Abstract
Ceftizoxime sodium is a third-generation cephalosporin available for parenteral administration, which is mainly excreted through urine. A rapid and sensitive ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS-MS) method was developed and validated for the determination of ceftizoxime in human serum and urine. The samples were purified by protein precipitation and separated on an XTerra Phenyl column (4.6×50 mm, 5 µm). Electrospray ionization in the positive ion mode and multiple reaction monitoring were used to monitor the ion transitions at m/z 383.9/227.0. The results revealed that the method had excellent selectivity. The linear range covered from 2.50 to 10,000 ng/mL in serum and from 0.500 to 50.0 µg/mL in urine, respectively. Intra-batch and inter-batch precisions (in terms of relative standard deviation) were all <15% and the accuracies (in terms of relative error) were within the range of ±15%. The lower limit of quantification, stability and extraction recovery were also validated and satisfied the criteria of validation. Finally, the method was successfully applied to a pharmacokinetic study of Chinese elderly healthy subjects after intravenous administration. The Cmax values in serum were 34,721.3 ± 5,697.3 ng/mL. Serum concentrations declined with t1/2 of 2.57 ± 0.22 h.
Introduction
Ceftizoxime sodium is a parenteral β-lactamic anti-bacterial drug applied to treat with both the gram-positive and gram-negative bacterial infection. The molecular formula of ceftizoxime is C13H13N5O5S2 and the relative molecular weight is 383.4. Ceftizoxime rather resembles cefotaxime in its structure and properties, but is not sensitive to the hydrolytic enzymes because its C-3 side chain is removed. After parenteral administration, ∼80% amount of ceftizoxime is excreted through urine in forms of parent drug in 24 h (1). As a result, both serum and urine concentration of the parent drug should be analyzed if the pharmacokinetic profile would be described and the safety of this drug would be evaluated.
So far, several analytical methods have been reported for ceftizoxime determination, including bioassays (1, 2), HPLC combined with UV spectrophotometer (3–7) and flow injection analysis (FIA) with a UV spectrophotometer (8). However, these methods all have their limitations. The method of bioassay using Bacillus subtilis ATCC 6633 was time-consuming and showed poor sensitivity, with the lower limit of quantification (LLOQ) of 1.25 µg/mL. The method of FIA was even less sensitive than bioassay. These methods could not meet the requirement for measuring the drug concentration today. The application of LC-UV for determining ceftizoxime with a 0.1 µg/mL sensitivity limit made the serum samples at 12 h post-infusion measurable (3); however, the serum samples collected after that time-point would be below LLOQ. Recent years, liquid chromatography–mass spectrometry (LC–MS) was also applied to ceftizoxime detection; however, the method was mainly used in identifying the impurities (9–11), and had not been validated for determining the drug in human serum. Besides, the method of determining ceftizoxime in human urine has not been reported in detail.
Although ceftizoxime has been approved to be used as an anti-bacterial drug for more than 30 years, a few of clinical studies are still being conducted in some special populations, such as people of different races, ages and with different renal functions. If a more sensitive method could be developed, the profile of the elimination phase would be described more exactly, which will help us analyze the pharmacokinetic differences between different populations. Thus, a rapid method of high sensitivity is required for determining the ceftizoxime concentration in human serum and urine today.
In this article, an analytical method of LC–MS-MS to determine ceftizoxime concentration in human serum and urine was developed and fully validated. The method was applied to analyze the samples from Chinese healthy elderly subjects administrated with ceftizoxime, the results proved that the method performed reliably and met the requirement of analysis. Besides, this sensitive method was simple and had a widely linear range and a short analytical run-time, thus provided a possibility of mass determination.
Experimental
Reagents and chemicals
The standards of ceftizoxime (purity 98.2%, batch no.130504–200702, chemical structure shown in Figure 1A) and cefotaxime (Internal standard, IS, purity 90.6%, batch no. 130483–200904, chemical structure shown in Figure 1B) were purchased from National Institutes for Food and Drug Control (Beijing, China). Acetonitrile (ACN; HPLC grade) was purchased from Burdick & Jackson, Korea. Formic acid (FA; A.R. grade) was purchased from Sigma, USA. Ammonium acetate, dimethyl sulphoxide (DMSO) and aqueous ammonia (all A.R. grade) was purchased from Sinopharm Chemical Reagent Co., Ltd, China. XTerra Phenyl column (5 µm, 4.6×50 mm) was purchased from Waters Co., Ltd, USA.
