Abstract
The current studies describe the Quality by Design (QbD)-based development and validation of a LC–MS-MS method for quantification of fluoxetine in human plasma using fluoxetine-D5 as an internal standard (IS). Solid-phase extraction was employed for sample preparation, and linearity was observed for drug concentrations ranging between 2 and 30 ng/mL. Systematic optimization of the method was carried out by employing Box-Behnken design with mobile phase flow rate (X1), pH (X2) and mobile phase composition (X3) as the method variables, followed by evaluating retention time (Rt) (Y1) and peak area (Y2) as the responses. The optimization studies revealed reduction in the variability associated with the method variables for improving the method robustness. Validation studies of the developed method revealed good linearity, accuracy, precision, selectivity and sensitivity of fluoxetine in human plasma. Stability studies performed in human plasma through freeze–thaw, bench-top, short-term and long-term cycles, and autosampler stability revealed lack of any change in the percent recovery of the drug. In a nutshell, the developed method demonstrated satisfactory results for analysis of fluoxetine in human plasma with plausible utility in pharmacokinetic and bioequivalence studies.
Introduction
Fluoxetine ((±)-N-methyl-3-phenyl-3-[(α,α,α-trifluoro-ρ-tolyl)oxy]propylamine) is a selective serotonin reuptake inhibitor (SSRI) and frequently prescribed as an antidepressant (1, 2). Although fluoxetine has been marketed for decades, it continues to be one of the most commonly prescribed antidepressants worldwide. It is also considered to be one of the few among the antidepressants approved for use in children and adolescents, as well as during pregnancy. Fluoxetine is also approved by USFDA for the treatment of depression and administered in the form of racemic mixture. It is extensively metabolized in the liver to norfluoxetine, which exhibit similar potency to that of the parent compound. However, the generation of active metabolite from the analyte takes 7–8 days owing to the longer elimination half-life of the fluoxetine. This makes the analysis highly inconvenient to make blood sampling from the human volunteers for longer days for detection of the analyte. Thus, the majority of the bioanalytical methods tend to focus on detection of the fluoxetine in biological samples for the purpose (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM244384.pdf) (3–5).
Several analytical methods have been developed for estimating the SSRIs, including paroxetine and fluoxetine in human plasma using HPLC–UV, GC–MS and LC–MS-MS (6–12). However, these analytical methods of fluoxetine described in the literature always involve extraction of the analyte from the biological samples before chromatographic separation (13–17). In such cases, sample preparation is considered to be highly crucial for the analysis of such compounds in biological samples, thus making the analysis highly tedious and time-consuming, and at times, leads to reduced accuracy and precision of the analyte.
Currently, the analytical methods used for analysis of fluoxetine describe its determination in various biological samples such as human plasma, urine, serum and necessitate extensive use of high cost solvents, and maintenance of multitude of parameters for chromatographic separation (18). Besides, the majority of methods tend to involve high degree of variability owing to involvement of multiple method parameters for the purpose. This requires apt optimization of the method variables for attaining the desired method performance.
The development of bioanalytical methods based on the holistic principles of Quality by Design (QbD) has been gaining immense popularity for enhanced understanding of the high degree of variability associated with the LC–MS method development and attaining optimal chromatographic separation. As per the QbD approach, the Design of Experiments (DoE) is considered as a vital tool, which helps in systematic execution of the chromatographic method development. Not only DoE helps in identifying the “vital few” method variables critically influencing the method performance, but also optimizes them with minimal investment of time, efforts and cost (19, 20). Literature reports, in this regard, are testimony to the higher fruition of the QbD approach for efficient development of the liquid chromatographic methods with greater flexibility and enhanced method performance (21–24).
Thus attempts, therefore, were made for the development of a simple, fast, sensitive and accurate LC–MS-MS method of fluoxetine followed by optimization of the selected method variables using Box-Behnken design (BBD) for effective, cost-effective and efficient chromatographic separation. The developed method was extensively evaluated as per the ICH guideline followed by evaluating the drug stability in human plasma.
Materials and methods
Test compounds and ISs
Fluoxetine was purchased from Vivan Life Sciences Pvt. Ltd., Mumbai, India, and Fluoxetine D5 was obtained from Sigma-Aldrich, Mumbai, India.
Biological matrix
The biological matrix was purchased from Yash Laboratories, Shop no. 9, Louiswadi, Thane (Mumbai India). For preparation of calibration standards (CC) and quality control (QC) samples, human plasma with K3-EDTA was used and the analyte was suitably extracted to remove the interfering substances. For this, blank plasma was run each time to confirm the absence of interfering substance.
Reagents
HPLC-grade acetonitrile, methanol, ammonium formate, water, liquid ammonia (∼25% NH3; analytical grade) and orthophosphoric acid (OPA, analytical grade) were procured from the Fischer Scientific (Mumbai, India) and used as obtained throughout the studies.
