Abstract
Many potential health benefits of raspberry (Rubus idaeus L.) leaves were attributed to polyphenolic compounds, especially flavonoids. In this study, the methanol extract of R. idaeus leaves showed significant protein tyrosine phosphatase-1B (PTP1B) inhibitory activity with IC50 value of 3.41 ± 0.01 µg mL−1. Meanwhile, a rapid and reliable method, employed high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry, was established for structure identification of flavonoids from PTP1B inhibitive extract of R. idaeus leaves using accurate mass measurement and characteristic fragmentation patterns. A total of 16 flavonoids, including 4 quercetin derivatives, 2 luteolin derivatives, 8 kaempferol derivatives and 2 isorhamnetin derivatives, were identified. Compounds 3 and 4, Compounds 6 and 7 and Compounds 15 and 16 were isomers with different aglycones and different saccharides. Compounds 8, 9 and 10 were isomers with the same aglycone and the same saccharide but different substituent positions. Compounds 11 and 12 were isomers with the same aglycone but different saccharides. Compounds 2, 8, 9 and 10 possessed the same substituent saccharide of glycuronic acid. Most of them were reported in R. idaeus for the first time.
Introduction
Rubus idaeus L. (raspberry) leaves have received considerable attention for its human health benefits in treating fever, influenza, diabetes, menstrual pain, diarrhea and colic pain. Many of these potential health benefits may be attributed to polyphenolic compounds, especially flavonoids. In previous studies, R. idaeus leaves were a rich source of flavonoid derivatives, as well as phenolic acids, triterpenes (1–3). Although the beneficial activities of R. idaeus fruits have been established to some extent, there is little information about the biological activity and action mechanism of R. idaeus leaves. To provide a better understanding of pharmacological functions of R. idaeus leaves, characterization of bioactive compounds from R. idaeus leaves is essential.
Isolation and purification of compounds need a time-consumed chromatographic procedure. MS promoted the detection of minor constituents that were not possible by the classical way. HPLC–MS was successfully used to characterize a number of components from plants (4). Quadrupole time-of-flight tandem mass spectrometry (QTOF-MS) can provide accurate mass and formulae and distinguish the isobaric compounds according to the different molecular formulae. QTOF–MS-MS can provide fragmentation analysis with accurate mass measurement for structural characterization and identification (5–8). In the past, fragmentation behaviors of flavonoids have been extensively investigated (9–20). A great number of rules were summarized and used for the identification of unknown compounds even without reference standards.
Protein-tyrosine phosphatase-1B (PTP1B) is an enzyme of the protein-tyrosine phosphatase (PTP) family. It is considered a promising potential therapeutic target, in particular for treatment of type 2 diabetes. In this study, the antioxidant and PTP1B inhibitory activity of methanol extract of R. idaeus leaves were assayed. Meanwhile, a simple method of HPLC–ESI–QTOF–MS-MS was established to identify flavonoids in the methanol extract of R. idaeus leaves based on accurate mass and formulae provided by QTOF–MS, and based on the fragmentation analysis provided by QTOF–MS-MS. A total of 16 flavonoids, including 4 quercetin derivatives, 2 luteolin derivatives, 8 kaempferol derivatives and 2 isorhamnetin derivatives were identified. Most of them were reported in R. idaeus for the first time.
Experimental
Materials and reagents
Leaves of R. idaeus L. were collected in August 2013 in Shihezi, Xinjiang, China, and identified by Dr Wen-Bin XU. A voucher specimen (RIL 136521) was deposited at College of Life Science, Shihezi University, Shihezi, Xinjiang, China.
Acetonitrile (Fisher, optima®, LC/MS-grade; Fair Lawn, NJ, USA) and formic acid (Merck, EMSURE®, analytical grade; Darmstadt, Germany) were also obtained. Water used in the experiment was deionized and further purified by Milli-Q Plus water purification system (Millipore Ltd., Bedford, MA, USA). Isorhamnetin-3-O-rutinoside, quercetin, quercetin-3-O-glucoside, luteolin, luteolin-3-O-glucoside, kaempferol and kaempferol-3-O-glucose-glucose (Manst, Chengdu, Sichuan, China) were used as standards. Other reagents and chemicals were of analytical grade.
Extraction and sample preparation
R. idaeus leaves were natural dried for 30 days in the laboratory room under a constant temperature of 24°C and a relative humidity of 40%. Dried leaves were porphyrized by a LD-500 grinder (Changhong Technologies, Changsha, China) with a rotating speed of 25,000 revolutions per minute, and then sieved by a 100 mesh sifter. One gram of sieved leaves powder were transferred to a glass vial (15 mL), and then 5 mL methanol was added to macerate for 36 h and agitated adequately for 1 min every 12 h. After 36 h, the supernatant was moved out. Another 5 mL methanol was added, and two more times repeated. The supernatant was combined and concentrated under 38°C by BÜCHI Rotavapor R-100 (BÜCHI Labortechnik AG, Flawil, Switzerland) and BÜCHI Vacuum Pump V-100 (BÜCHI Labortechnik AG). After concentrated, the dark brown residue was further dissolved in 50% aqueous acetonitrile (5 mL). Samples were stored under 4°C in brown glass bottles and under nitrogen. Each sample was filtered through a Minispike syringe filter (Polypropylene, 0.2 µm, 13 mm; PALL, Radnor, PA, USA) before LC–MS analysis.
