Abstract
The intercalated cells of the kidney collecting duct are specialized for physiologically regulated proton transport. In these cells, a vacuolar H(+)-ATPase is expressed at enormous levels in a polarized distribution on the plasma membrane, enabling it to serve in transepithelial H+ transport. In contrast, in most eukaryotic cells, vacuolar H(+)-ATPases reside principally in intracellular compartments to effect vacuolar acidification. To investigate the basis for the selective amplification of the proton pump in intercalated cells, we isolated and sequenced cDNA clones for two isoforms of the approximately 56-kDa subunit of the H(+)-ATPase and examined their expression in various tissues. The predicted amino acid sequence of the isoforms was highly conserved in the internal region but diverged in the amino and carboxyl termini. mRNA hybridization to a cDNA probe for one isoform (the "kidney" isoform) was detected only in kidney cortex and medulla, whereas mRNA hybridization to the other isoform of the approximately 56-kDa subunit and to the H(+)-ATPase 31-kDa subunit was found in the kidney and other tissues. Immunocytochemistry of rat kidney with an antibody specific to the kidney isoform revealed intense staining only in the intercalated cells. Staining was absent from proximal tubule and thick ascending limb, where H(+)-ATPase was detected with a monoclonal antibody to the 31-kDa subunit of the H(+)-ATPase. This example of specific amplification of an isoform of one subunit of the vacuolar H(+)-ATPase being limited to a specific cell type suggests that the selective expression of the kidney isoform of the approximately 56-kDa subunit may confer the capacity for amplification and other specialized functions of the vacuolar H(+)-ATPase in the renal intercalated cell.
Full text
PDF

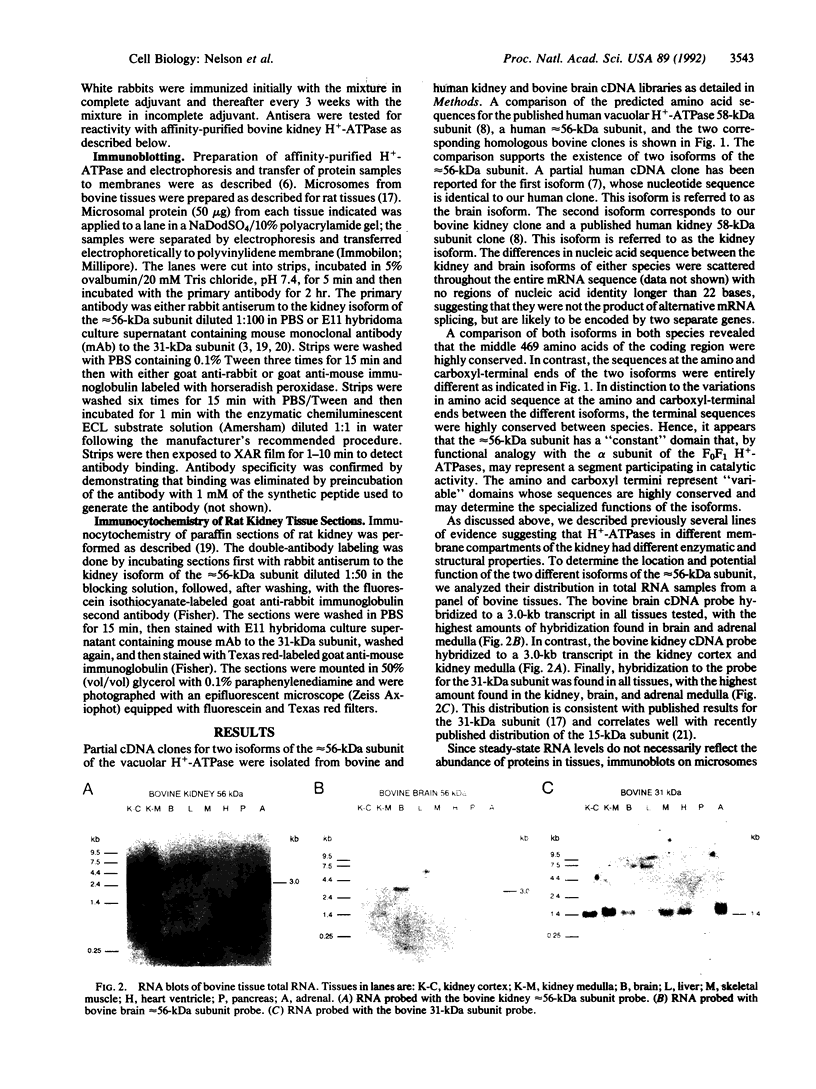


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastani B., Purcell H., Hemken P., Trigg D., Gluck S. Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest. 1991 Jul;88(1):126–136. doi: 10.1172/JCI115268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi P., Rausch T., Struve I., Morgan L., Taiz L. An mRNA from human brain encodes an isoform of the B subunit of the vacuolar H(+)-ATPase. J Biol Chem. 1990 Oct 15;265(29):17428–17431. [PubMed] [Google Scholar]
- Blair H. C., Teitelbaum S. L., Ghiselli R., Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989 Aug 25;245(4920):855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- Bowman E. J., Tenney K., Bowman B. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-1 encoding the 67-kDa subunit reveals homology to other ATPases. J Biol Chem. 1988 Oct 5;263(28):13994–14001. [PubMed] [Google Scholar]