Figure 1.
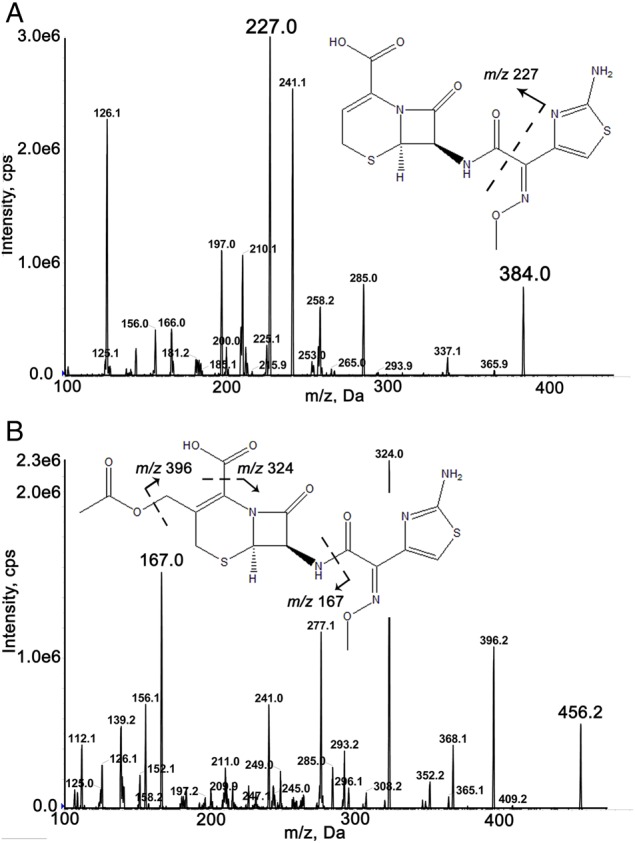
Product ion spectra: (A) ceftizoxime and (B) cefotaxime.
Instrumentation and experimental conditions
Liquid chromatography
UPLC–MS-MS was performed on an ACQUITY UPLC system (Waters Corp., USA) and a X Terra Phenyl column (5 µm, 4.6×50 mm) with a gradient elution by a mobile phase consisting of 25 mM ammonium acetate (pH 7.5) (A) and acetonitrile (B), with the flow rate of 0.7 mL/min. The mobile phase gradient commenced at 10% Solvent B which increased slowly to 60% by 0.8 min and then was maintained for 0.5 min. Next, the proportion of Solvent B in the mobile phase was returned to 10% over a 0.1 min period and maintained for 0.9 min until the end of the chromatographic run. The column temperature was maintained at 40°C. The injection volume was set to be 10 µL.
Mass spectrometry conditions
Mass spectrometric detection was carried out on a QTrap 5500 (AB Sciex, USA). The electrospray ionization (ESI) source was operated in the positive mode. The curtain, nebulizer and turbo-gas (all nitrogen) pressure were set at 45, 60 and 60 psi, respectively. The source temperature was 500°C and the ion spray needle voltage was 5,000 V. The collision-activated dissociation gas level was set at 7. Other optimized ionization conditions were referred to Table I. The product ion spectra of ceftizoxime and cefotaxime (IS) are shown in Figure 1. Acquisition in the multiple reaction monitoring (MRM) mode was performed with a dwell time of 100 ms. Data were collected and analyzed by the Analyst Data Acquisition and Processing software (Version 1.5.1, AB Sciex, USA).
Table I.