PTP1B enzymatic assay and inhibition screening
Protein-tyrosine phosphatase-1B (PTP1B, human, recombinant) was purchased from Sigma-Aldrich Chemical Co. The enzyme activity was measured using p-nitrophenyl phosphate (pNPP) as a substrate. Each 96-well (final volume: 100 µL) was added 10 mM pNPP and 100 nM PTP1B in a buffer containing 25 mM tris(hydroxymethyl)aminomethane–HCl (Tris–HCl, pH 7.5), 1 mM ethylenediaminetetraacetic acid (EDTA), 2 mM β-mercaptoethanol and 1 mM dithiothreitol (DTT) with or without test compounds. After incubating at 37°C for 30 min, the reaction was terminated with 2 M sodium hydroxide. Dephosphorylation of p-NPP generated product p-nitrophenyl, which can be monitored at 405 nm. The nonenzymatic hydrolysis of 10 mM pNPP was corrected by measuring the increase in absorbance at 405 nm obtained in the absence of PTP1B enzyme. The known PTP1B inhibitor NaVO4 20 µM was used as a positive control. The inhibition assay was performed in triplicate (21, 22).
Instrumentation and condition
HPLC analysis was performed on an Agilent 1200 series HPLC system (Agilent Technologies, Waldbronn, Germany), coupled with an autosampler and a quaternary solvent delivery system with an online degasser.
Chromatographic separation was performed on a high strength silica (HSS) T3 column (250 mm × 4.6 mm i.d., 5 µm; Waters, MA, USA) with a VanGuard pre-column (20 mm × 4.6 mm i.d., 5 µm; Waters). HSS T3 columns contain the first and only 100% silica particle designed. The first ligand chemistry in this column family utilizes T3 bonding in order to retain and separate polar organic compounds. HSS T3 columns possess the superior polar-compound retention, aqueous mobile phase compatibility and ultra-low MS bleed. The system dead volume was 1.45 mL. The mobile phase consisted of acetonitrile (A) and 0.1% (v/v) formic acid/water (B) with a gradient elution of 5–65% A at 0–60 min. The flow rate of mobile phase was 1.000 mL min−1, and the injection volume was 5.00 µL.
Mass spectrometry was performed using a QSTAR Elite LC–MS-MS system from Applied Biosystems/MDS Sciex (Concord, ON, Canada) coupled with an electrospray ionization (ESI) interface. Nitrogen was used in all cases. Under the negative ESI mode, molecules with phenol hydroxyl could produce strong and stable [M–H]−, which could be much more helpful for identification. As a result, most of the research on polyphenols, especially on flavonoids, were taken by the negative ESI mode. In this study, the parameters were optimized as follows: ESI voltage, −4,000 V; nebulizer gas, 60; auxiliary gas, 50; curtain gas, 35; turbo gas temperature, 500°C; declustering potential, −60 V; focusing potential, −350 V; and declustering potential, −10 V. The samples were analyzed with an information-dependent acquisition (IDA) method, which can automatically select candidate ions for the MS–MS study. The TOF mass range was set from m/z 100 to 1,000, and the mass range for product ion scan was m/z 100–1000. The collision energy (CE) was set to 30 eV to observe the pseudomolecular [M–H]− ion and the losses of substituent groups, and 70 eV to obtain information about the basic skeletons. The mass analyzer was calibrated using Taurocholic acid (2 ng µL−1) by direct injection at a flow rate of 5 µL min−1. The data were acquired and processed using Analyst QS 2.0 software.
Results
The methanol extract of R. idaeus leaves showed significant PTP1B inhibitory activity with IC50 value of 3.41 ± 0.01 µg mL−1, which was almost the equal inhibition with that of positive control (NaVO4, IC50 value of 1.46 ± 0.40 µg mL−1).
