin), where there was no statistically significant difference in activity against PSSP and PISP (P = 0.111).
CLSI guidelines indicate that isolates susceptible to penicillin G (MIC ≤0.06 mg/L) can be reported as susceptible to amoxicillin, amoxicillin/clavulanic acid, cefaclor and cefuroxime. Data from this study confirmed this, as all penicillin-susceptible S. pneumoniae were also susceptible to cefaclor and all isolates except one were also susceptible to amoxicillin/clavulanic acid (amoxicillin) and cefuroxime. However, the reverse was not always found, with 86.2% and 45.7% of penicillin non-susceptible isolates susceptible to amoxicillin/clavulanic acid (amoxicillin) and cefuroxime, respectively. However, only 5.7% of penicillin-susceptible S. pneumoniae were cefaclor susceptible. A similar ‘expert rule’ is provided by EUCAST but for penicillins only, i.e. amoxicillin/clavulanic acid (amoxicillin) in this study. However, unlike CLSI, individual breakpoints are not provided by EUCAST for amoxicillin/clavulanic acid to make this comparison.
S. pyogenes susceptibility
Only India provided isolates of S. pyogenes. Summary MIC and susceptibility data and MIC distributions are shown in Tables 4 and 5.
Table 4.
MIC and susceptibility results for S. pyogenes (India only)
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| India | ||||||||||||
| AMCa | 78 | 0.03 | 0.25 | ≤0.015 | 1 | 100b | 0.0 | 0.0 | 100 (100) | 100b | 0.0 | 0.0 |
| azithromycin | 78 | 64 | >256 | 0.12 | >256 | NA | NA | NA | NA | NA | NA | NA |
| cefixime | 78 | 0.25 | 0.5 | 0.03 | 32 | 100b | 0.0 | 0.0 | 98.7 | 100b | 0.0 | 0.0 |
| cefpodoxime | 78 | 0.03 | 0.03 | ≤0.015 | 4 | 100b | 0.0 | 0.0 | 98.7 | 100b | 0.0 | 0.0 |
| cefuroxime | 78 | ≤0.015 | 0.03 | ≤0.015 | 1 | 100b | 0.0 | 0.0 | 100 | 100b | 0.0 | 0.0 |
| clarithromycin | 78 | 32 | >256 | 0.06 | >256 | NA | NA | NA | NA | NA | NA | NA |
| erythromycinc | 76 | 16 | >256 | 0.06 | >256 | 23.7 | 7.9 | 68.4 | NA | NA | NA | NA |
| levofloxacin | 78 | 1 | >32 | 0.5 | >32 | 79.5 | 9.0 | 11.5 | 79.5 | 55.1 | 24.4 | 20.5 |
| ofloxacin | 78 | 2 | >32 | 1 | >32 | 52.6 | 24.4 | 23.1 | NA | NA | NA | NA |
| penicillin | 78 | 0.015 | 0.03 | ≤0.015 | 0.12 | 100 | 0.0 | 0.0 | NA | 100 | 0.0 | 0.0 |
| SXT | 78 | >32 | >32 | ≤0.015 | >32 | NA | NA | NA | 33.3 | 33.3 | 0.0 | 66.7 |
| erythromycind | 78 | — | — | — | — | 21.8 | 7.7 | 70.5 | — | 21.8 | 3.8 | 74.4 |
min, minimum; max, maximum; S, susceptible; I, intermediate; AMC, amoxicillin/clavulanic acid; NA, no breakpoint data available (NA for macrolides by EUCAST or PK/PD because Etest® breakpoints in CO2 not available); SXT, trimethoprim/sulfamethoxazole.
aPK/PD susceptibility at high dose is shown in parentheses.
bCLSI and EUCAST guidelines assumes susceptibility to amoxicillin/clavulanic acid and cephalosporins based on penicillin susceptibility.
cbioMérieux Etest® breakpoints for incubation in CO2.
dUsing S/I/R zone diameters (mm) of CLSI (≤15/16–20/≥21) and EUCAST (≤18/19–21/≥22).
Table 5.
Distribution of S. pyogenes MICs (India only)
| Number of isolates at MIC (mg/L) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | n | ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >32 | 64 | 128 | 256 | >256 |
| India | ||||||||||||||||||
| AMC | 78 | 23 | 38 | 6 | 3 | 2 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 78 | 0 | 0 | 0 | 1 | 0 | 3 | 12 | 7 | 2 | 1 | 6 | 6 | 0 | 14 | 6 | 6 | 14 |
| cefixime | 78 | 0 | 1 | 7 | 17 | 44 | 7 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| cefpodoxime | 78 | 23 | 52 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefuroxime | 78 | 43 | 32 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 78 | 0 | 0 | 4 | 8 | 9 | 0 | 2 | 3 | 2 | 1 | 9 | 18 | 0 | 8 | 0 | 0 | 14 |
| erythromycin | 76 | 0 | 0 | 1 | 10 | 7 | 0 | 0 | 6 | 4 | 3 | 11 | 17 | 0 | 4 | 0 | 0 | 13 |
| levofloxacin | 78 | 0 | 0 | 0 | 0 | 0 | 24 | 19 | 19 | 7 | 1 | 0 | 0 | 8 | 0 | 0 | 0 | 0 |
| ofloxacin | 78 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 21 | 19 | 5 | 3 | 0 | 10 | 0 | 0 | 0 | 0 |
| penicillin | 78 | 42 | 34 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SXT | 78 | 1 | 3 | 8 | 5 | 1 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 52 | 0 | 0 | 0 | 0 |
AMC, amoxicillin/clavulanic acid (2:1); SXT, trimethoprim/sulfamethoxazole (1:19).
All isolates were fully susceptible to penicillin and therefore, according to CLSI and EUCAST guidelines, may be considered susceptible to amoxicillin/clavulanic acid (amoxicillin) and cephalosporins. However, susceptibility to levofloxacin was 79.5% by CLSI and 55.1% by EUCAST and ofloxacin susceptibility (CLSI only) was 52.6%. Trimethoprim/sulfamethoxazole breakpoints are only available by EUCAST and PK/PD, which showed that susceptibility was low, at 33.3%. Erythromycin susceptibility was the lowest of all, at 21.8%–23.7% (Table 4).
H. influenzae susceptibility in individual countries
Summary MIC and susceptibility data for all H. influenzae isolates are shown in Table 6 and the complete MIC distribution data in Table 7.
Table 6.