Optimized Parameters on Mass to Charge (m/z) Transition and DP, EP, CE and CXP of Ceftizoxime and IS
| Analytes | m/z | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|
| Ceftizoxime | 383.9/227.0 | 161 | 10 | 27 | 18 |
| Cefotaxime | 456.0/167.0 | 175 | 10 | 27 | 14 |
Preparation of calibration standards and quality control samples
Stock solution (1.0 mg/mL) of ceftizoxime was prepared in duplicate by dissolving the accurately weighed standard in the mixture of DMSO and water (1:50, v/v) for preparation of calibration standards and quality controls (QCs), respectively. The final concentrations of ceftizoxime standard calibration serum samples were 2.50, 5.00, 10.0, 100, 500, 1,000, 5,000 and 10,000 ng/mL. The final concentrations of ceftizoxime in the low-, medium- and high-QC serum samples were 7.50, 750 and 7,500 ng/mL, respectively. For the matrix of urine, the final concentrations of ceftizoxime standard calibration were 0.500, 1.00, 2.00, 4.00, 8.00, 20.0 and 50.0 µg/mL and the concentrations of the QC samples were 1.50, 6.00 and 40.0 µg/mL.
Stock solution (1.0 mg/mL) of cefotaxime (IS) was prepared in water. The internal standard working solution (ISws, 1 µg/mL for serum samples; ISwu, 20 µg/mL for urine samples) was diluted with mobile phase A.
Sample preparation
A total of 50 µL aliquot serum sample were spiked with 50 µL of ISws (1 µg/mL) solution and 300 µL acetonitrile. After a thorough vortex mixing for 1 min, mixtures were centrifuged at 13,000 rpm for 10 min. Aliquots of the supernatant (350 µL) were transferred to the glass tubes and evaporated at 40°C under a gentle stream of nitrogen. The residues were dissolved in 100 µL of the mixture of mobile phase A and B (9:1, v/v). Finally, 10 µL of the solution was injected to UPLC–MS-MS system.
To prepare the urine samples, a total of 20 µL aliquot urine sample were spiked with 50 µL of ISwu (20 µg/mL) solution and 930 µL of mobile phase A/acetonitrile (9:1, v/v). After a thorough vortex mixing for 1 min, mixtures were centrifuged at 13,000 rpm for 10 min. Then, 10 µL of the supernatant was injected to UPLC–MS-MS system directly.
Method validation
The method was validated for the selectivity, linearity, LLOQ, accuracy, precision, matrix effect, recovery, stability and dilution integrity.
Selectivity
Six different lots of human blank serum and urine were individually analyzed to exclude the interference of endogenous substances to evaluate the selectivity.
Linearity of calibration curve, LLOQ, precision and accuracy
In both the serum and urine validation, the calibration curves were performed with eight concentrations. The linearity of each calibration curve was determined by plotting the peak area ratio (y) of the analyte to IS versus the nominal concentration (x) of the analyte with weighed (1/x2) least square linear regression. The lowest quantification level of ceftizoxime on the calibration curves (2.50 ng/mL in serum and 0.500 µg/mL in urine, respectively) were recognized as LLOQ. The accuracy was expressed as relative error (RE) and the precision was expressed as relative standard deviation (RSD). Both the RE% and RSD% of LLOQ were considered acceptable when they are within ±20%, whereas the RE% and RSD% of other measured calibration concentrations should be within ±15%. The correlation coefficient (r) should be >0.99.
The accuracy and precision of the assay were assessed on three different days using five replicates of low, medium and high QCs. Concentrations were measured by the calibration curve. RE% and RSD% ≤15% were considered acceptable. Both the QC samples in human serum and urine were assessed.
Matrix effect and extraction recovery
The matrix effect for ceftizoxime was estimated using five different individual lots of human serum or urine, by calculating the ratio of the peak area in the presence of matrix (extracted blank matrix spiked with the analyte), to the peak area in the absence of matrix (pure solution of the analyte at the corresponding concentrations). The RSD% of the matrix effect recoveries calculated from the five different lots of matrix should not be >15%. The extraction recovery was evaluated by comparing peak areas obtained from extracted spiked samples with those of the post-extracted spiked samples.
Stability
Freeze–thaw stability was evaluated after three freeze (−80°C) and thaw (room temperature) cycles before sample preparation. Short-term stability was assessed by analyzing QC samples kept at room temperature for 4 h in serum or 24 h in urine. Auto-sampler stability was evaluated after the processed QC samples were placed in auto-sampler (10°C) for 24 h. Long-term stability was investigated by analyzing QC samples after storage at −80°C for 2 months.
Dilution integrity
In order to analyze the samples at the concentration above the upper limit of quantification (ULOQ), serum samples spiked with ceftizoxime at 30.0 µg/mL and urine samples spiked with ceftizoxime at 100 µg/mL were exerted 10-fold dilution with pooled serum and urine, respectively, and then analyzed. The RE% and RSD% for five replicates after dilution should be ≤15%.