Preparation of buffer solution and mobile phase
Buffer-1 (2 mM ammonium formate solution)
Approximately 0.126 g of ammonium formate was weighed in a 1,000 mL reagent bottle, and 1,000 mL of HPLC grade water was added to dissolve it by mixing and sonication in an ultrasonic water bath. The prepared solution was used within 5 days from the date of its preparation, followed by dilution to prepare the solutions of varying concentrations.
Mobile phase (acetonitrile: buffer-1 solution: 95:5 v/v)
The mobile phase was prepared by transferring 50 mL of buffer-1 into a 1,000 mL reagent bottle, followed by addition of 950 mL of HPLC-grade acetonitrile. The mixture was mixed well, sonicated and degassed in an ultrasonic water bath. The mobile phase was used within 3 days from the date of its preparation, followed by its use for preparing appropriate dilutions.
OPA solution (5% v/v)
An accurately measured, 5 mL of OPA solution was transferred into a 100 mL volumetric flask. The volume was adjusted with HPLC grade water and mixed well, followed by sonication and degassing in an ultrasonic water bath. The prepared solution was stored at room temperature and used within 3 days from the date of its preparation.
Washing solution
An accurately measured, 2 mL of formic acid was transferred into a 100 mL volumetric flask, and the volume was adjusted with HPLC grade water and mixed well, followed by sonication and degassing in an ultrasonic water bath. The prepared solution was stored at room temperature and used within 3 days from the date of preparation.
Elution solution
An accurately measured, 5 mL of ammonia solution was transferred into a 100 mL volumetric flask, and the volume was adjusted with HPLC grade water. The solution was mixed well, followed by sonication and degassing in an ultrasonic water bath. The prepared solution was stored at room temperature and used within 3 days from the date of its preparation.
Diluent solution
An accurately measured, 500 mL of HPLC grade acetonitrile was added to a 1,000 mL reagent flask, and the volume was adjusted by adding HPLC grade water. The mixture was thoroughly mixed well, sonicated and degassed in an ultrasonic bath. The prepared solution was stored at room temperature and used within 3 days from the date of its preparation.
Rinsing solution
The HPLC grade methanol was used as rinsing solution, which was thoroughly sonicated for 15 min before use.
Stock solutions
Stock solution of fluoxetine was prepared in the diluent by the spiking method to obtain the working standard solution of concentration 1 mg/mL. From the stock, dilutions of 50 ng were prepared for spiking of CC and QC standards of fluoxetine. The CC and QC samples were used to evaluate the accuracy and precision as well as used for the determination of low limit of quantification (LLOQ). Fluoxetine-D5 stock solution was used as an IS solution and subjected to chromatographic analysis by mass spectrometry for interfering elements.
LC–MS-MS conditions
Fluoxetine was analyzed using a HPLC instrument (Shimadzu LC10A, Tokyo, Japan), which was connected with an AB SCIEX API-4000 triple quadruple mass spectrometer (Ontario, Canada). Chromatographic separation was performed on an Ascentis express C18 analytical column (75 × 4.6 mm, 2.7 µm; Sigma Aldrich, Mumbai, India), where the mixture of ammonium formate and acetonitrile solution in 5:95 ratio was used as the mobile phase at a flow rate of 0.8 mL/min. The injection volume of 10 µL was constant throughout the study, and the run time was fixed at 3.5 min. By applying the above-mentioned conditions, the retention time (Rt) of fluoxetine and fluoxetine-D5 (IS) were reported. The mass spectrometer was used in positive ion mode having a turbo ion spray ionization pattern. Mass spectrometer parameters were fixed as curtain gas on 35, nebuliser on 50, heater gas on 50, focusing potential on 12, collision cell exit potential on 12, ion spray voltage on 5,000 V, temperature on 500°C, collision gas on 9, entrance potential at 8 V and dwell time at 200 ms, respectively. The multiple reaction monitoring (MRM) conditions were monitored for both the analyte and IS, where fluoxetine was kept at 310/44, and fluoxetine D5 at 315/44. The spectrometric data analysis was carried out by the system built analyst 1.5 software.
CC and QC samples
The spiked plasma with fluoxetine was used for CC and QC samples. The concentration of CC and QC samples ranging between 2 and 30 ng/mL was explored for the purpose. A set of nine CC and QC standards of pooled plasma were prepared and stored at −22°C. Each validation run consisted of CC and six replicates of QC samples at low (5.8 ng/mL), medium (12.5 ng/mL) and high (25.0 ng/mL) concentration levels, respectively.
Method validation studies
The method validation studies were carried out by preparing three analytical runs including nine CC with QC samples having two standard zero (with IS) and blank (without IS). Two-thirds of CC standards must pass individually, while individual accuracy of the standard must be within ±15% of nominal and ±20% of LLOQ. Among these, three analytical runs, at least one of the two CC standards at the LLOQ and ULOQ level must pass, and the CC coefficient of the determinant (r2) must be >0.998. The within-run and between-run accuracy must be within ±15% at QC levels, and individual accuracy at each concentration must be within ±15%.