MIC and susceptibility results for H. influenzae isolates by country
| Susceptibility |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
||||||||||
| Antimicrobial | Isolate group | n | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| India | |||||||||||||
| AMCa,b | All | 135 | 0.5 | 4 | ≤0.015 | >256 | 97.0 | 0.0 | 3.0 | 89.6 (97.0) | 89.6 | 0.0 | 10.4 |
| BL− | 124 | 0.5 | 2 | ≤0.015 | 8 | 99.2 | 0.0 | 0.8 | 91.9 (99.2) | 91.9 | 0.0 | 8.1 | |
| BL+ | 11 | 2 | >256 | 0.03 | >256 | 72.7 | 0.0 | 27.3 | 63.6 (72.7) | 63.6 | 0.0 | 36.4 | |
| ampicillinc | All | 135 | 0.25 | 1 | ≤0.015 | 32 | 91.1 | 1.5 | 7.4 | NA | 91.1 | 0.0 | 8.9 |
| BL− | 124 | 0.25 | 0.5 | ≤0.015 | 2 | 99.2 | 0.8 | 0.0 | NA | 99.2 | 0.0 | 0.8 | |
| BL+ | 11 | 4 | 16 | 2 | 32 | 0.0 | 9.1 | 90.9 | NA | 0.0 | 0.0 | 100 | |
| azithromycind | All | 94 | 4 | 8 | ≤0.015 | >256 | 94.7 | 0.0 | 5.3 | NA | NA | NA | NA |
| BL− | 87 | 4 | 8 | ≤0.015 | >256 | 94.3 | 0.0 | 5.8 | NA | NA | NA | NA | |
| BL+ | 7 | 4 | 8 | ≤0.015 | 8 | 100 | 0.0 | 0.0 | NA | NA | NA | NA | |
| cefixime | All | 135 | 0.06 | 0.5 | ≤0.015 | >256 | 97.0 | 0.0 | 3.0 | 97.0 | 85.2 | 0.0 | 14.8 |
| BL− | 124 | 0.06 | 0.5 | ≤0.015 | >256 | 96.8 | 0.0 | 3.2 | 96.8 | 84.7 | 0.0 | 15.3 | |
| BL+ | 11 | 0.03 | 0.12 | ≤0.015 | 0.5 | 100 | 0.0 | 0.0 | 100 | 90.9 | 0.0 | 9.1 | |
| cefpodoxime | All | 134 | 0.06 | 1 | ≤0.015 | >256 | 97.0 | 0.0 | 3.0 | 89.5 | 78.4 | 11.2 | 10.5 |
| BL− | 123 | 0.12 | 1 | ≤0.015 | >256 | 96.7 | 0.0 | 3.3 | 88.6 | 78.1 | 10.6 | 11.4 | |
| BL+ | 11 | 0.06 | 0.5 | 0.03 | 0.5 | 100 | 0.0 | 0.0 | 100 | 81.8 | 18.2 | 0.0 | |
| cefuroximee,b | All | 134 | 0.5 | 1 | ≤0.015 | >256 | 99.3 | 0.0 | 0.8 | 90.3 | 17.9 | 72.4 | 9.7 |
| BL− | 123 | 0.5 | 1 | ≤0.015 | >256 | 99.2 | 0.0 | 0.8 | 91.9 | 18.7 | 73.2 | 8.1 | |
| BL+ | 11 | 1 | 2 | ≤0.015 | 4 | 100 | 0.0 | 0.0 | 72.7 | 9.1 | 63.6 | 27.3 | |
| ciprofloxacin | All | 135 | 0.5 | 8 | ≤0.015 | >32 | 76.3 | 0.0 | 23.7 | 76.3 | 59.3 | 0.0 | 40.7 |
| BL− | 124 | 0.5 | 8 | ≤0.015 | >32 | 75.0 | 0.0 | 25.0 | 75.0 | 58.1 | 0.0 | 41.9 | |
| BL+ | 11 | 0.25 | 1 | ≤0.015 | 8 | 90.9 | 0.0 | 9.1 | 90.9 | 72.7 | 0.0 | 27.3 | |
| clarithromycind | All | 108 | 16 | 64 | 0.06 | >256 | 66.7 | 19.4 | 13.9 | NA | NA | NA | NA |
| BL− | 99 | 16 | 64 | 0.06 | >256 | 65.7 | 19.2 | 15.2 | NA | NA | NA | NA | |
| BL+ | 9 | 8 | 32 | 1 | 32 | 77.8 | 22.2 | 0.0 | NA | NA | NA | NA | |
| levofloxacin | All | 135 | 0.5 | 8 | ≤0.015 | >32 | 85.2 | 0.0 | 14.8 | 85.2 | 79.3 | 0.0 | 20.7 |
| BL− | 124 | 0.5 | 8 | ≤0.015 | >32 | 84.7 | 0.0 | 15.3 | 84.7 | 78.2 | 0.0 | 21.8 | |
| BL+ | 11 | 0.5 | 1 | 0.03 | 8 | 90.9 | 0.0 | 9.1 | 90.9 | 90.9 | 0.0 | 9.1 | |
| ofloxacin | All | 135 | 0.5 | 8 | ≤0.015 | >32 | 80.7 | 0.0 | 19.3 | NA | 53.3 | 0.0 | 46.7 |
| BL− | 124 | 0.5 | >32 | ≤0.015 | >32 | 79.8 | 0.0 | 20.2 | NA | 51.6 | 0.0 | 48.4 | |
| BL+ | 11 | 0.5 | 2 | 0.06 | 8 | 90.9 | 0.0 | 9.1 | NA | 72.7 | 0.0 | 27.3 | |
| SXT | All | 135 | 4 | >32 | ≤0.015 | >32 | 23.0 | 13.3 | 63.7 | 23.0 | 23.0 | 5.2 | 71.9 |
| BL− | 124 | 4 | >32 | ≤0.015 | >32 | 25.0 | 13.7 | 61.3 | 25.0 | 25.0 | 4.8 | 70.2 | |
| BL+ | 11 | >32 | >32 | 1 | >32 | 0.0 | 9.1 | 90.9 | 0 | 0.0 | 9.1 | 90.9 | |
| South Korea | |||||||||||||
| AMCa,b | All | 72 | 4 | 32 | 0.25 | >256 | 62.5 (56.9)f | 0.0 0.0 | 37.5 (43.1)f | 38.9 (62.5) | 38.9 (31.9)f | 0.0 | 61.1 (68.1)f |
| BL− | 30 | 4 | 16 | 0.25 | >256 | 66.7 | 0.0 | 33.3 | 36.7 (66.7) | 36.7 | 0.0 | 63.3 | |
| BL+ | 42 | 4 | 32 | 0.5 | >256 | 59.5 | 0.0 | 40.5 | 40.5 (59.5) | 40.5 | 0.0 | 59.5 | |
| ampicillinc | All | 72 | 64 | >256 | 0.25 | >256 | 16.7 | 13.9 | 69.4 | NA | 16.7 | 0.0 | 83.3 |
| BL− | 30 | 2 | 16 | 0.25 | >256 | 26.7 | 30.0 | 43.3 | NA | 26.7 | 0.0 | 73.3 | |
| BL+ | 42 | >256 | >256 | 0.25 | >256 | 9.5 | 2.4 | 88.1 | NA | 9.5 | 0.0 | 90.5 | |
| azithromycind | All | 72 | 2 | 8 | 0.5 | >256 | 94.4 | 0.0 | 5.6 | NA | NA | NA | NA |
| BL− | 30 | 2 | 8 | 1 | >256 | 93.3 | 0.0 | 6.7 | NA | NA | NA | NA | |
| BL+ | 42 | 2 | 4 | 0.5 | 64 | 95.2 | 0.0 | 4.8 | NA | NA | NA | NA | |
| cefaclorb | All | 72 | 64 | >256 | 0.5 | >256 | 29.2 (27.8)f | 8.3 | 62.5 (63.9)f | 1.4 | NA | NA | NA |
| BL− | 30 | 32 | >256 | 0.5 | >256 | 30.0 | 13.3 | 56.7 | 3.3 | NA | NA | NA | |
| BL+ | 42 | 64 | >256 | 2 | >256 | 28.6 | 4.8 | 66.7 | 0 | NA | NA | NA | |
| cefuroximee,b | All | 72 | 16 | >256 | 0.5 | >256 | 40.3 (37.5)f | 4.2 | 55.6 (58.3)f | 16.7 | 0.0 | 16.7 | 83.3 |
| BL− | 30 | 4 | >256 | 0.5 | >256 | 50.0 | 6.7 | 43.3 | 16.7 | 0.0 | 16.7 | 83.3 | |
| BL+ | 42 | 32 | >256 | 0.5 | >256 | 33.3 | 2.4 | 64.3 | 16.7 | 0.0 | 16.7 | 83.3 | |