Method application
This validated LC–MS-MS method would be applied to determination of serum and urine concentrations of ceftizoxime in a clinical study of ceftizoxime pharmacokinetics in Chinese elderly people. The study was approved by the Ethical Committee of Peking Union Medical College Hospital (approved on 31 October 2012, Beijing, permission number S-477) and was performed in accordance with the Declaration of Helsinki. Five Chinese healthy elderly volunteers (more than 65 years old) were given intravenous infusion of 0.5 g ceftizoxime over a 30 min period. The blood samples (4 mL) were collected before administration and at 0.0833, 0.25, 0.50, 0.75 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, 12.0 and 24.0 h after dosing. These samples were placed for 20 min at room temperature and centrifuged at 3,000 g for 10 min at 4°C to obtain serum. The urine samples were collected before administration and at intervals of 0–4, 4–8, 8–12 and 12–24 h thereafter. Urine volumes were recorded for each collection. All these serum and urine samples were frozen at −80°C until analysis.
Results
Method validation
Selectivity
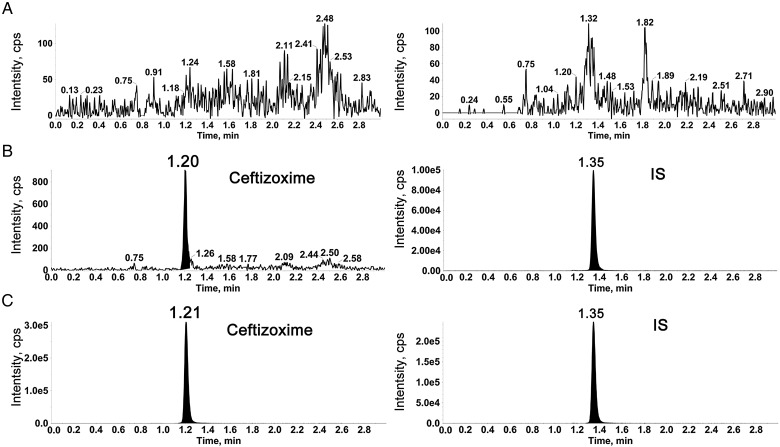
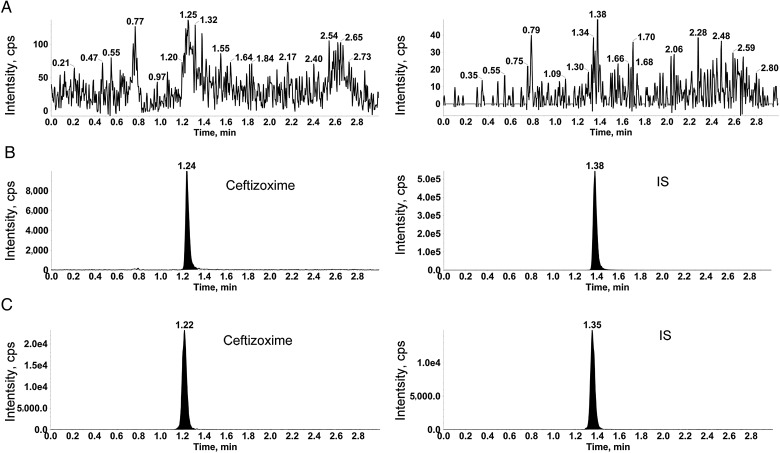
The validated method was highly selective for ceftizoxime as no significant interference was observed in the blank serum and urine samples from six different lots. The typical chromatograms of ceftizoxime and cefotaxime (IS) in a blank human serum sample, a blank serum sample spiked with ceftizoxime at the LLOQ level (2.50 ng/mL) and IS (1 µg/mL), and a serum sample collected at 2 h after parenteral administration of 0.5 g of ceftizoxime to healthy subjects are shown in Figure 2. The typical chromatograms of ceftizoxime and IS in human urine samples are shown in Figure 3.
Figure 2.
Typical MRM chromatograms of serum samples: (A) blank serum; (B) serum spiked with ceftizoxime at LLOQ and IS and (C) serum sample obtained 2 h after parenteral administration of 0.5 g ceftizoxime.