For method selectivity, 20 blank plasma sample batches were screened to check the interference at the Rt of analytes and IS for its acceptance. It must be <20% of the mean peak response calculated from the analysis of LLOQ along with QC samples at the expected Rt of analytes and <5% of IS.
Stability was checked by analyzing the QC samples of analytes at lower and higher concentrations (n = 6), which were stored under various conditions. For acceptance, two-thirds of the stability QC samples must have individual accuracy and mean accuracy within ±15%. The stability of the analytes in stored stock solution during long-term stability for 43 days was determined by comparing it with the fresh stock solution. It would pass the test if the mean difference between the two is <5%.
At different room temperature, the samples were refrigerated for 13.5 h and 5 days to demonstrate the stability, while 24 h storage was used for reinjection reproducibility.
Dilution integrity was determined to establish the accurate quantification within the range of CC at a concentration greater than the ULOQ, which can be diluted with the matrix. Individually, two-third dilution QC samples must pass the individual and mean accuracy <±15%.
Evaluation of method robustness using experimental design
The robustness testing of the developed method was carried out by applying a 3-factor 3-level BBD with the help of Design-Expert 8.0.7.1 software (Stat-Ease Inc., Minneapolis, USA). The selected factors include the composition of the mobile phase (ammonium formate buffer–acetonitrile) from 0:100 to 10:90, flow rate from 0.6 to 1.0 and pH from 6.6 to 6.8. The obtained runs were evaluated for the response variables, namely peak area and Rt. Response surface analysis was carried out for understanding the factor–response relationship(s) and plausible interaction(s) among them. Search for identification of optimum chromatographic solution was carried out by numerical optimization and desirability function by “trading-off” the responses for maximizing the peak area and Rt.
Extraction procedure
One set of CC and QC samples were removed from the freezer and allowed to thaw at normal room temperature. Thawed samples were vortexed to ensure complete mixing of contents. An aliquot 50 µL of IS (∼50 ng/mL of Fluoxetine-D5) was pipetted out into appropriately labeled polypropylene tubes (except blank). Further, 200 µL of plasma was added to each sample and subjected to centrifugation in microcentrifuge tubes and vortexed. Then, 1 mL of methanol was added twice and passed through solid cartridges under pressure. Also 1 mL of elution solution was passed through cartridges under pressure. The cartridges (Oasis MCX 30 mg/cc) were conditioned using 1 mL of methanol followed by 1 mL of water with the help of positive pressure during the solid-phase extraction process. The loaded samples onto the cartridges were passed under pressure and washed with 1 mL of washing solution twice, followed by drying under a stream of nitrogen at pressure not more than 20 psi and a temperature of 50 ± 2°C. The samples were reconstituted in 0.5 mL of mobile phase and transferred to vials for analysis. The sample processing was carried out under dark conditions throughout the study period.
Results
Sample extraction
The solid-phase extraction was used during the entire study period to minimize the matrix effect at the LLOQ level (i.e., 2 ng/mL). All the available extraction procedures were used during extraction of samples, but among them solid-phase extraction gave good results, thus it was considered to be the best procedure for the extraction.
The samples were eluted using elution solution, which yields excellent recovery of 79.6% for fluoxetine and 71% for fluoxetine-D5, indicating aptness of the developed method. The shape of chromatographic peak was found to be symmetric, and recovery was found to be consistent for the analyte and IS at the LLOQ level.
Tandem mass spectrometry
The product ion mass spectra and scanning of fluoxetine and fluoxetine-D5 were obtained using positive ion mode. The scanned mass spectra of fluoxetine and fluoxetine-D5 gave signals for the protonated molecular ions at m/z = 310.0 and m/z = 315.0, respectively. The product ion mass spectra for the above compounds were observed at 44.0. Transition from m/z 310 to m/z 44, and m/z 315 to 44 were monitored in MRM mode for quantification.
Separation
In preclinical volunteers, different metabolites of fluoxetine were present and these metabolites may cause problem in separation. To triumph over this problem, Ascentis Express C 18 (75 × 4.6, 2.7 µm) particle size and flow rate of 0.8 mL/min with a suitable post-column splitter were used.
