Abstract
A new simple, rapid stability indicating assay method has been developed and validated for the determination of emtricitabine, tenofovir disoproxil fumarate, elvitegravir and cobicistat using reverse-phase high-performance liquid chromatography in their pharmaceutical dosage form. The chromatographic separation was performed on an ODS column (250 × 4.6 mm, 5 µm) using mobile phase A (potassium dihydrogen orthophosphate, pH adjusted to 2.5) and mobile phase B (acetonitrile) in the ratio of 55:45% v/v at a flow rate of 1 mL/min. The analytes were detected at 250 nm. The method was found to be linear in the concentration range of 2–12 µg/mL for EMT, 3–18 µg/mL for TNDF, 1.5–9 µg/mL for ELV and COB, with the coefficient value (R2) of >0.9990. The accuracy was measured via recovery studies and found to be acceptable, and the percentage recoveries were found in the range of 99.93–100.08 ± 0.5%. Forced degradation studies were also conducted, and the drugs were subjected to various stress conditions such as acid hydrolysis, base hydrolysis, oxidative, photolytic and thermal degradation. The proposed method was successfully validated and applied for the quantitative estimation of these drugs in both bulk and tablet dosage forms.
Introduction
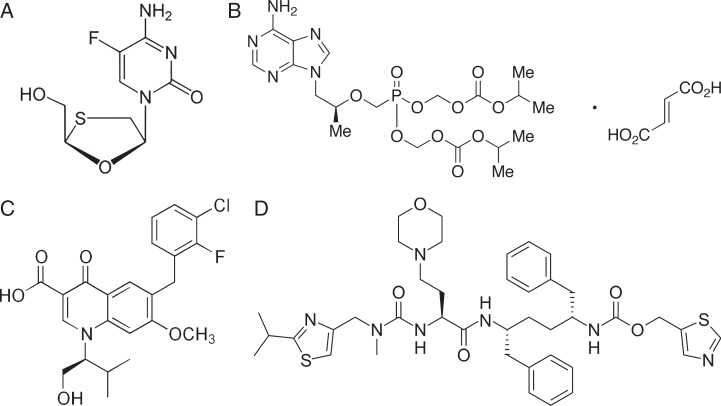
Emtricitabine (EMT) is synthetic deoxycitidine analogue and has been approved as an anti-retroviral agent in the treatment of HIV-type 1 infections (1, 2). It is a nucleoside reverse transcriptase inhibitor that competitively inhibits the HIV reverse transcriptase enzyme and blocks the HIV replication. The chemical name of the EMT (Figure 1A) is 5-fluoro-1-[(2R,5S)-2(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine (3). Tenofovir disoproxil fumarate (TNDF) is an adenine derivative and is a pro-drug of tenofovir, chemically known as (Figure 1B) 9-[(R)[(bis)[(isopropoxy carbonyl)oxy]-phosphinyl]methoxy]propyl] adenine fumarate (4). It blocks the HIV reverse transcriptase enzyme and incorporated into viral DNA (5, 6). Elvitegravir (ELV) is a novel class of anti-retroviral agents, belongs to integrase strand transferase inhibitor, inhibits the integrase enzyme and prevents the virus replication (7). The chemical name of ELV (Figure 1C) is 6(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutane-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (4). Cobicistat (COB) was used in patients with HIV infection. Co-administration of COB (Figure 1D) inhibits the cytochrome p450 3A4 enzyme and helps in improving bioavailability and half-life of ELV in HIV-infected patients (8–12). A literature survey revealed that there are various analytical methods such as UV (13), HPLC (14–16), HPTLC (17), UPLC (18) and LC–MS (19), which were reported for the estimation of individual drugs and in combination with other drugs. However, to the best of our knowledge, a simple, rapid method has not been published for the above-mentioned components. The reason for selecting this combination is to provide a validated HPLC method which can separate four different drugs in a single formulation that may be useful in the pharmaceutical industry. This study also deals with stability studies for developing a degradation pathway and to isolate the impurities which are likely to be present.
Figure 1.