Figure 3.
Typical MRM chromatograms of urine samples: (A) blank urine; (B) urine spiked with ceftizoxime at LLOQ and IS; (C) urine sample obtained 4–8 h after parenteral administration of 0.5 g ceftizoxime.
Linearity of calibration curve, LLOQ, precision and accuracy
Linearity for ceftizoxime was obtained over the concentration range of 2.50–10,000 ng/mL in human serum and 0.5–50.0 µg/mL in human urine. The typical linear regression equation of the calibration curves generated during the serum validation was as follows: y = 0.00255x + 0.00183 (r = 0.9995), whereas the typical equation in human urine was y = 0.0000338x + 0.0000933 (r = 0.9989), where y represents the peak area ratio of ceftizoxime to the IS and x is the nominal concentration of ceftizoxime.
The LLOQ of ceftizoxime was determined to be 2.47 ± 0.22 ng/mL in human serum and 0.494 ± 0.013 µg/mL in human urine. Both the RSD% and RE% were within ±20%. The RSD% and RE% of other QCs (intra-batch, n = 5; inter-batch, n = 15) in human serum were ≤4.4% and 6.0%, respectively. In human urine, the data were ≤6.4 and 6.7%, respectively (Table II).
Table II.
Precision and Accuracy of the QC Samples in Human Serum and Urine (n = 5, Three Batches)
| Matrix | Concentration (ng/mL) | Intra-batch |
Inter-batch |
||
|---|---|---|---|---|---|
| RE (%) | RSD (%) | RE (%) | RSD (%) | ||
| Serum | 2.50 | −1.2 | 0.9 | NAa | NAa |
| 7.50 | 6.0 | 3.6 | 1.3 | 4.4 | |
| 750 | −5.1 | 1.3 | −0.8 | 3.9 | |
| 7,500 | 4.9 | 0.7 | 3.7 | 2.7 | |
| Urine | 500 | −1.2 | 2.7 | NAa | NAa |
| 1,500 | 6.7 | 5.2 | −0.7 | 6.4 | |
| 6,000 | 5.8 | 1.7 | −1.0 | 5.3 | |
| 40,000 | 6.0 | 1.6 | −0.5 | 5.3 | |
aThe LLOQ samples were analyzed in only one batch, RE and RSD of inter-batch were not applicable.
Matrix effect and extraction recovery
The matrix effect recoveries for ceftizoxime in human serum determined at 7.50, 750 and 7,500 ng/mL were 70.1, 66.4 and 68.5%, respectively, with RSD% <4.0%. The matrix effect recoveries for ceftizoxime in human urine determined at 1.50, 6.00 and 40.0 µg/mL were 106.3, 103.6 and 92.0%, respectively, with RSD% <5.3%. The matrix effect recoveries of IS were 71.3% in serum and 88.7% in urine, respectively, with RSD% <4.0%.
The overall mean recoveries of ceftizoxime in serum at three different concentration levels were found to be 83.5–92.9% with RSD% <4.8%. The mean recoveries in urine at three different concentration levels were found to be 93.9–105.8% with RSD% <3.5%. Besides, the extraction recovery of IS was found to be 88.7% in serum and 93.6% in urine, respectively, with the RSD% <5.3%.
The detail information of matrix effect and extraction recovery was shown in Supplementary material S1.
Stability
The results of short-term stability, auto-sampler stability, freeze–thaw stability and long-term stability are shown in Table III. It revealed that ceftizoxime performed good stability after leaving untreated in human serum at room temperature for 4 h as well as in human urine for 24 h. It was also stable in frozen human serum (−80°C) for 60 days and in frozen human urine (−80°C) for 56 days. The serum and urine samples remained stable after suffering three freeze–thaw cycles from −80°C to 25°C. Moreover, the processed samples remained stable after placed in the auto-sampler at least for 24 h.
Table III.