Sensitivity
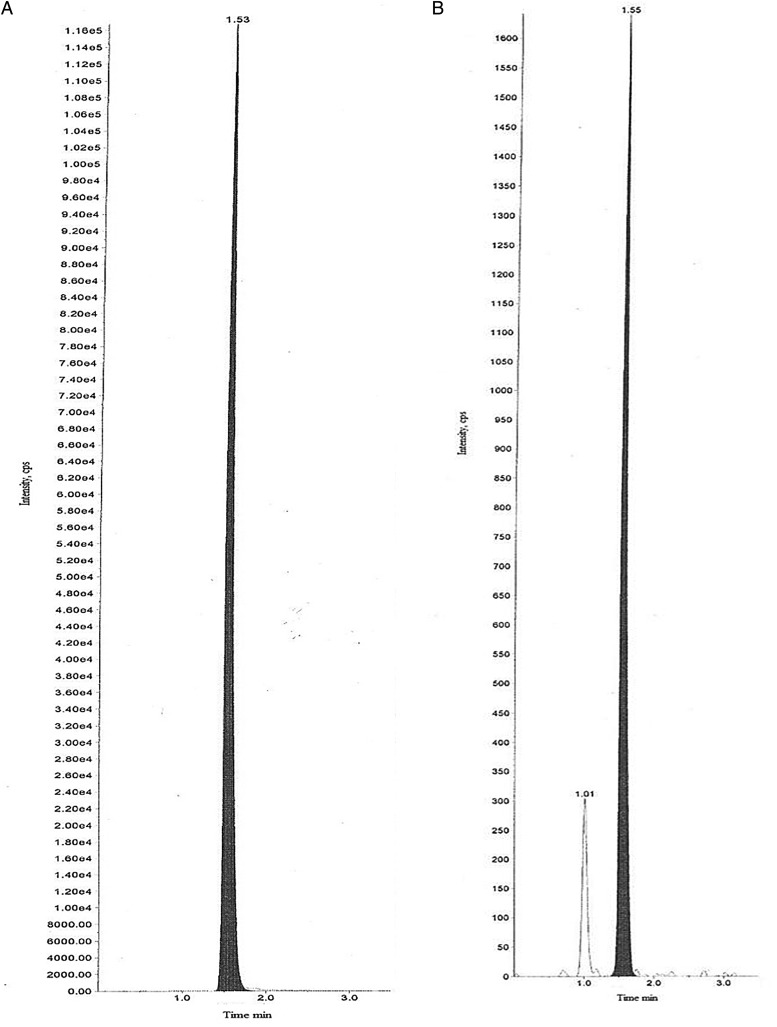
Sensitivity of the method was determined in one of the validation runs at the LLOQ (2 ng/mL) level by processing six replicates for fluoxetine. The batch precision and accuracy at LLOQ levels using the IS ratio was obtained as 2.5 and 101%, respectively. Chromatograms of fluoxetine and fluoxetine-D5 at the LLOQ are shown in Figure 1A and B.
Figure 1.
Chromatogram of fluoxetine alone (A), and chromatogram of internal standard (i.e., Fluoxetine-D5) (B).
Matrix effect
Matrix effect is the suppression or enhancement of ionization of analytes in the presence of matrix in the biological samples. Thus, the method was designed in a way to avoid any matrix effect and the percent matrix factor at 2, 12.7 and 25.5 was found to be 0.9, 1.0 and 1.0, respectively. This showed that the matrix effect was found to be negligible in the presence of plasma.
Selectivity
Selectivity was performed in 20 different lots of plasma having K3-EDTA as an anticoagulant. No significant interference was observed at Rt of Fluoxetine and Fluoxetine-D5 (IS) in all the batches of screened K3-EDTA plasma lots.
Calibration curve regression
The observed responses for fluoxetine were subjected to quadratic regression (1/concentration), which showed best-fit results for the coefficient of determination (r2) greater than 0.998.
Accuracy and precision
Table I illustrates the accuracy (% nominal) data for fluoxetine within batch and between batches. The data showed that accuracy was found between 101 and 100.7%, while % CV within batch and between batches was reported as 2.5 and 2.7%, respectively.
Table I.
Precision and Accuracy of Fluoxetine in Human Plasma
| Concentration nominal (ng/mL) | Precision (%) |
Accuracy (%) |
||
|---|---|---|---|---|
| Within batch | Between batch | Within batch | Between batch | |
| 2.0 | 5.1 | 4.9 | 94.3 | 94.3 |
| 5.89 | 3.2 | 3.8 | 109.6 | 109 |
| 12 | 1.3 | 1.5 | 100.2 | 99.8 |
| 25 | 0.6 | 0.8 | 100.1 | 99.9 |
Recovery
It was determined by comparing the peak area of the QC sample before extraction to the peak area after extraction. Fluoxetine recoveries at the low, medium and high QC level were found to be 89.4, 75.6 and 73.8%, respectively, while the recovery of fluoxetine-D5 was 71%. The results suggested good recovery of both the analyte and IS.
Integrity of dilution
This dilution integrity quality control (DIQC) samples were prepared and diluted two and four times with a blank matrix. The % nominal of DIQC samples after two to four times dilution was found to be 94.9 and 94.2%, while % CV was reported as 2.9 and 6.8%, respectively. The observed results were found to be well within the acceptable range.