| clarithromycind | All | 72 | 16 | 64 | 4 | >256 | 50.0 | 37.5 | 12.5 | NA | NA | NA | NA |
| BL− | 30 | 16 | 64 | 8 | 64 | 53.3 | 33.3 | 13.3 | NA | NA | NA | NA | |
| BL+ | 42 | 32 | 64 | 4 | >256 | 47.6 | 40.5 | 11.9 | NA | NA | NA | NA | |
| levofloxacin | All | 72 | 0.015 | 0.03 | ≤0.015 | 1 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 |
| BL− | 30 | 0.015 | 0.03 | ≤0.015 | 1 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 | |
| BL+ | 42 | 0.015 | 0.03 | ≤0.015 | 0.5 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 | |
| SXT | All | 72 | 0.12 | >32 | ≤0.015 | >32 | 59.7 | 1.4 | 38.9 | 59.7 | 59.7 | 0.0 | 40.3 |
| BL− | 30 | 0.06 | >32 | 0.03 | >32 | 73.3 | 3.3 | 23.3 | 73.3 | 73.3 | 0.0 | 26.7 | |
| BL+ | 42 | 0.25 | >32 | ≤0.015 | >32 | 50.0 | 0.0 | 50.0 | 50.0 | 50.0 | 0.0 | 50.0 | |
| Singapore | |||||||||||||
| AMCa,b | All | 60 | 1 | 4 | 0.06 | 16 | 93.3 | 0.0 | 6.7 | 86.7 (93.3) | 86.7 (76.7)f | 0.0 | 13.3 (23.3)f |
| BL− | 49 | 1 | 4 | 0.06 | 16 | 91.8 | 0.0 | 8.2 | 85.7 (91.8) | 85.7 | 0.0 | 14.3 | |
| BL+ | 11 | 1 | 2 | 0.5 | 4 | 100 | 0.0 | 0.0 | 90.9 (100) | 90.9 | 0.0 | 9.1 | |
| ampicillinc | All | 60 | 0.5 | >256 | 0.03 | >256 | 63.3 | 15.0 | 21.7 | NA | 63.3 | 0.0 | 36.7 |
| BL− | 49 | 0.5 | 2 | 0.03 | 32 | 77.6 | 18.4 | 4.1 | NA | 77.6 | 0.0 | 22.5 | |
| BL+ | 11 | >256 | >256 | 64 | >256 | 0.0 | 0.0 | 100 | NA | 0.0 | 0.0 | 100 | |
| azithromycind | All | 60 | 2 | 4 | 0.12 | 16 | 98.3 | 0.0 | 1.7 | NA | NA | NA | NA |
| BL− | 49 | 2 | 4 | 0.12 | 8 | 100 | 0.0 | 0.0 | NA | NA | NA | NA | |
| BL+ | 11 | 2 | 4 | 1 | 16 | 90.9 | 0.0 | 9.1 | NA | NA | NA | NA | |
| cefaclorb | All | 60 | 4 | 256 | 0.03 | >256 | 75.0 (73.3)f | 1.7 | 23.3 (25.0)f | 5.0 | NA | NA | NA |
| BL− | 49 | 4 | >256 | 0.03 | >256 | 71.4 | 2.0 | 26.5 | 2.0 | NA | NA | NA | |
| BL+ | 11 | 4 | 8 | 0.12 | 256 | 90.9 | 0.0 | 9.1 | 18.2 | NA | NA | NA | |
| cefuroximee,b | All | 60 | 1 | 8 | 0.06 | >256 | 85.0 | 10.0 | 5.0 | 60.0 | 1.7 | 58.3 | 40.0 |
| BL− | 49 | 1 | 8 | 0.06 | >256 | 85.7 | 10.2 | 4.1 | 57.1 | 2.0 | 55.1 | 42.9 | |
| BL+ | 11 | 1 | 8 | 0.5 | 16 | 81.8 | 9.1 | 9.1 | 72.7 | 0.0 | 72.7 | 27.3 | |
| ciprofloxacin | All | 60 | 0.015 | 0.25 | ≤0.015 | 0.5 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 |
| BL− | 49 | 0.015 | 0.25 | ≤0.015 | 0.5 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 | |
| BL+ | 11 | 0.015 | 0.25 | ≤0.015 | 0.5 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 | |
| clarithromycind | All | 60 | 8 | 32 | 0.25 | >256 | 83.3 | 8.3 | 8.3 | NA | NA | NA | NA |
| BL− | 49 | 8 | 32 | 0.25 | 64 | 87.8 | 6.1 | 6.1 | NA | NA | NA | NA | |
| BL+ | 11 | 8 | 64 | 2 | >256 | 63.6 | 18.2 | 18.2 | NA | NA | NA | NA | |
| levofloxacin | All | 60 | 0.015 | 0.12 | ≤0.015 | 0.5 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 |
| BL− | 49 | 0.015 | 0.12 | ≤0.015 | 0.25 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 | |
| BL+ | 11 | 0.015 | 0.03 | ≤0.015 | 0.5 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 | |
| moxifloxacin | All | 60 | 0.03 | 0.06 | ≤0.015 | 2 | 98.3 | 0.0 | 1.7 | 98.3 | 98.3 | 0.0 | 1.7 |
| BL− | 49 | 0.03 | 0.12 | ≤0.015 | 0.25 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 | |
| BL+ | 11 | 0.015 | 0.06 | ≤0.015 | 2 | 90.9 | 0.0 | 9.1 | 90.9 | 90.9 | 0.0 | 9.1 | |
| ofloxacin | All | 60 | 0.06 | 0.06 | ≤0.015 | 2 | 100 | 0.0 | 0.0 | NA | 98.3 | 0.0 | 1.7 |
| BL− | 49 | 0.06 | 0.25 | ≤0.015 | 0.5 | 100 | 0.0 | 0.0 | NA | 100 | 0.0 | 0.0 | |
| BL+ | 11 | 0.06 | 0.06 | 0.03 | 2 | 100 | 0.0 | 0.0 | NA | 90.9 | 0.0 | 9.1 | |
| SXT | All | 60 | 0.12 | >32 | ≤0.015 | >32 | 63.3 | 3.3 | 33.3 | 63.3 | 63.3 | 1.7 | 35.0 |
| BL− | 49 | 0.12 | >32 | ≤0.015 | >32 | 67.4 | 4.1 | 28.6 | 67.4 | 67.4 | 2.0 | 30.6 | |
| BL+ | 11 | 8 | >32 | 0.03 | >32 | 45.5 | 0.0 | 54.6 | 45.5 | 45.5 | 0.0 | 54.6 | |
| Thailand | |||||||||||||
| AMCa,b | All | 263 | 1 | 2 | 0.06 | 16 | 97.7 (93.5)f | 0.0 | 2.3 (6.5)f | 90.5 (97.7) | 90.5 (81.4)f | 0.0 | 9.5 (18.6)f |
| BL− | 167 | 1 | 2 | 0.06 | 16 | 98.2 | 0.0 | 1.8 | 93.4 (98.2) | 93.4 | 0.0 | 6.6 | |
| BL+ | 96 | 1 | 4 | 0.12 | 16 | 96.9 | 0.0 | 3.1 | 85.4 (96.9) | 85.4 | 0.0 | 14.6 | |
| ampicillinc | all | 263 | 1 | >256 | 0.03 | >256 | 51.7 | 8.8 | 39.5 | na | 51.7 | 0.0 | 48.3 |
| BL− | 167 | 0.5 | 2 | 0.03 | 32 | 80.8 | 12.0 | 7.2 | na | 80.8 | 0.0 | 19.2 | |
| BL+ | 96 | >256 | >256 | 0.5 | >256 | 1.0 | 3.1 | 95.8 | na | 1.0 | 0.0 | 99.0 | |
| azithromycind | all | 263 | 2 | 4 | 0.06 | 16 | 99.6 | 0.0 | 0.4 | na | na | na | na |
| BL− | 167 | 2 | 4 | 0.06 | 8 | 100 | 0.0 | 0.0 | na | na | na | na | |