Chemical structures of (A) emtricitabine, (B) tenofovir disoproxil fumarate, (C) elvitegravir and (D) cobicistat.
Experimental section
Chemicals and reagents
A reference standard sample of ELV, COB, EMT and tenofovir was obtained as a gift sample from Spectrum Pharma Research Solution (Hyderabad, India), and the fixed dose combination was purchased from a local market. Acetonitrile (ACN) and water of HPLC grade were supplied by E. Merck (Mumbai, India). Potassium dihydrogen orthophosphate (PDOP), hydrochloric acid, sodium hydroxide and hydrogen peroxide (HP) of analytical grade were obtained from RANKEM Chemical (Mumbai, India). All other reagents of analytical grade were purchased from RANKEM (Mumbai, India).
Instrument and chromatographic separation
Chromatography was performed on a WATERS 2695 HPLC column (Waters Corporation, Milford, USA) with an autosampler and equipped with a waters 2996 series of PDA detector with a spectral bandpass of 1.2 nm. Components were detected using 250 nm, and data processing was achieved by Empower 2 software. A hot air oven was used for thermal degradation of the samples, and a UV crossinker, with series of 234100 model UV chamber, equipped with a UV fluorescence lamp with the wavelength range between 200 and 300 nm was selected for photolytic degradation. The chromatographic separation was performed on an Intertsil ODS C18 (250 × 4.6 mm, 5 µm) column at an ambient column temperature. The samples were eluted using phosphate buffer (pH 2.5):ACN (55:45% v/v) as the mobile phase at a flow rate of 1 mL/min. The mobile phase was filtered through a 0.45-µm nylon filter, and it was degassed before use. Then, 10 µL of sample solutions were injected into the HPLC system.
Standard and working solutions
EMT (8 mg), TNDF (12 mg), ELV (6 mg) and COB (6 mg) were accurately weighed and transferred into a 10-mL clean and dry volumetric flask. Then, 7 mL of the diluent (water–ACN, 50:50) was added. It was sonicated for ∼30 min and diluted to the final volume with the diluent. From the above stock solution, 1 mL of the solution was transferred into another 10 mL volumetric flask and then diluted to the final volume with the diluent.
Sample solution preparation
Twenty tablets were weighed and powdered finely. The average weight of each tablet was calculated, and the weight of the powder equivalent to one tablet was transferred into a 100-mL volumetric flask. Then, 70 mL of the diluents were added, the flask was sonicated for 25 min and the volume was made up with the diluent and filtered. Finally, 0.4 mL of the filtered solution was pipetted out, transferred into a 10-mL volumetric flask and made up the final volume with diluents.
Results
Analytical method validation
The validation of an analytical method confirms the accuracy and characteristics of the method to satisfy requirements of the application. The proposed method was validated according to the ICH guidelines (20, 21) for specificity, recovery, precision, linearity, system suitability, robustness, limit of detection (LOD) and limit of quantification (LOQ). Under the validation study, the following parameters were studied.
Recovery
The percentage recovery was calculated by preparing standard drug concentrations of EMT, tenofovir, ELV and COB with concentration levels of 50%, 100% and 150%. A known amount of the standard drug was added to the blank sample at each level. Good recovery of the spiked drugs was obtained at each added concentration, and the mean percentage recovery of EMT, TNDF, ELV and COB was achieved between 99.93–100.08 ± 0.5%. The results are given in Table I.
Table I.
Results of Percentage Recovery Data
| Compound | Quantity (mg/mL) |
Mean percentage recovery | % RSD | |
|---|---|---|---|---|
| Amount added | Amount found | |||
| EMT | 4 | 4.03 | 100.08 | 1.11 |
| 8 | 7.95 | 99.43 | ||
| 12 | 11.98 | 99.86 | ||
| TNDF | 6 | 5.99 | 99.93 | 0.99 |
| 12 | 11.98 | 99.84 | ||
| 18 | 18.10 | 100.61 | ||
| ELV | 3 | 2.96 | 98.68 | 1.28 |
| 6 | 6.04 | 100.69 | ||
| 9 | 9.01 | 100.16 | ||
| COB | 3 | 2.98 | 99.34 | 1.18 |
| 6 | 5.94 | 99.61 | ||
| 9 | 9.00 | 100.06 | ||
EMT, emtricitabine; TNDF, tenofovir disoproxil fumarate; ELV, elvitegravir; COB, cobicistat; RSD, relative standard deviation.