Stability
It was assessed to determine that whether the analyte and IS are stable under different storage and processing conditions. Furthermore, the analyte stability during sample transport, storage and preparation were concerned. It was done by QC samples (n = 6) at the lower quality control, medium quality control and higher quality control level and assessed for the mean value of the stability samples at each level with the mean value of the same QC pooled freshly. It was found that fluoxetine was stable in human plasma. The obtained values of fluoxetine at different stability studies such as freeze–thaw stability, bench-top stability, bench-top extraction stability, injector stability, long-term stability using K3-EDTA below −15°C (5 days) are shown in Table II. It is clear and evident that stability samples of the analytes and IS were stable at various stability conditions listed above.
Table II.
Stability of Fluoxetine in Human Plasma
| Storage condition | Concentration (ng/mL) | % Accuracy |
|---|---|---|
| Bench-top stability (13.5 h) | 5 | 98.1 |
| 25 | 100.2 | |
| Bench-top extraction stability | 5 | 96.4 |
| 25 | 100 | |
| Long-term stability (5 days) | 5 | 101.8 |
| 25 | 102.6 | |
| Freeze–thaw stability (after 3 cycles) | 5 | 102.8 |
| 25 | 99.3 | |
| In injector stability (50.15 h) | 5 | 96.3 |
| 25 | 99.5 |
Ruggedness
The ruggedness determined by different analysts running one precision and accuracy batch by a different analyst using the same and/or different instruments. The within batch accuracy (% nominal) for fluoxetine was 100.2%, while within batch precision (% CV) was 4.3%. The above reported values for fluoxetine indicated that the method is highly rugged for routine applications.
Robustness testing using experimental design
The capabilities to remain impervious by small and premeditated changes in chromatographic conditions such as mobile phase composition, flow rate and column oven temperature are known as robustness. BBD was applied to test the robustness for three dependent variables, i.e., flow rate (A) (mL/min), pH (B) and mobile phase (% v/v ammonium formate buffer: ACN) (C), where their likely impact was studied on two-dependent variables (response), namely peak area (cm2) (Y1) as well as Rt (min) (Y2) as the robustness parameters. A total of 17 runs were obtained, and each suggested combination was run on the system to observe the results.
The method perfectness was elaborated on the basis of results obtained as per the experimental design. Out of the trial combinations suggested by the selected design, BBD showed that a combination of 5:95 ratio of mobile phase (ammonium formate buffer: ACN), flow rate of 0.8 mL/min and pH of 6.7 were found to be the optimum solution with responses as peak area of 300,134 and Rt of 1.5 min, respectively (Table III).
Table III.
Summary of Design Matrix as per the BBD
| Run | Factor 1 Flow rate (mL/min) |
Factor 2 pH |
Factor 3 Mob. ph composition (%) |
Rt (min) | Peak area (cm2) |
|---|---|---|---|---|---|
| 1 | 0.6 | 6.7 | 0 | 3.4 | 301,547 |
| 2 | 1 | 6.6 | 5 | 3.5 | 296,427 |
| 3 | 0.8 | 6.7 | 5 | 1.54 | 418,865 |
| 4 | 0.8 | 6.7 | 5 | 1.54 | 418,865 |
| 5 | 0.8 | 6.7 | 5 | 1.54 | 418,865 |
| 6 | 1 | 6.7 | 10 | 5.2 | 334,678 |
| 7 | 0.6 | 6.7 | 10 | 4.6 | 283,573 |
| 8 | 0.8 | 6.6 | 10 | 5.4 | 382,341 |
| 9 | 0.6 | 6.8 | 5 | 4.3 | 321,378 |
| 10 | 0.8 | 6.8 | 0 | 5.9 | 321,378 |
| 11 | 1 | 6.7 | 0 | 5.9 | 301,463 |
| 12 | 1 | 6.8 | 5 | 5.4 | 393,542 |
| 13 | 0.8 | 6.7 | 5 | 1.54 | 418,865 |
| 14 | 0.6 | 6.6 | 5 | 3.6 | 301,243 |
| 15 | 0.8 | 6.6 | 0 | 3.1 | 281,438 |
| 16 | 0.8 | 6.7 | 5 | 1.54 | 418,865 |
| 17 | 0.8 | 6.8 | 10 | 4.2 | 273,256 |
To minimize the effects of uncontrolled factors on the responses, experiments were performed in a randomized order. Among the experimental runs suggested by BBD, the second-order model with a quadratic experimental domain was selected for both the responses, Y1 (r2= 0.9842) and response Y2 (r2= 0.9145) as compared with other models suggested by the design through a lack-of-fit test. A higher P-value when compared with the model F-value was recorded, which indicated insignificant lack-of-fit values. This was validated and analyzed by applying analysis of variance (ANOVA) to both the response variables to examine the model significance. The analysis of results showed that both these responses achieved significant differences in their values.