Stability of Ceftizoxime in Human Serum and Urine (n = 5)
| Matrix | Stability types | Concentration (nominal, ng/mL) | Concentration (measured, ng/mL) | Accuracy (RE, %) | RSD (%) |
|---|---|---|---|---|---|
| Serum | Short-term (at room temperature for 4 h) | 7.5 | 7.22 | −3.7 | 4.0 |
| 750 | 738 | −1.7 | 4.7 | ||
| 7,500 | 7,442 | −0.8 | 2.2 | ||
| Auto-sampler (24 h) | 7.5 | 7.00 | −6.6 | 4.1 | |
| 750 | 717 | −4.4 | 2.7 | ||
| 7,500 | 7,166 | −4.5 | 3.4 | ||
| Freeze–thaw (3 cycles) | 7.5 | 7.60 | 1.4 | 5.9 | |
| 750 | 765 | 2.1 | 3.3 | ||
| 7,500 | 7,570 | 0.9 | 1.1 | ||
| Long-term (60 days) | 7.5 | 7.42 | −1.1 | 2.9 | |
| 750 | 740 | −1.2 | 1.7 | ||
| 7,500 | 6,786 | −9.5 | 0.8 | ||
| Urine | Short-term (at room temperature for 24 h) | 1.50 | 1.41 | −5.9 | 3.3 |
| 6.00 | 5.62 | −6.3 | 1.1 | ||
| 40.0 | 37.9 | −5.3 | 0.4 | ||
| Auto-sampler (24 h) | 1.50 | 1.55 | 3.1 | 1.7 | |
| 6.00 | 5.94 | −1.0 | 2.0 | ||
| 40.0 | 39.3 | −1.8 | 1.9 | ||
| Freeze–thaw (3 cycles) | 1.50 | 1.38 | −8.1 | 2.0 | |
| 6.00 | 5.68 | −5.3 | 2.3 | ||
| 40.0 | 38.7 | −3.3 | 5.6 | ||
| Long-term (56 days) | 1.50 | 1.63 | 8.9 | 2.4 | |
| 6.00 | 6.30 | 5.0 | 2.6 | ||
| 40.0 | 39.5 | −1.3 | 1.7 |
Dilution integrity
Following a 10-fold dilution of a serum sample at 30.0 µg/mL with pooled human serum and a 10-fold dilution of a urine sample at 100 µg/mL with pooled human urine, the RSD% and RE% were <3.3%. The results indicated that samples could be diluted by 10-fold when their concentrations were higher than ULOQ levels. This expanded the quantification range to 2.50–100,000 ng/mL in human serum and 0.5–500 µg/mL in human urine.
Method application
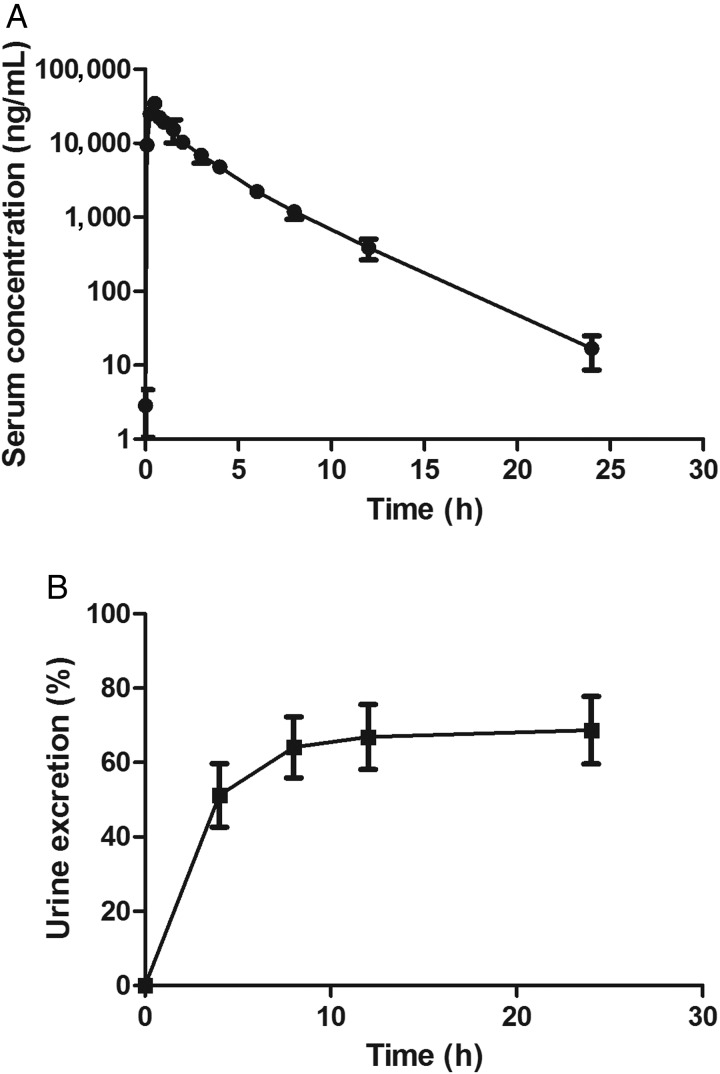
The validated method was applied to the pharmacokinetic study of ceftizoxime after intravenous infusion (0.5 g over a 30 min period) to five Chinese healthy elderly subjects. The sensitivity and specificity of the method were found to be sufficient for accurately characterizing the pharmacokinetics of ceftizoxime in this study; the samples collected at 24 h post-infusion were measurable. The mean serum concentration versus time profile of ceftizoxime is depicted in Figure 4A and the corresponding pharmacokinetic parameters of non-compartment model (NCA) are given in Table IV. The Cmax values of ceftizoxime in serum were found to be 34,721.3 ± 5,697.3 ng/mL, the AUC0-∞ was 68,700.1 ± 12,254.1 µg h/L. Serum concentrations declined with t1/2 of 2.57 ± 0.22 h, and the total clearance was 7.48 ± 1.41 L/h. The total urine excretion is shown in Figure 4B; nearly, 70% of the total drug was excreted through urine within 24 h of administration.
Figure 4.
Concentration–time profile (mean ± SD) of (A) ceftizoxime in human serum and (B) the total percentage of urine excretion after intravenous administration of 0.5 g (n = 5).
Table IV.