- Brown D., Gluck S., Hartwig J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J Cell Biol. 1987 Oct;105(4):1637–1648. doi: 10.1083/jcb.105.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Hirsch S., Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest. 1988 Dec;82(6):2114–2126. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Anderson R. G., Faust J. R., Goldstein J. L., Brown M. S. Appearance of crystalloid endoplasmic reticulum in compactin-resistant Chinese hamster cells with a 500-fold increase in 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1185–1189. doi: 10.1073/pnas.79.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forgac M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol Rev. 1989 Jul;69(3):765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- Gillespie G. A., Somlo S., Germino G. G., Weinstat-Saslow D., Reeders S. T. CpG island in the region of an autosomal dominant polycystic kidney disease locus defines the 5' end of a gene encoding a putative proton channel. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4289–4293. doi: 10.1073/pnas.88.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck S., Caldwell J. Immunoaffinity purification and characterization of vacuolar H+ATPase from bovine kidney. J Biol Chem. 1987 Nov 15;262(32):15780–15789. [PubMed] [Google Scholar]
- Hirsch S., Strauss A., Masood K., Lee S., Sukhatme V., Gluck S. Isolation and sequence of a cDNA clone encoding the 31-kDa subunit of bovine kidney vacuolar H+-ATPase. Proc Natl Acad Sci U S A. 1988 May;85(9):3004–3008. doi: 10.1073/pnas.85.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman J. S., Stahl P. D., Gluck S. Distribution and structure of the vacuolar H+ ATPase in endosomes and lysosomes from LLC-PK1 cells. Exp Cell Res. 1991 Feb;192(2):445–452. doi: 10.1016/0014-4827(91)90063-z. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Fried V. A., Stone D. K., Johnston P. A., Xie X. S. Human endomembrane H+ pump strongly resembles the ATP-synthetase of Archaebacteria. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6067–6071. doi: 10.1073/pnas.86.16.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Q., Gluck S. Isolation and properties of bovine kidney brush border vacuolar H(+)-ATPase. A proton pump with enzymatic and structural differences from kidney microsomal H(+)-ATPase. J Biol Chem. 1990 Dec 15;265(35):21957–21965. [PubMed] [Google Scholar]
- Yurko M. A., Gluck S. Production and characterization of a monoclonal antibody to vacuolar H+ATPase of renal epithelia. J Biol Chem. 1987 Nov 15;262(32):15770–15779. [PubMed] [Google Scholar]
- Zimniak L., Dittrich P., Gogarten J. P., Kibak H., Taiz L. The cDNA sequence of the 69-kDa subunit of the carrot vacuolar H+-ATPase. Homology to the beta-chain of F0F1-ATPases. J Biol Chem. 1988 Jul 5;263(19):9102–9112. [PubMed] [Google Scholar]