| BL+ | 96 | 2 | 4 | 0.5 | 16 | 99.0 | 0.0 | 1.0 | na | na | na | na | |
| cefuroximee,b | all | 263 | 0.5 | 2 | 0.03 | 32 | 96.2 (92.4)f | 1.5 | 2.3 (6.1)f | 79.5 | 7.2 | 72.4 (66.9)f | 20.5 (25.9)f |
| BL− | 167 | 0.5 | 4 | 0.03 | 16 | 95.2 | 2.4 | 2.4 | 79.0 | 10.1 | 69.1 | 20.8 | |
| BL+ | 96 | 0.5 | 2 | 0.06 | 32 | 97.9 | 0.0 | 2.1 | 80.2 | 2.1 | 78.1 | 19.8 | |
| clarithromycind | all | 263 | 16 | 32 | 1 | 128 | 79.5 | 19.8 | 0.8 | na | na | na | na |
| BL− | 167 | 16 | 32 | 1 | 128 | 81.4 | 17.4 | 1.2 | na | na | na | na | |
| BL+ | 96 | 16 | 32 | 1 | 32 | 76.0 | 24.0 | 0.0 | na | na | na | na | |
| levofloxacin | all | 263 | 0.015 | 0.03 | ≤0.015 | >256 | 99.6 | 0.0 | 0.4 | 99.6 | 99.2 | 0.0 | 0.8 |
| BL− | 167 | 0.015 | 0.03 | ≤0.015 | >256 | 99.4 | 0.0 | 0.6 | 99.4 | 98.8 | 0.0 | 1.2 | |
| BL+ | 96 | 0.015 | 0.03 | ≤0.015 | 1 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 | |
min, minimum; max, maximum; S, susceptible; I, intermediate; AMC, amoxicillin/clavulanic acid; BL−, β-lactamase negative; BL+, β-lactamase positive; NA, no breakpoint data available (NA for azithromycin and clarithromycin by EUCAST because Etest® breakpoints in CO2 not available); SXT, trimethoprim/sulfamethoxazole.
aPK/PD susceptibility at high dose is shown in parentheses.
bIn clinical settings, isolates of BLNAR are considered resistant to amoxicillin/clavulanic acid, cefaclor and cefuroxime (see main text).
cClinically all β-lactamase-positive H. influenzae should be considered resistant.
dbioMérieux Etest® breakpoints for incubation in CO2.
eBreakpoints used are for cefuroxime axetil.
fClinical susceptibility to amoxicillin/clavulanic acid or cefuroxime reduced (data in parenthesis) due to corrections according to BLNAR (see main text).
Table 7.
Distribution of H. influenzae MICs by country
| Number of isolates at MIC (mg/L) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial | na | ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >32 | 64 | 128 | 256 | >256 |
| India | ||||||||||||||||||
| AMC | 135 | 2 | 2 | 3 | 17 | 20 | 32 | 34 | 11 | 10 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| ampicillin | 135 | 1 | 1 | 9 | 45 | 32 | 27 | 8 | 2 | 7 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| azithromycin | 94 | 3 | 6 | 7 | 5 | 1 | 2 | 0 | 14 | 36 | 15 | 4 | 0 | 0 | 0 | 0 | 0 | 1 |
| cefixime | 135 | 24 | 37 | 45 | 9 | 3 | 11 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| cefpodoxime | 134 | 5 | 22 | 41 | 29 | 8 | 15 | 4 | 6 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| cefuroxime | 134 | 9 | 9 | 5 | 1 | 11 | 48 | 38 | 8 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| ciprofloxacin | 135 | 21 | 5 | 10 | 10 | 16 | 18 | 23 | 10 | 5 | 5 | 2 | 0 | 10 | 0 | 0 | 0 | 0 |
| clarithromycin | 108 | 0 | 0 | 1 | 2 | 0 | 1 | 3 | 10 | 14 | 14 | 27 | 21 | 0 | 8 | 0 | 0 | 7 |
| levofloxacin | 135 | 13 | 14 | 6 | 9 | 14 | 26 | 25 | 8 | 5 | 5 | 2 | 0 | 8 | 0 | 0 | 0 | 0 |
| ofloxacin | 135 | 7 | 11 | 11 | 8 | 18 | 17 | 19 | 18 | 8 | 5 | 0 | 0 | 13 | 0 | 0 | 0 | 0 |
| SXT | 135 | 4 | 7 | 10 | 4 | 5 | 1 | 7 | 11 | 22 | 10 | 2 | 2 | 50 | 0 | 0 | 0 | 0 |
| South Korea | ||||||||||||||||||
| AMC | 72 | 0 | 0 | 0 | 0 | 2 | 4 | 8 | 14 | 17 | 12 | 6 | 5 | 0 | 0 | 0 | 0 | 4 |
| ampicillin | 72 | 0 | 0 | 0 | 0 | 5 | 2 | 5 | 10 | 5 | 5 | 2 | 0 | 0 | 4 | 1 | 0 | 33 |
| azithromycin | 72 | 0 | 0 | 0 | 0 | 0 | 1 | 8 | 40 | 14 | 5 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| cefaclor | 72 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 13 | 4 | 6 | 6 | 0 | 8 | 1 | 2 | 28 |
| cefuroxime | 72 | 0 | 0 | 0 | 0 | 0 | 3 | 9 | 9 | 8 | 3 | 6 | 8 | 0 | 5 | 1 | 1 | 19 |
| clarithromycin | 72 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 8 | 26 | 27 | 0 | 5 | 2 | 0 | 2 |
| levofloxacin | 72 | 51 | 15 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SXT | 72 | 1 | 1 | 28 | 9 | 4 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 26 | 0 | 0 | 0 | 0 |
| Singapore | ||||||||||||||||||
| AMC | 60 | 0 | 0 | 2 | 2 | 4 | 9 | 22 | 13 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| ampicillin | 60 | 0 | 1 | 0 | 4 | 7 | 19 | 7 | 9 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 3 | 7 |
| azithromycin | 60 | 0 | 0 | 0 | 1 | 0 | 0 | 9 | 32 | 16 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefaclor | 60 | 0 | 1 | 0 | 1 | 0 | 1 | 4 | 10 | 20 | 8 | 1 | 3 | 0 | 3 | 1 | 1 | 6 |
| cefuroxime | 60 | 0 | 0 | 1 | 0 | 4 | 10 | 21 | 12 | 3 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| ciprofloxacin | 60 | 49 | 1 | 0 | 3 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 60 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 7 | 21 | 19 | 5 | 0 | 4 | 0 | 0 | 1 |