Linearity, LOD and LOQ
Linearity of an analytical method was evaluated over the concentration range of standard solutions. A minimum of six standard drug concentrations ranging between 2 and 18 µg/mL were prepared, and their peak areas were recorded. The linearity of the calibration curve was checked by constructing a plot of area versus concentration. The LOD and LOQ were measured from the calibration curve method. The LOD value of EMT, TNDF, ELV and COB was found to be 0.25, 0.13, 0.02 and 0.17 µg/mL, respectively. The LOQ values were found to be 0.77 µg/mL of EMT, 0.40 µg/mL of TNDF, 0.07 µg/mL of ELV and 0.51 µg/mL of COB. The statistical linearity data are presented in Table II.
Table II.
Statistical Data of Calibration Curve
| Parameter | EMT | TNDF | ELV | COB |
|---|---|---|---|---|
| Linearity range | 2–12 µg/mL | 3–18 µg/mL | 1.5–9 µg/mL | 1.5–9 µg/mL |
| Regression equation | y = 12,681x+ 4,187 | y= 8,574x + 4,187 | y= 17,059x + 2,953 | y= 1,792x+ 227.7 |
| Limit of detection | 0.25 µg/mL | 0.13 µg/mL | 0.02 µg/mL | 0.17 µg/mL |
| Limit of quantification | 0.77 µg/mL | 0.40 µg/mL | 0.07 µg/mL | 0.15 µg/mL |
EMT, emtricitabine; TNDF, tenofovir disoproxil fumarate; ELV, elvitegravir; COB, cobicistat; RSD, relative standard deviation.
Precision
Precision is expressed as the closeness of agreement between a series of measurements obtaining from multiple sampling of the same homogeneous sample. Six replicate injections of a known concentration of EMT (80 µg/mL), TNDF (120 µg/mL), ELV (60 µg/mL) and COB (60 µg/mL) have been analyzed by injecting them into a HPLC column on the same day and on consecutive days. From the results obtained, % RSD was calculated and was found to be within the limits (<2). The results of precision are given in Table III.
Table III.
Results of Intra-Day and Inter-Day Precision
| Sample | Concentration (µg/mL) | Mean area |
% RSD |
||
|---|---|---|---|---|---|
| Intra-day | Inter-day | Intra-day | Inter-day | ||
| EMT | 80 | 1,133,022 | 1,131,519 | 0.914 | 1.101 |
| TNDF | 120 | 1,014,157 | 1,018,501 | 0.664 | 1.113 |
| ELV | 60 | 1,010,134 | 1,011,482 | 0.821 | 1.336 |
| COB | 60 | 102,633 | 104,258 | 1.327 | 1.766 |
EMT, emtricitabine; TNDF, tenofovir disoproxil fumarate; ELV, elvitegravir; COB, cobicistat; RSD, relative standard deviation.
Robustness
Robustness of the proposed analytical method is a measure of its capacity to remain unaffected, and it reflects the reliability of the analysis with respect to deliberate changes in the parameters such as flow rate (0.8 ± 0.2 mL), column temperature (30 ± 5°C), mobile phase ratio and pH of the mobile phase. The parameters chosen for the study of robustness is the flow rate and column temperature. From the results obtained, there were no significant changes observed at the end of the study.
Forced degradation studies
The assay method was used to test the drug stability by conducting forced degradation studies for the drug substances under various stress conditions. Stress degradation studies were carried out for acid hydrolysis (1 mL HCl heated for 30 min at 60°C), alkali hydrolysis (2 N NaOH heated for 30 min at 60°C), oxidative degradation (20% H2O2 heated at 60°C for 30 min) and thermal degradation (samples placed in an oven at 105°C for 6 h). For photolytic stress studies, samples were exposed to UV light by keeping them in a UV chamber for 7 days.