The predicted values for all factors, i.e., flow rate (A), pH (B) and mobile phase composition (C), are under the satisfactory value having a predicted model F-value of 48.51 which represented that the model is highly significant with a P-value of 0.0001, indicating only 0.01% chance that the model F-value could be large due to noise. The ANOVA results predicted for response Y2 with model F-value was found to be 8.32, where the model tends to be significant owing to 0.54% chances that the model F-value may be large due to noise. Further, the model recommended that predicted values for both the response are closer to the actual values indicating higher accuracy and precision of the obtained values.
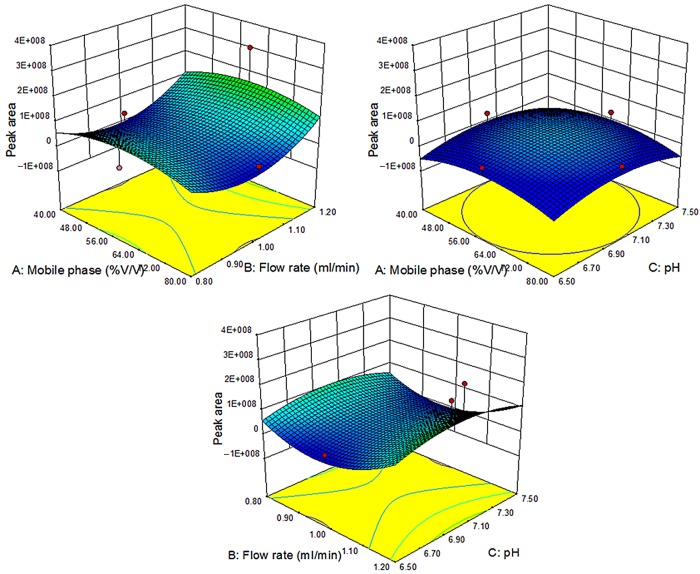
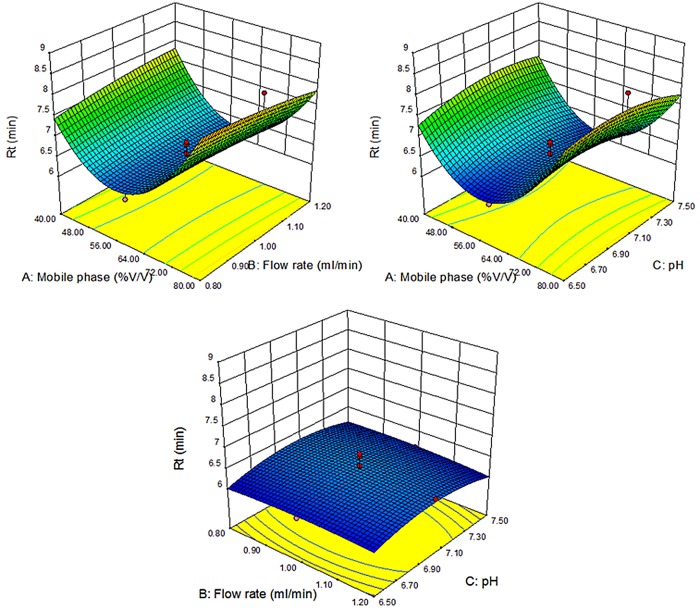
The effect of individual factors of the model was evaluated on the responses in the form of contour plots indicating response surfaces for both the responses Y1 and Y2 as shown in Figures 2 and 3. This suggested that the effect of both the responses are dependent on factor A (flow rate) and B (mobile phase composition % age), while C (pH) has no effect on the obtained responses. By studying the interaction of different factors on the responses, the model was validated. The effect of different factors on response Y1 (Rt) and Y2 (peak area) was evaluated by one factor interaction study, which revealed that the flow rate (A) exhibited two-factor interaction effect with the study that revealed that both factor A (flow rate) and factor B (pH) showed multiple interactions on the obtained response, which can be clearly evident from the contour plots for the purpose. On the other hand, factor C has no effect on either of the responses (Table IV).
Figure 2.
3D-response surface plots depicting the influence of mobile phase ratio, flow rate and pH on the peak area as the response variable (Y1). This figure is available in black and white in print and in color at JCS online.
Figure 3.
3D-response surface plots depicting the influence of mobile phase ratio, flow rate and pH on retention time (Rt) as the response variable (Y1). This figure is available in black and white in print and in color at JCS online.
Table IV.