| levofloxacin | 60 | 34 | 19 | 0 | 4 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| moxifloxacin | 60 | 23 | 24 | 7 | 2 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ofloxacin | 60 | 3 | 25 | 26 | 0 | 4 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SXT | 60 | 1 | 3 | 21 | 13 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 6 | 11 | 0 | 0 | 0 | 0 |
| Thailand | ||||||||||||||||||
| AMC | 263 | 0 | 0 | 4 | 7 | 16 | 72 | 94 | 45 | 19 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| ampicillin | 263 | 0 | 1 | 4 | 7 | 44 | 52 | 28 | 23 | 11 | 7 | 18 | 10 | 0 | 5 | 1 | 0 | 52 |
| azithromycin | 263 | 0 | 0 | 1 | 1 | 2 | 21 | 40 | 108 | 82 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| cefuroxime | 263 | 0 | 2 | 6 | 10 | 43 | 85 | 63 | 32 | 12 | 4 | 5 | 1 | 0 | 0 | 0 | 0 | 0 |
| clarithromycin | 263 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 7 | 28 | 77 | 93 | 52 | 0 | 1 | 1 | 0 | 0 |
| levofloxacin | 263 | 168 | 78 | 6 | 4 | 1 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
AMC, amoxicillin/clavulanic acid (2:1); SXT, trimethoprim/sulfamethoxazole (1:19).
aNot all isolates were tested.
India
For all H. influenzae (n = 135), susceptibility to amoxicillin/clavulanic acid by CLSI and PK/PD high-dose criteria was 97.0%, and 89.6% by EUCAST or PK/PD low-dose criteria. Susceptibility to ampicillin was 91.1% by CLSI and EUCAST criteria, which reflects the prevalence of β-lactamase-positive isolates (n = 11, 8.1%). None was β-lactamase negative and ampicillin resistant (BLNAR) according to the CLSI definition (ampicillin MIC ≥4 mg/L), but one isolate was BLNAR according to the EUCAST definition (ampicillin MIC ≥2 mg/L).
Using CLSI breakpoints, susceptibility to cephalosporins was high, at 97%–99%. However, using EUCAST breakpoints, rates of susceptibility were lower for cefixime (85.2%) and cefpodoxime (78.4%) and much lower for cefuroxime (17.9%), therefore showing considerable difference in cephalosporin susceptibility depending on the breakpoints used (Table 6).
Azithromycin susceptibility was high, at 94.7%, but clarithromycin was much lower, at 66.7% (CLSI breakpoints in CO2).
As would be expected, none of the β-lactamase-positive isolates was susceptible to ampicillin, but also none was susceptible to trimethoprim/sulfamethoxazole. Among the β-lactamase-positive isolates, 27.3% were resistant to amoxicillin/clavulanic acid by CLSI breakpoints (36.4% by EUCAST breakpoints) (Table 6).
South Korea
Of the 72 H. influenzae isolates from South Korea, 42 (58.3%) were β-lactamase positive. In addition, 13 isolates (18.1%) were BLNAR according to CLSI definition (ampicillin MIC ≥4 mg/L) and 22 isolates (30.6%) were BLNAR according to the EUCAST definition (ampicillin MIC ≥2 mg/L). As a consequence, ampicillin activity was very low (16.7% susceptibility by CLSI and EUCAST criteria). Susceptibility to amoxicillin/clavulanic acid in vitro was 62.5% by CLSI or high-dose PK/PD breakpoints and 38.9% by EUCAST or low-dose PK/PD pharmacokinetics. However, taking BLNAR (considered to be resistant) into account, the clinical susceptibility was reduced further to 56.9% and 31.9%, respectively.
Susceptibility to cefuroxime and cefaclor was also low by CLSI, at 40.3% and 29.2%, respectively. None of the isolates was susceptible to cefuroxime by EUCAST breakpoints and 16.7% were susceptible by PK/PD breakpoints. Cefaclor susceptibility by PK/PD was 1.4% (Table 6).
Azithromycin susceptibility was high, at 94.4%, but much lower for clarithromycin, at 50% (CLSI breakpoints in CO2) (Table 6).
Singapore
Eleven of the 60 isolates (18.3%) were β-lactamase positive and 2 (3.3%) were BLNAR according to the CLSI definition (ampicillin MIC ≥4 mg/L) and 11 isolates (18.3%) were BLNAR according to the EUCAST definition (ampicillin MIC ≥2 mg/L). Ampicillin susceptibility was 63.3% by both CLSI and EUCAST criteria. Susceptibility to amoxicillin/clavulanic acid was 93.3% by CLSI and PK/PD high-dose breakpoints and 86.7% by EUCAST and low-dose PK/PD breakpoints. Using CLSI breakpoints, the rates of susceptibility to cefuroxime and cefaclor were reduced to 85% and 75%, respectively, but were even lower using PK/PD or EUCAST breakpoints: 1.7% susceptible to cefuroxime by EUCAST and 5.0% susceptible to cefaclor by PK/PD breakpoints (Table 6).
Azithromycin susceptibility was high (98.3%), as was susceptibility to clarithromycin (83.3%) by CLSI breakpoints in CO2 (Table 6).