Discussion
Method development and optimization of chromatographic conditions
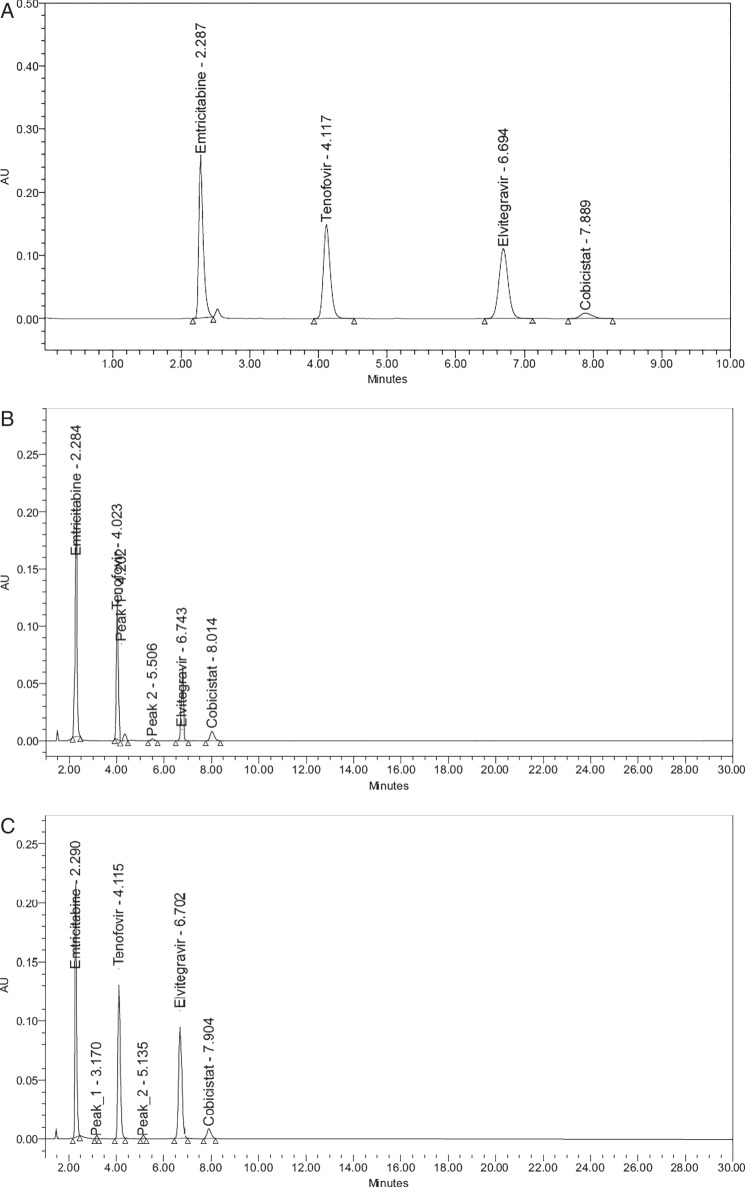
The main objective of this study is to develop and validate a stability indicating assay method for simultaneous estimation of EMT, TNDF, ELV and COB by reverse-phase high-performance liquid chromatography. Several chromatographic trials were conducted using various solvents such as methanol, ACN, water at different ratios, but the peak purity and resolution were not achieved properly. During the mobile phase selection, it was found that buffer could help in separating four drugs with good resolution. Then, the trials were conducted at different buffer pH levels 2.5, 3.5, 4.5 and 6. The best results were achieved by using the Inertsil ODS C18 asymmetry column with mobile phase A consisting of phosphate buffer (pH 2.5) and mobile phase B consisting of ACN in the ratio of 55:45% v/v at 250 nm using a PDA detector. The retention time of EMT, TNGF, ELV and COB was found to be 2.28, 4.11, 6.69 and 7.88 min, respectively. The optimized chromatographic conditions are given in Table IV.
Table IV.