Chromatographic Conditions and Range Investigated During Robustness Testing
| Factor | Range | Low level | High level | Optimized value |
|---|---|---|---|---|
| A = flow rate | 0.6–1.0 | 0.6 | 1.0 | 0.8 |
| B = pH | 6.6–6.8 | 6.6 | 6.8 | 6.7 |
| C = composition of AF | 0/100–10/90 | 0.0 | 10.0 | 5.0 |
Discussion
Overall, this study embarks upon the application of QbD principles for developing the LC–MS-MS method of fluoxetine in human plasma. Systematic method development was carried out by employing BBD for evaluating the method robustness using mobile phase composition, buffer pH and flow rate as the method variables, followed by optimization of their effects on the peak area and Rt as the responses (Table V). The response surface analysis helped in critical understanding of the method parameters followed by critical understanding of the relationship between them. Method validation studies revealed high degree of sensitivity, selectivity and specificity of the obtained method. Evaluation of the stability in plasma through freeze–thaw, short-term, long-term and bench-top stability studies revealed no significant influence of the experimental conditions on the drug stability. By and large, the method was found to be highly robust and reliable enough for routine analysis of the drug in bioanalytical samples.
Table V.
Numerical Optimization Parameters
| Constraints | ||||||
|---|---|---|---|---|---|---|
| Name | Goal | Lower limit | Upper limit | Lower weight | Upper weight | Importance |
| A: Flow rate | is in range | 0.6 | 1 | 1 | 1 | 3 |
| B: pH | is in range | 6.6 | 6.8 | 1 | 1 | 3 |
| C: AF:ACN composition | is in range | 0 | 10 | 1 | 1 | 3 |
| Solutions | ||||||
| Number | Flow rate | pH | AF composition | Desirability | ||
| 1 | 0.80 | 6.70 | 5.00 | 1 | Selected | |
| 2 | 1.00 | 6.70 | 10.00 | 1 | ||
| 3 | 0.80 | 6.60 | 10.00 | 1 | ||
Conclusion
The proposed method of fluoxetine was found to be simple, specific, accurate and linear and validated in human plasma over a range of 2–25 ng/mL. The results obtained from the validation of the method were satisfactory and offer a rapid and simple sample preparation which can facilitate the biostudies of fluoxetine. This vouched the routine applicability of the method in pharmacokinetic analysis of samples. The regulatory requirements for accuracy, precision, sensitivity, selectivity, stability and ruggedness were found to be excellent. The applied BBD design for optimization of robustness parameters was found to be highly suitable for validation and able to predict minor changes in the flow rate and mobile phase composition for the responses, i.e., peak area and Rt for the purpose.
Acknowledgments
The authors are very thankful to the management of Fortis Clinical Research Limited, Faridabad, for supporting this work.
References
- 1.Oakes K.D., Coors A., Escher B.I., Fenner K., Garric J., Gust M. et al. ; An environmental risk assessment for the serotonin re-uptake inhibitor fluoxetine—a case study utilizing the European risk assessment framework; Integrated Environmental Assessment and Management, (2010); 6(1): 524–539. [DOI] [PubMed] [Google Scholar]
- 2.Levine S.; Comparative trial of a new antidepressant; British Journal of Psychiatry, (1987); 151: 861. [DOI] [PubMed] [Google Scholar]
- 3.Pan R., Chen T., Huang C.S., Hsiong C.; Pharmacokinetics and bioequivalent study of generic fluoxetine capsules preparation; Journal of Food and Drug Analysis, (2002); 10(1): 13–17. [Google Scholar]
- 4.Altamura A.C., Moro A.R., Percudani M.; Clinical pharmacokinetics of fluoxetine; Clinical Pharmacokinetics, (1994); 26: 201–214. [DOI] [PubMed] [Google Scholar]
- 5.Lemberger L., Bergestrom R.F., Wolen R.L., Farid N.A., Enas G.G., Aronoff G.R.; Fluoxetine: clinical pharmacology and physiologic disposition; Journal of Clinical Psychiatry, (1985); 46: 14–19. [PubMed] [Google Scholar]
- 6.Zhu Z.M., Neirinck L.; High-performance liquid chromatography–mass spectrometry method for the determination of paroxetine in human plasma; Journal of Chromatography B, (2002); 780(2): 295–300. [DOI] [PubMed] [Google Scholar]
- 7.Eerkes A., Weng N., King M., Du A., Shou W.Z.; A sensitive and high-throughput LC/MS/MS method using a silica column and an aqueous–organic mobile phase for the analysis of fluoxetine and norfluoxetine in human plasma; Journal of Liquid Chromatography and Related Technology, (2002); 25: 1215. [Google Scholar]
- 8.Sutherland F.C., Badenhorst D., de Jager A.D., Scanes T., Hundt H.K., Swart K.J. et al. ; Sensitive liquid chromatographic-tandem mass spectrometric method for the determination of fluoxetine and its primary active metabolite norfluoxetine in human plasma; Journal of Chromatography A, (2001); 914(1-2): 45–51. [DOI] [PubMed] [Google Scholar]