Thailand
Ninety-six out of 263 isolates were β-lactamase positive (36.5%). Twelve (4.6%) were BLNAR according to the CLSI definition (ampicillin MIC ≥4 mg/L) and 32 (12.2%) were BLNAR according to the EUCAST definition (ampicillin MIC ≥2 mg/L). Therefore, ampicillin susceptibility was low, at 51.7%, whereas 97.7% were susceptible to amoxicillin/clavulanic acid by CLSI and high-dose PK/PD breakpoints or 90.5% by EUCAST and low-dose PK/PD breakpoints. Cefuroxime susceptibility was high with CLSI breakpoints (96.2%) but 79.5% by PK/PD and 7.2% by EUCAST (Table 6).
Virtually all isolates were susceptible to azithromycin (99.6%) but only 79.5% were susceptible to clarithromycin (CLSI breakpoints in CO2). More than 99% of isolates were susceptible to levofloxacin by all three breakpoints (Table 6).
Comparative susceptibility of H. influenzae isolates by country
Some clear differences between the countries were apparent: susceptibility to amoxicillin/clavulanic acid, ampicillin, cefuroxime and clarithromycin were significantly lower in South Korea than in other countries. In addition, susceptibility to levofloxacin and trimethoprim/sulfamethoxazole was significantly lower in India (data not shown).
M. catarrhalis susceptibility in individual countries
Summary MIC and susceptibility data for all M. catarrhalis isolates are shown in Table 8 and the complete MIC distribution data are in Table 9.
Table 8.
MIC and susceptibility results for M. catarrhalis isolates by country
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) |
CLSI |
PK/PD |
EUCAST |
|||||||||
| Antimicrobial | na | 50% | 90% | min | max | %S | %I | %R | %S | %S | %I | %R |
| India | ||||||||||||
| AMCb | 61 | 0.25 | 2 | 0.03 | 16 | 98.4 | 0.0 | 1.6 | 93.4 (98.4) | 80.3 | 0.0 | 19.7 |
| azithromycin | 61 | 0.5 | 8 | ≤0.015 | >256 | NA | NA | NA | NA | NA | NA | NA |
| cefixime | 37 | 0.5 | 0.5 | 0.06 | 1 | NA | NA | NA | 100 | 97.3 | 2.7 | 0.0 |
| cefpodoxime | 61 | 1 | 2 | 0.12 | 8 | NA | NA | NA | 45.9 | NA | NA | NA |
| cefuroximec | 59 | 2 | 16 | 0.25 | 64 | 81.4 | 6.8 | 11.9 | 23.7 | 0.0 | 81.4 | 18.6 |
| ciprofloxacin | 61 | 1 | 8 | ≤0.015 | >32 | 67.2 | 0.0 | 32.8 | 67.2 | 34.4 | 0.0 | 65.6 |
| clarithromycin | 49 | 0.5 | 8 | ≤0.015 | 64 | NA | NA | NA | NA | NA | NA | NA |
| levofloxacin | 61 | 1 | 8 | 0.03 | >32 | 80.3 | 0.0 | 19.7 | 80.3 | 57.4 | 0.0 | 42.6 |
| South Korea | ||||||||||||
| AMCb | 18 | 0.12 | 0.25 | ≤0.015 | 0.25 | 100 | 0.0 | 0.0 | 100 (100) | 100 | 0.0 | 0.0 |
| azithromycin | 4 | 0.12 | >256 | 0.06 | >256 | NA | NA | NA | NA | NA | NA | NA |
| cefaclor | 18 | 0.5 | 1 | ≤0.015 | 1 | 100 | 0.0 | 0.0 | 50.0 | NA | NA | NA |
| cefuroximec | 18 | 1 | 2 | 0.03 | 2 | 100 | 0.0 | 0.0 | 50.0 | 5.6 | 94.4 | 0.0 |
| ciprofloxacin | 18 | 0.06 | 0.12 | 0.03 | 0.12 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 |
| clarithromycin | 18 | 0.5 | 2 | 0.12 | >256 | NA | NA | NA | NA | NA | NA | NA |
| levofloxacin | 18 | 0.03 | 0.06 | 0.03 | 0.06 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 |
| Singapore | ||||||||||||
| AMCb | 15 | 0.06 | 0.25 | ≤0.015 | 0.5 | 100 | 0.0 | 0.0 | 100 (100) | 100 | 0.0 | 0.0 |
| azithromycin | 20 | 0.12 | 0.25 | 0.12 | 0.5 | NA | NA | NA | NA | NA | NA | NA |
| cefaclor | 20 | 2 | 4 | 1 | 4 | 100 | 0.0 | 0.0 | 0.0 | NA | NA | NA |
| cefuroximec | 14 | 1 | 2 | 0.12 | 4 | 100 | 0.0 | 0.0 | 57.1 | 7.1 | 92.9 | 0 |
| ciprofloxacin | 20 | 0.06 | 0.06 | 0.03 | 0.5 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 |
| clarithromycin | 20 | 0.25 | 0.25 | 0.03 | 0.5 | NA | NA | NA | NA | NA | NA | NA |
| levofloxacin | 20 | 0.06 | 0.06 | 0.03 | 0.06 | 100 | 0.0 | 0.0 | 100 | 100 | 0.0 | 0.0 |
| moxifloxacin | 20 | 0.06 | 0.06 | 0.06 | 0.06 | NA | NA | NA | 100 | 100 | 0.0 | 0.0 |
| Thailand | ||||||||||||
| AMCb | 49 | 0.25 | 0.5 | 0.03 | 0.5 | 100 | 0.0 | 0.0 | 100 (100) | 100 | 0.0 | 0.0 |
| azithromycin | 49 | 0.25 | 1 | ≤0.015 | >256 | NA | NA | NA | NA | NA | NA | NA |
| cefuroximec | 49 | 1 | 2 | 0.06 | 4 | 100 | 0.0 | 0.0 | 63.3 | 2.0 | 98.0 | 0.0 |
| clarithromycin | 49 | 0.25 | 1 | 0.03 | >256 | NA | NA | NA | NA | NA | NA | NA |
| levofloxacin | 49 | 0.06 | 0.12 | 0.03 | >32 | 98.0 | 0.0 | 2.0 | 98.0 | 98.0 | 0.0 | 2.0 |
min, minimum; max, maximum; S, susceptible; I, intermediate; AMC, amoxicillin/clavulanic acid; NA, no breakpoint data available (NA for azithromycin and clarithromycin by CLSI, PK/PD or EUCAST because Etest® breakpoints in CO2 not available).
aNot all isolates were tested.
bPK/PD susceptibility at high dose is shown in parentheses.
cBreakpoints used are for cefuroxime axetil.
For South Korea, Singapore and Thailand, 100% susceptibility was observed according to CLSI breakpoints for all antimicrobial agents tested (except 98.0% for levofloxacin in Thailand). Amoxicillin/clavulanic acid and fluoroquinolone susceptibility was also 100% (except 98.0% for levofloxacin in Thailand) for both PK/PD and EUCAST breakpoints (Table 8). As noted previously for other bacterial pathogens, susceptibility to cefaclor and cefuroxime was significantly lower according to EUCAST and/or PK/PD breakpoints.