Optimized Chromatographic Conditions
| Instrumentation | Optimized chromatographic conditions |
|---|---|
| Equipment | RP-HPLC Waters 2695 equipped with auto PDA detector |
| Column | Symmetry ODS 18 (250×4.6 mm, 5 µm) |
| Mobile phase | Phosphate buffer (pH 2.5) and ACN in the ratio of 55:45% v/v |
| Wavelength | 250 nm |
| Flow rate | 1 mL/min |
| Injection volume | 10 µL |
| Runtime | 10 min |
Forced degradation studies
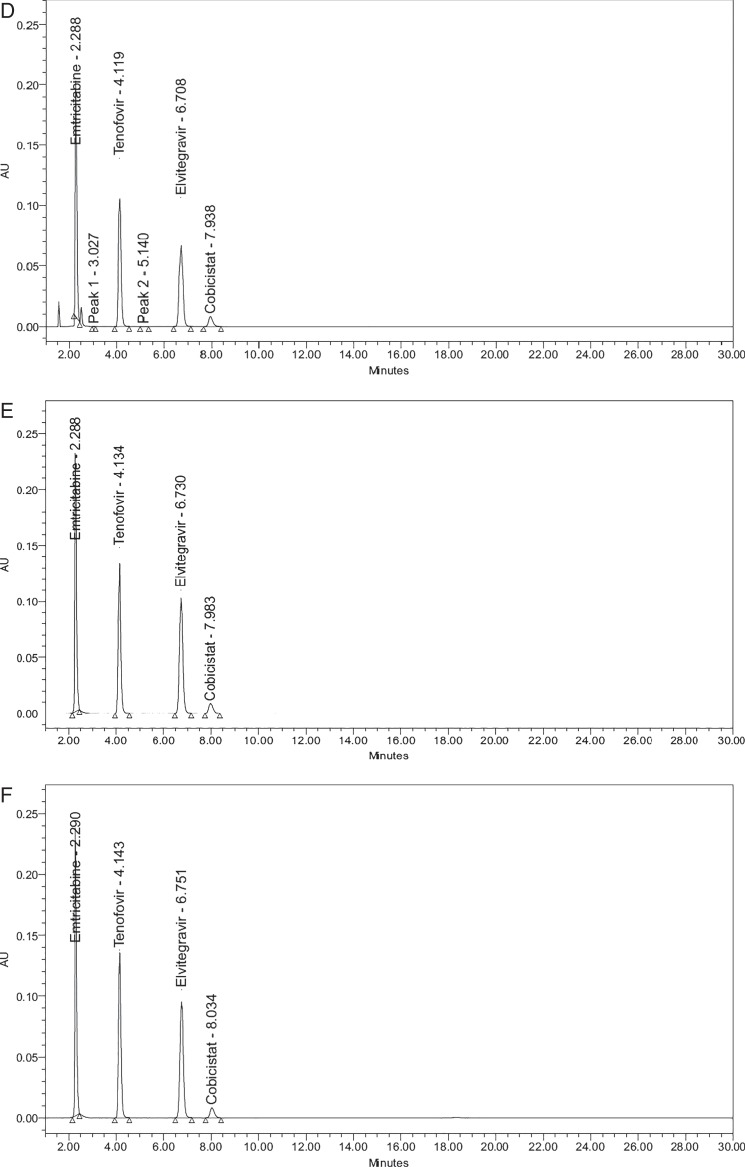
Degradation studies were performed and compared with undegraded samples (Figure 2A) to demonstrate the stability of the drug substances. Forced degradation studies of EMT, TNDF, ELV and COB were conducted under various stress conditions. In all the stress conditions, EMT was found to be stable. However, tenofovir and ELV were undergone degradation only under acid, base and peroxide conditions. Under acid and alkali conditions (Figure 2B and C), both tenofovir and ELV have undergone slightly higher degradation with degradation products, retention time (RT) value of 4.202 min of tenofovir and 5.506 min of ELV. The peroxide degradation was carried out at different concentrations (3,10 and 20%) of hydrogen peroxide, and finally at 20% concentration (Figure 2D), it was observed that tenofovir and ELV were comparatively more susceptible to undergo degradation with degradation products with RT values of tenofovir and 3.027 min of ELV and whereas the ELV and COB were less susceptible. Under photolysis and dry heat conditions (Figure 2E and F), all the four drugs showed very less degradation. In all the degradation studies, the purity of angle was found to be less than the purity of threshold. The degradation results are given in Table V.