- 9.Maya M.T., Domingos C.R., Guerreiro M.T., Morais J.A.; Determination of the antidepressant fluoxetine in human plasma by LC with UV detection; Journal of Pharmaceutical and Biomedical Analysis, (2000); 23(6): 989–996. [DOI] [PubMed] [Google Scholar]
- 10.Raggi M.A., Mandrioli R., Casamenti G., Volterra V., Desiderio C., Fanali S.; Improved HPLC determination of fluoxetine and norfluoxetine in human plasma; Chromatographia, (1999); 50(7-8): 423–427. [Google Scholar]
- 11.Crifasi J.A., Le N.X., Long C.; Simultaneous identification and quantitation of fluoxetine and its metabolite, norfluoxetine, in biological samples by GC–MS; Journal of Analytical Toxicology, (1997); 21(6): 415. [DOI] [PubMed] [Google Scholar]
- 12.Urichuk L.J., Aspeslet L.J., Holt A., Silverstone P.H., Coutts R.T., Baker G.B.; Determination of p-trifluoromethylphenol, a metabolite of fluoxetine, in tissues and body fluids using an electron-capture gas chromatographic procedure; Journal of Chromatography B, (1997); 698(1–2): 103–109. [DOI] [PubMed] [Google Scholar]
- 13.Kovacevic I., Pokrajac M., Miljkovic B., Jovanovic D., Prostran M.; Comparison of liquid chromatography with fluorescence detection to liquid chromatography-mass spectrometry for the determination of fluoxetine and norfluoxetine in human plasma; Journal of Chromatography B Analytical Technology in Biomedical Life Sciences, (2006); 830(2): 372–376. [DOI] [PubMed] [Google Scholar]
- 14.Kirchherr H., Kuhn-Velten W.N.; Quantitative determination of forty-eight antidepressants and antipsychotics in human serum by HPLC tandem mass spectrometry: a multi-level, single-sample approach; Journal of Chromatography B, (2006); 843: 100–113. [DOI] [PubMed] [Google Scholar]
- 15.Wille S.M., Maudens K.E., Van Peteghem C.H., Lambert W.E.; Development of a solid phase extraction for 13 ‘new’ generation antidepressants and their active metabolites for gas chromatographic–mass spectrometric analysis; Journal of Chromatography A, (2005); 1098(1–2): 19–29. [DOI] [PubMed] [Google Scholar]
- 16.Chaves A.R., Silva S.M., Queiroz R.H.C., Lanc F.M., Queiroz M.E.C.; Stir bar sorptive extraction and liquid chromatography with UV detection for determination of antidepressants in plasma samples; Journal of Chromatography B, (2007); 850(1–2): 295–302. [DOI] [PubMed] [Google Scholar]
- 17.Chow T.W., AndrásSzeitz D.W., Rurak K., Riggs W.; A validated enantioselective assay for the simultaneous quantitation of (R)-, (S)-fluoxetine and (R)-, (S)-norfluoxetine in ovine plasma using liquid chromatography with tandem mass spectrometry (LC/MS/MS); Journal of Chromatography B, (2011); 879: 349–358. [DOI] [PubMed] [Google Scholar]
- 18.Singh B., Kumar R., Ahuja N.; Optimizing drug delivery systems using systematic “design of experiments.” Part I: fundamental aspects; Critical Reviews in Therapeutic Drug Carrier Systems, (2005); 22: 27–105. [DOI] [PubMed] [Google Scholar]
- 19.Singh B., Raza K., Beg S.; Developing “optimized” drug products employing “designed” experiments; Chemical Industry Digest, (2013); 6: 70–76. [Google Scholar]
- 20.Singh B., Kapil R., Nandi M., Ahuja N.; Developing oral drug delivery systems using formulation by design: vital precepts, retrospect and prospects; Expert Opinion on Drug Delivery, (2011); 8: 1341–1360. [DOI] [PubMed] [Google Scholar]
- 21.Hasnain M.S., Rao S., Singh M.K., Vig N., Gupta A., Ansari A. et al. ; Development and validation of LC–MS/MS method for the quantitation of lenalidomide in human plasma using Box–Behnken experimental design; Analyst, (2013); 138: 1581. [DOI] [PubMed] [Google Scholar]
- 22.Beg S., Kohli K., Swain S., Hasnain M.S.; Development and validation of RP-HPLC method for quantitation of amoxicillin trihydrate in bulk and pharmaceutical formulation using Box–Behnken experimental design; Journal of Liquid Chromatography and Related Technology, (2012); 35: 393–406. [Google Scholar]
- 23.Beg S., Sharma G., Katare O.P., Lohan S., Singh B.; Development and validation of a stability-indicating liquid chromatographic method for estimating olmesartan medoxomil using Quality by Design; Journal of Chromatographic Science, (2015); 53(7): 1048–1059. [DOI] [PubMed] [Google Scholar]
- 24.Panda S.S., Bera V.V., Beg S., Sahu S.K.; Ultrafast liquid chromatographic method development and its validation for quantification of telaprevir in pharmaceutical dosage form by using Quality by Design approach; Journal of Chromatographic Science, (2015); 53(7): 1193–1202. [DOI] [PubMed] [Google Scholar]