Isolates of M. catarrhalis from India were less susceptible to antimicrobial agents, but these were still 98.4% susceptible to amoxicillin/clavulanic acid by CLSI or PK/PD high-dose breakpoints and 93.4% by PK/PD low-dose breakpoints. Amoxicillin/clavulanic acid susceptibility by EUCAST breakpoints was 80.3%. Susceptibility to cefuroxime was 81.4% by CLSI breakpoints, 23.7% by PK/PD and 0% by EUCAST. Cefpodoxime has PK/PD breakpoints that indicated 45.9% susceptibility. Levofloxacin susceptibility was 80.3% by CLSI or PK/PD and 57.4% by EUCAST. Lower susceptibility was observed for ciprofloxacin, at 67.2% and 34.4%, respectively (Table 8).
Age group analysis
Using CLSI breakpoints, susceptibility to tested antimicrobial agents was compared across age groups in each of the countries (data not shown). Insufficient isolates from the individual age groups were obtained from India for H. influenzae or from all countries for M. catarrhalis to allow an analysis to be performed.
Generally, there was no significant difference when comparing antimicrobial agent susceptibility between age groups for each country for S. pneumoniae, S. pyogenes or H. influenzae. In the few cases where a significant difference in susceptibility by age group was observed (P < 0.05) there tended to be lower susceptibility in paediatric patients compared with adults and/or the elderly: amoxicillin/clavulanic acid versus S. pneumoniae in South Korea—paediatric (57.1%) versus adult (92.0%); trimethoprim/sulfamethoxazole versus S. pneumoniae in South Korea—paediatric (7.1%) versus adult (48.0%) or elderly (52.2%); penicillin versus S. pneumoniae in Thailand—paediatric (32.4%) versus elderly (57.3%); and ampicillin versus H. influenzae in South Korea—paediatric (0%) versus adult (20.0%) or elderly (27.3%). In one case there was a difference between adults and the elderly: ofloxacin versus S. pneumoniae in India—adults (73.4%) versus elderly (93.9%).
Discussion
SOAR is an ongoing surveillance study (which began in 2002) of key respiratory pathogens in various parts of the world. The data presented here concern the susceptibility of S. pneumoniae, S. pyogenes, H. influenzae and M. catarrhalis isolated during the 2012–14 period in India, South Korea, Singapore and Thailand.
Antimicrobial agent resistance in respiratory pathogens is considered to be a worldwide problem, although susceptibility can differ widely among countries. The SOAR study has previously evaluated S. pneumoniae, H. influenzae and M. catarrhalis from Singapore (2009–11) and S. pneumoniae from Thailand (2007–09).19,20 It would appear that antibiotic susceptibility in S. pneumoniae and M. catarrhalis from Singapore has not varied significantly between 2009–11 and the current study period (2012–14) (data not shown). For H. influenzae in Singapore, ampicillin susceptibility reduced from 72% in 2009–11 to 63.3% in 2012–14, but this is not a statistically significant change (P = 0.252). Similar insignificant changes in susceptibility occurred for other antibiotics against H. influenzae in Singapore, except for cefuroxime, where susceptibility reduced from 99% in 2009–11 to 85% in 2012–14, which is statistically significant (P = 0.0004). In the Thailand 2007–09 study, the testing was done in various phases, which makes it difficult to make direct comparisons for most antibiotics.20 However, macrolide susceptibility based on erythromycin disc testing showed a reduction in susceptibility from 60.4% in 2007–09 to 51.9% in 2012–14, which is just marginally short of being significant (P = 0.063).
Overall, antimicrobial resistance was not a major issue for S. pyogenes (all isolates from India), which were all fully susceptible to penicillin and, by association, other β-lactam agents such as cephalosporins and amoxicillin/clavulanic acid. Nevertheless, erythromycin susceptibility was very low, at around 20%, thereby reducing the clinical utility of macrolides for respiratory infections caused by S. pyogenes in India.
Antimicrobial agent susceptibility was very high in M. catarrhalis from South Korea, Singapore and Thailand based on CLSI breakpoints. Based on EUCAST and PK/PD breakpoints, prevalence of resistance to cefuroxime and cefaclor was high. Following EUCAST guidelines, amoxicillin/clavulanic acid or fluoroquinolones would be the only viable treatment option. However, it is important to note that we were unable to assess the activity of macrolides against M. catarrhalis due to a lack of breakpoints in the presence of CO2. M. catarrhalis from India showed reduced susceptibility to fluoroquinolones and cefuroxime.
Antimicrobial susceptibility in H. influenzae was generally high in India, Singapore and Thailand, with significantly lower antibiotic susceptibility in South Korea for all antimicrobial agents, except for azithromycin, levofloxacin and trimethoprim/sulfamethoxazole. Trimethoprim/sulfamethoxazole susceptibility was amongst the lowest in each country where it was tested, but was significantly lower in India than elsewhere. This may relate to reported extensive use of trimethoprim/sulfamethoxazole in India.21 Levofloxacin susceptibility was also significantly lower in India than other countries, which complements the susceptibility data for M. catarrhalis from India. The prevalence of β-lactamase varied from country to country and was highest in South Korea (58.3%) and lowest in India (8.1%). However, amoxicillin/clavulanic acid susceptibility was >90% in all countries except South Korea (62.5%). Low antimicrobial agent susceptibility is a major problem in H. influenzae from South Korea, as found previously,14 and the data from this current study suggest that only azithromycin and levofloxacin are treatment options for H. influenzae from this country.
For S. pneumoniae, susceptibility to macrolides, trimethoprim/sulfamethoxazole and penicillin was very low in each of the four Asian countries, as found in previous publications.11–13 Overall, only amoxicillin/clavulanic acid and levofloxacin had >80% susceptibility in all four countries. As observed with H. influenzae, low antimicrobial agent susceptibility is a particular problem in South Korea. Penicillin non-susceptibility is also a problem in this region of the world because of associated cross-resistance to macrolides and also, but to a lesser extent, to amoxicillin/clavulanic acid (amoxicillin). Data from this study indicate that only levofloxacin has good activity against penicillin-resistant pneumococci.
The data from this study confirm that isolates of S. pneumoniae susceptible to penicillin G are also susceptible to other penicillins as inferred by CLSI and EUCAST guidelines and cephalosporins as inferred by CLSI guidelines. Interestingly, the data from this study found the reverse was not always correct using CLSI breakpoints; i.e. most penicillin-non-susceptible S. pneumoniae were susceptible to amoxicillin/clavulanic acid (amoxicillin) and cefuroxime. Therefore, either these β-lactam agent breakpoints are not correct or the CLSI cross-resistance statement within the β-lactam class is not correct. This warrants further investigation.