Figure 2.
Chromatograms of forced degradation studies. (A) Undegraded standard chromatogram of EMT, TNDF, ELV and COB. (B) Acid degradation. (C) Alkali degradation. (D) Peroxide degradation. (E) Thermal degradation. (F) Photolytic degradation.
Table V.
Results of Stress-Induced Degradation Studies
| Stress condition | % Degradation |
|||
|---|---|---|---|---|
| EMT | TNDF | ELV | COB | |
| Acid degradation | 6.04 | 6.25 | 6.70 | 6.51 |
| Base degradation | 5.19 | 5.08 | 5.76 | 5.57 |
| Oxidative degradation | 6.99 | 7.05 | 7.55 | 7.48 |
| Thermal degradation | 3.59 | 2.54 | 3.04 | 3.42 |
| Photolytic degradation | 1.16 | 1.85 | 2.32 | 1.77 |
EMT, emtricitabine; TNDF, tenofovir disoproxil fumarate; ELV, elvitegravir; COB, cobicistat; RSD, relative standard deviation.
Analysis of Stribild tablet dosage form
The developed method was applied for the estimation of drugs in the commercial tablet dosage form (STRIBLID tablets: EMT 200 mg/TNDF 300 mg/ELV 150 mg/COB 150 mg). The chromatograms show good separation of the samples with acceptable limits such as tailing factor and USP plate counts. The % assay was calculated and found to be satisfactory, and the results are given in Table VI.
Table VI.
Percentage Assay Results of Stribild Tablets
| Tablet | Drug | Dosage (mg) | Amount found (mg) | % Assay |
|---|---|---|---|---|
| STRIBILD | Emtricitabine | 200 | 198.42 mg | 99.21 |
| Tenofovir | 300 | 299.16 mg | 99.72 | |
| Elvitegravir | 150 | 149.46 mg | 99.64 | |
| Cobicistat | 150 | 147.54 mg | 98.36 |
EMT, emtricitabine; TNDF, tenofovir disoproxil fumarate; ELV, elvitegravir; COB, cobicistat.
Conclusion
In this study, a simple, specific, accurate and robust stability indicating assay method was developed by using RP-HPLC. The developed stability indicating method was validated successfully for simultaneous estimation of EMT, TNDF, ELV and COB. The proposed method was tested for its accuracy, linearity, precision, robustness, LOD and LOQ. The forced degradation studies were carried out, and the reported method effectively separates the drug substances without interference of excipients and degradation products. Hence, it can be concluded that the developed method can be used for the routine analysis of EMT, TNDF, ELV and COB in the pharmaceutical dosage form.
Acknowledgments
The authors are thankful to spectrum research laboratory for providing gift samples and dosage form and Joginpally B.R. Pharmacy College for providing necessary facilities for preparation of the article.
References
- 1.Bang L.M., Scott L.J.; Emtricitabine and antiretroviral agent for HIV infections; Drugs, (2003); 63: 2413–2424. [DOI] [PubMed] [Google Scholar]
- 2.Saag M.S.; Emtricitabine, a new antiviral agent with activity against HIV and Hepatitis B virus; Reviews of Anti-Infective Agents, (2006); 42: 126–131. [DOI] [PubMed] [Google Scholar]
- 3.Budawari S.; The Merck Index. 13th ed Merck and Co. Inc., Whitehouse Station, NJ, (2001); pp. 1631–1632. [Google Scholar]
- 4.Martindale W.; Martindale: TheComplete DrugReference, 33rd edn. Pharmaceutical Press, London, (2002); pp. 620–642.