Pharmacokinetic Parameters of Ceftizoxime in Serum
| t1/2 (h) | Cmax (µg/L) | AUCinf (h µg/L) | Vz (L) | CL (L/h) | Age (year) | Weight (kg) | |
|---|---|---|---|---|---|---|---|
| Mean ± SD (n = 5) | 2.57 ± 0.22 | 34,721.3 ± 5,697.3 | 68,636.1 ± 12,231.8 | 27.5 ± 4.2 | 7.48 ± 1.41 | 70.8 ± 1.8 | 69.3 ± 5.9 |
Discussion
Optimization of LC–MS-MS conditions
The most abundant ion of cefotaxime in the MRM transition was observed at m/z 456.0 → 125.0; however, there was a small peak at the specific retention time of cefotaxime in the urine blank sample. Therefore, the MRM transition of m/z 456.0 → 167.0 was chosen to ensure that there were no interferences from the endogenous substances.
During the optimization of chromatographic conditions, several kinds of columns were tried to obtain an optimal peak shape and good separation. Ceftizoxime was retained on both Waters Acquity BEH C18 (3.5 µm) and XTerra Phenyl columns. In all previous studies, C18 columns were selected to separate ceftizoxime; however, finally, XTerra Phenyl column was chosen because the peak shape of ceftizoxime was narrow and more symmetric than that on BEH C18 column (Supplementary material S2).
The stock solution solubility
Ceftizoxime has poor solubility; it does not dissolve in water or in methanol. We found that ceftizoxime could dissolve in 10 mM sodium hydroxide solution or 1% aqueous ammonia (pH = 12), and would not re-precipitate when neutralized with acetic acid. However, although the standard could be dissolved in this method, the stock solution was not stable because the acidic or alkaline conditions might affect its stability. Finally, ceftizoxime was found to be solvable in DMSO/water (1:50, v/v); this stock solution was demonstrated to be stable at −80°C for 2 months.
Pharmacokinetic
The total clearance in our study was 0.109 ± 0.027 L/h/kg, smaller than that of the previous study (0.160 ± 0.047 L/h/kg) (3), and the half-life in the β-phase exhibited to be 2.57 ± 0.22 h, longer than previous report (2.05 ± 0.44 h) (3). The differences may be partially due to the improved sensitivity of this method because the serum samples at 24 h post-infusion that were not measurable in previous studies were determined successfully in our study; the involvement of the additional concentrations in NCA analysis would lead to more exact results. Besides, two other potential reasons may also be responsible for the differences. First, drug disposition in Chinese subjects may be different from the Caucasian; second, these data were obtained from elderly subjects (range from 69 to 73 years) whose renal elimination could be weaker than the young man. Thus, in order to describe the pharmacokinetics of ceftizoxime in elderly people more exactly, further study would be required and more volunteers grouped by ages would be involved.