Our analysis would indicate that generally there is little difference in antimicrobial agent susceptibility between isolates from varying patient age groups. Where differences did occur, generally the susceptibility of paediatric isolates was lower than that of adults or the elderly. A global demographic analysis study found similar results for penicillin and erythromycin susceptibility in infants compared with both adults and the elderly,22 and SOAR data from Turkey also found this to be true for penicillin but not macrolides.23 Interestingly, within the same geographical region, SOAR Vietnam paediatric isolates were less susceptible to most antibiotics than isolates from the elderly, but there was no difference compared with adults (except for azithromycin).24
In this analysis we measured susceptibility by three breakpoint criteria. In general these produced quite similar results. However, there were notable exceptions. The first of these was considerably lower cefaclor and ofloxacin susceptibility by EUCAST compared with CLSI with S. pneumoniae. To a lesser extent this was also the case for cefuroxime and cefpodoxime. Similarly, in H. influenzae cefuroxime and ciprofloxacin susceptibility was considerably lower with EUCAST than with CLSI. This has been noted elsewhere25 and in other SOAR publications.22,23 As has been stated previously, considerable harmonization of breakpoints is necessary to avoid confusion and potentially poor therapeutic decisions.
In summary, we have analysed antibiotic susceptibility in community-acquired respiratory pathogens. There are quite large country-specific differences in antibiotic susceptibility even within the same region, with overall antibiotic resistance being the highest in S. pneumoniae and isolates from South Korea. These data reinforce the need for regular antibiotic resistance surveillance to track changes in susceptibility over time.
Funding
This study was funded by GlaxoSmithKline.
Transparency declarations
This article forms part of a Supplement sponsored by GlaxoSmithKline. D. Torumkuney, S. Kanapura, V. Chareonphaibul, J. H. Kim, and P. Mukherjee are employees of GlaxoSmithKline. D. Torumkuney and J. H. Kim also hold shares in GlaxoSmithKline.
I. Morrissey is an employee of IHMA, a medical communication and consultancy company, who participated in the exploration, interpretation of the results and preparation of this manuscript on behalf of GlaxoSmithKline. IHMA also provided medical writing support in the form of writing assistance, collating author's comments, grammatical editing and referencing that was paid for by GlaxoSmithKline. All other authors declare that they have no conflict of interest.
Editorial assistance was provided by Tracey Morris, Livewire Editorial Communications.
Acknowledgements
We would like to thank Dr Keith Barker (GSK) for reviewing the manuscript and Krutika Patil, Anjali Shetty (P. D. Hinduja Hospital and Medical Research Center), Associate Professor P. Santanirand (Head, Microbiology Laboratory, Ramathibodi Hospital), Seungok Lee (Korea) and Soo Young Kim (Korea) for their participation in this study.
References
- 1.World Health Organization World Health Part III. Global Health Indicators. http://www.who.int/gho/publications/world_health_statistics/EN_WHS2014_Part3.pdf?ua=1.
- 2.Torres A, Blasi F, Peetermans WE et al. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: a literature review. Eur J Clin Microbiol Infect Dis 2014; 33: 1065–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riedel S, Beekmann SE, Heilmann KP et al. Antimicrobial use in Europe and antimicrobial resistance in Streptococcus pneumoniae. Eur J Clin Microbiol Infect Dis 2007; 26: 485–90. [DOI] [PubMed] [Google Scholar]
- 4.Goossens H, Ferech M, Vander Stichele R et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005; 365: S79–87. [DOI] [PubMed] [Google Scholar]
- 5.Bronzwaer SL, Cars O, Buchholz U et al. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis 2002; 8: 278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67: 71–9. [DOI] [PubMed] [Google Scholar]
- 7.Liam C-K, Pang Y-K, Poosparajah S et al. Community-acquired pneumonia: an Asia Pacific perspective. Respirology 2007; 12: 162–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tleyjeh IM, Tlaygeh HM, Hejal R et al. The impact of penicillin resistance on short-term mortality in hospitalized adults with pneumococcal pneumonia: a systematic review and meta-analysis. Clin Infect Dis 2006; 42: 788–97. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs MR, Johnson CE. Macrolide resistance: an increasing concern for treatment failure in children. Pediatr Infect Dis J 2003; 22: S131–8. [DOI] [PubMed] [Google Scholar]
- 10.Kang CI, Song JH, Kim SH. Association of levofloxacin resistance with mortality in adult patients with invasive pneumococcal diseases: a post hoc analysis of a prospective cohort. Infection 2013; 41: 151–7. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Song JH, Chung DR et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother 2012; 56: 1418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Kim SH, Park M et al. High prevalence of multiresistance in levofloxacin-nonsusceptible Streptococcus pneumoniae isolates in Korea. Diagn Microb Infect Dis 2013; 76: 227–31. [DOI] [PubMed] [Google Scholar]
- 13.Chawla K, Gurung B, Mukhopadhyay C et al. Reporting emerging resistance of Streptococcus pneumoniae from India. J Glob Infect Dis 2010; 2: 10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae S, Lee J, Kim E et al. Serotype distribution and β-lactam resistance in Haemophilus influenzae isolated from patients with respiratory infections in Korea. J Microbiol 2010; 48: 84–8. [DOI] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disc Susceptibility Tests—Twelfth Edition: Approved Standard M02-A12. CLSI, Wayne, PA, USA, 2015. [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement M100-S25. CLSI, Wayne, PA, USA, 2015. [Google Scholar]
- 17.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 5.0, 2015 http://www.eucast.org.
- 18.Anon JB, Jacobs MR, Poole MD et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004; 130 Suppl 1: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien D, Tan TY, Lee I. Antibacterial resistance among Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis from Singapore. Results from the Survey of Antibiotic Resistance (SOAR) 2009–2011. In: Abstracts of the 8th International Symposium on Antimicrobial Agents and Resistance, Seoul, Korea, 2011. Abstract P3-109. [Google Scholar]
- 20.Khantawa B, Baosount V, Warachit B et al. Antibacterial resistance among Streptococcus pneumoniae from 4 centres in Thailand: results from the Survey of Antibiotic Resistance (SOAR) 2007–2009. In: Abstracts of the 20th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 2010. Abstract 2570. European Society for Clinical Microbiology and Infectious Diseases, Basel, Switzerland. [Google Scholar]
- 21.Chandy SJ, Thomas K, Mathai E et al. Patterns of antibiotic use in the community and challenges of antibiotic surveillance in a lower-middle-income country setting: a repeated cross-sectional study in Vellore, south India. J Antimicrob Chemother 2013; 68: 229–36. [DOI] [PubMed] [Google Scholar]
- 22.Hoban D, Baquero F, Reed V et al. Demographic analysis of antimicrobial resistance among Streptococcus pneumoniae: worldwide results from PROTEKT 1999–2000. Int J Infect Dis 2005; 9: 262–73. [DOI] [PubMed] [Google Scholar]
- 23.Soyletir G, Altinkanat G, Gur D et al. Results from the Survey of Antibiotic Resistance (SOAR) 2011–13 in Turkey. J Antimicrob Chemother 2016; 71 Suppl 1: i71–i83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van PH, Binh PT, Minh NHL et al. Results from the Survey of Antibiotic Resistance (SOAR) 2009–11 in Vietnam. J Antimicrob Chemother 2016; 71 Suppl 1: i93–i102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchese A, Esposito S, Barbieri R et al. Does the adoption of EUCAST susceptibility breakpoints affect the selection of antimicrobials to treat acute community-acquired respiratory tract infections? BMC Infect Dis 2012; 12: 181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