- 5.Fung H.B., Stone E.A., Piacenti F.J.; Tenofovir disoproxil fumarate: a nucleoside reverse transcriptase inhibitor for the treatment of HIV infections; Clinical Therapeutics, (2002); 24: 1515–1548. [DOI] [PubMed] [Google Scholar]
- 6.Porche D.J.; Tenofovir diproxil fumarate (viread); The Journal of the Association of Nurses in AIDS Care, (2002); 13: 100–102. [DOI] [PubMed] [Google Scholar]
- 7.Mouscadet J.F., Delelis O., Marcelin A.G., Tchertanov L.; Resistance to HIV-1 intregrase inhibitors: a structural perspective; Drug Resistance Updates, (2010); 13: 139–150. [DOI] [PubMed] [Google Scholar]
- 8.Zolopa A.R., Berger D.S., Lampiris H., Zhong L., Chuck S.L., Enejosa J.V. et al. Activity of elvitegravir a once daily integrase inhibitor against resistance HIV type 1; The Journal of Infectious Diseases, (2010); 201: 814–822. [DOI] [PubMed] [Google Scholar]
- 9.Janson J.S., Kathleen E.S.; Integrase inhibitors: a novel class of anti-retroviral agents; Annals of Pharmacotherapy, (2010); 44: 145–156. [DOI] [PubMed] [Google Scholar]
- 10.Deeks E.D.; Cobicistat: a review of its use as pharmacokinetic enhancer; Drugs, (2014); 74: 195–206. [DOI] [PubMed] [Google Scholar]
- 11.Nathan B., Bayley J., Waters L., Post F.A.; Cobicistat: a novel pharmacoenhancer for co-administration with HIV protease and integrase inhibitors; Infectious Disease & Therapy, (2013); 2: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sax P.E., DeJesus E., Mills A., Zolopa A., Cohen C., Wohl D. et al. Co-formulated elvitegravir, cobicistat, emtricitabine and tenofovir; Lancet, (2012); 30: 2439–2448. [DOI] [PubMed] [Google Scholar]
- 13.Anandakumar K., Kannan K., Vetrichelvan T.; Development and validation of emtricitabine and tenofovir disoproxil fumarate in pure and fixed dose combination by UV spectroscopy; Digest Journal of Nanomaterial & Biostructures, (2011); 6: 1085–1090. [Google Scholar]
- 14.Narendra D., Satyanarayana T., Ganga R.B.; HPLC method development and validation for simultaneous estimation of tenofovir and emtricitabine in combined pharmaceutical dosage form; International Journal of Research in Pharmaceutical and Biomedical Sciences, (2012); 3: 361–367. [Google Scholar]
- 15.Palavan C., Ramprasad L.A., Srinivasu P., Sheshagirirao J.V.L.N.; A new RP-HPLC method development for the simultaneous estimation of emtricitabine, efavirenz and tenofovir in tablet dosage form; American Journal of PharmaTech Research, (2013); 3: 547–554. [Google Scholar]
- 16.Ravendrababu V.V., Pankaj K.S., Singvi I.; A new gradient liquid chromatographic method for simultaneous estimation of tenofovir disoproxil fumarate, cobicistat, emtricitabine and elvitegravir in bulk and tablet dosage form; Asian Journal of Chemistry, (2014); 26: 6233–6237. [Google Scholar]
- 17.Joshi M., Nikalje A.P., Shahed M., Dehghan M.; HPTLC method for the simultaneous estimation of emtricitabine and tenofovir in tablet dosage form; Indian Journal of Pharmaceutical Sciences, (2009); 71: 95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manish G., Avinash K., Ravi Y., Tiwari P.; Development and validation of UPLC method for emtricitabine, tenofovir and efavirenz in pharmaceutical preparation; Analytical Chemistry, An Indian Journal, (2010); 9: 247–251. [Google Scholar]
- 19.Matt M.K., Pilli N.R., Rao J.V.N.L.; A validated liquid chromatography and tandem mass spectrometric method for simultaneous quantification of tenofovir, emtricitabine and efavirenz in human plasma and its pharmacokinetics application; Acta Chromatographica, (2015); 1: 27–39. [Google Scholar]
- 20.ICH Harmonised Tripartite Guideline, Validation of analytical procedures: text and methodology Q2(R1) current step 4 version, November (2005).
- 21.ICH Harmonised Tripartite Guideline, Stability testing of new drug substances and products Q1A (R2), ICH, Geneva, Switzerland, (2003).