Conclusion
In this study, a sensitive and rapid LC–MS-MS method was developed and fully validated for the quantification of ceftizoxime in human serum and urine. The method provided good and broad linearity with a low LLOQ (2.50 ng/mL in serum and 0.5 µg/mL in urine). The simple and economic preparation procedure and the short running time provide a possibility of analyzing the samples rapidly and conveniently.
Finally, this method was successfully applied to pharmacokinetic studies of ceftizoxime after intravenous administration to the Chinese healthy elderly subjects and the pharmacokinetic parameters were obtained. However, more details of the ceftizoxime pharmacokinetic profile of the elderly people require more investigation. Our method would be useful in these further clinical studies.
Supplementary Material
References
- 1.Nakashima N., Suzuki K., Hashimoto H., Nishijima K.; Phase I study of ceftizoxime, a new cephalosporin. Single-dose study; Journal of Clinical Pharmacology, (1981); 21(10): 388–395. [DOI] [PubMed] [Google Scholar]
- 2.Neuhauser M.M., McKinnon P.S., Hershberger E., Rybak M.J.; Pharmacokinetics and pharmacodynamics of ceftizoxime in patients with dosages adjusted for renal function; Pharmacotherapy, (2000); 20(5): 554–561. [DOI] [PubMed] [Google Scholar]
- 3.Francois V., Marc L.; Comparative study of pharmacokinetics and serum bactericidal activity of ceftizoxime and cefotaxime; Antimicrobial Agents and Chemotherapy, (1991); 35(10): 2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryan F., Bill F., Steve T.; Population pharmacokinetics of ceftizoxime administered by continuous infusion in clinically ill adult patients; Antimicrobial Agents and Chemotherapy, (1998); 42(7): 1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sar T.K., Mandal T.K., Das S.K., Chakraborty A.K.; Pharmacokinetics of ceftriaxone in carbontetrachloride-induced hepatopathic and uranyl nitrate-induced nephropathic goats following single dose intravenous administration; Drug Metabolism Letters, (2008); 2(1): 23–28. [DOI] [PubMed] [Google Scholar]
- 6.Péhourcq F., Jarry C.; Determination of third-generation cephalosporins by high-performance liquid chromatography in connection with pharmacokinetic studies; Journal of Chromatography. A, (1998); 812(1–2): 159–178. [DOI] [PubMed] [Google Scholar]
- 7.McCormick E.M., Echols R.M., Rosano T.G.; Liquid chromatographic assay of ceftizoxime in sera of normal and uremic patients; Antimicrobial Agents and Chemotheraphy, (1984); 25(3): 336–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Momani I.F.; Spectrophotometric determination of selected cephalosporins in drug formulations using flow injection analysis; Journal of Pharmaceutical and Biomedical Analysis, (2001); 25(5–6): 751–757. [DOI] [PubMed] [Google Scholar]
- 9.Ci L., Kusuhara H., Adachi M., Schuetz J.D., Takeuchi K., Sugiyama Y.; Involvement of MRP4 (ABCC4) in the luminal efflux of ceftizoxime and cefazolin in the kidney; Molecular Pharmacology, (2007); 71(6): 1591–1597. [DOI] [PubMed] [Google Scholar]
- 10.Bharathi C.H., Prasad C.H.S., Bharathi D.V., Shankar R., Rao V.J., Dandala R. et al. ; Structural identification and characterization of impurities in ceftizoxime sodium; Journal of Pharmaceutical and Biomedical Analysis, (2007); 43(2): 733–740. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z.Z., Zhang D.S., Wang N., Feng F., Hu C.Q.; Identification of impurity peaks in the HPLC chromatogram by LC-MS and two-dimensional chromatographic correlation spectroscop; Yao Xue Xue Bao = Acta pharmaceutica Sinica, (2012); 47(4): 492–497. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.