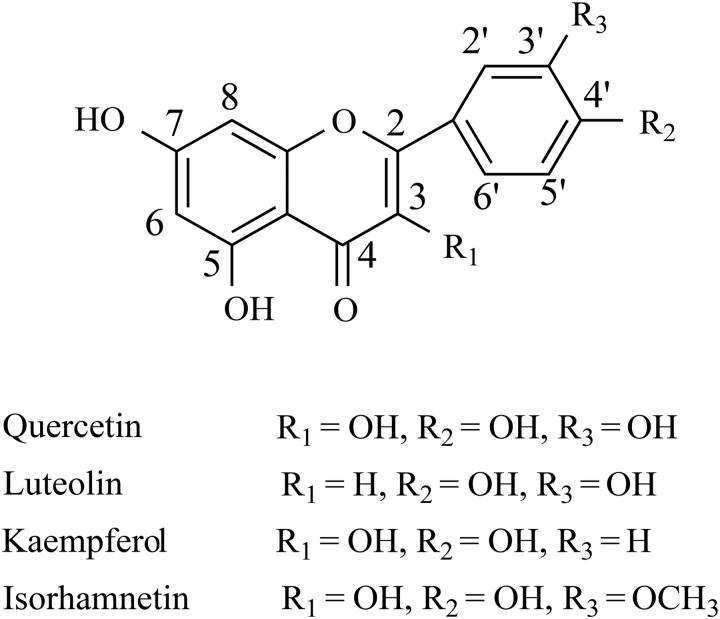
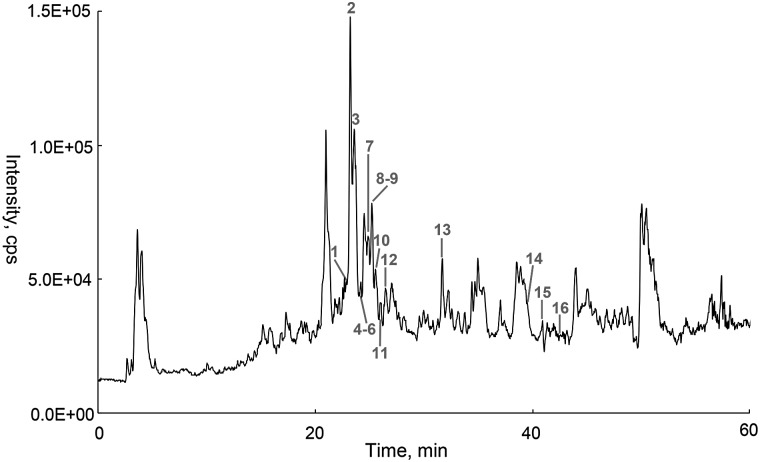
A total of 16 flavonoids were identified by HPLC–ESI–QTOF–MS-MS, and their structure skeletons are shown in Figure 1. The total ion chromatography (TIC) profiles are shown in Figure 2. Accurate mass measurements, retention time, formula and errors for all compounds are summarized in Table I as well as the main product ions observed in MS–MS spectrum.
Figure 1.
The structure skeletons of flavonoids identified from R. idaeus leaves.
Figure 2.
HPLC–QTOF–MS total ion chromatogram (TIC) of R. idaeus leaves in negative ESI mode.
Table I.
Identification of Flavonoids in R. idaeus Leaves by HPLC–ESI–QTOF–MS-MS in Negative Ion Mode
| No. | tR min | Formula | [M–H]− | Error, ppm | CE, eV | ESI–MS2 data | Identification |
|---|---|---|---|---|---|---|---|
| 1 | 22.84 | C21H20O12 | 463.0871 | −2.38 | 30 70 |
343 (2), 301 (61), 300 (100) 271 (100), 255 (58), 179 (7), 151 (12) |
Quercetin-3-O-glucoside |
| 2 | 23.24 | C21H18O13 | 477.0679 | 0.44 | 30 70 |
343 (3), 301 (100), 300 (24) 271 (79), 255 (33), 179 (32), 151 (100) |
Quercetin-7-O-glucuronic acid |
| 3 | 23.61 | C27H30O16 | 609.1473 | 1.96 | 30 70 |
447 (2), 285 (44), 284 (100) 255 (100), 227 (14) |
Kaempferol-3-O-glucose-glucose |
| 4 | 23.82 | C27H30O16 | 609.1435 | −4.28 | 30 70 |
505 (5), 463 (8), 343 (3), 301 (64), 300 (100) 271 (100), 255 (62), 179 (6), 151 (10) |
Quercetin-3-O-hexose-deoxyhexose |
| 5 | 24.05 | C28H32O16 | 623.1592 | −2.56 | 30 70 |
315 (42), 314 (100) 300 (100), 271 (81), 255 (4), 227 (2) |
Isorhamnetin-3-O-rutinoside |
| 6 | 24.13 | C21H20O11 | 447.0942 | 2.05 | 30 70 |
315 (100), 314 (57), 300 (2) 300 (100), 271 (8), 255 (3) |
Isorhamnetin-7-O-pentose |
| 7 | 24.86 | C21H20O11 | 447.0921 | −2.65 | 30 70 |
285 (100), 284 (55) 175 (9), 151 (100), 133 (31) |
Luteolin-7-O-glucoside |
| 8 | 25.21 | C21H18O12 | 461.0734 | 1.84 | 30 70 |
285 (50), 284 (100) 255 (100), 227 (8) |
Kaempferol-3-O-glucuronic acid |
| 9 | 25.29 | C21H18O12 | 461.0730 | 0.98 | 30 70 |
327 (5), 285 (100), 284 (70) 255 (100), 227 (6) |
Kaempferol-7-O-glucuronic acid |
| 10 | 25.56 | C21H18O12 | 461.0741 | 3.36 | 30 70 |
285 (100) 255 (100), 227 (8) |
Kaempferol-4′-O-glucuronic acid |
| 11 | 25.99 | C20H18O10 | 417.0843 | 3.79 | 30 70 |
285 (44), 284 (100) 255 (100), 227 (9) |
Kaempferol-3-O-pentose |
| 12 | 26.49 | C20H18O10 | 417.0823 | −1.01 | 30 70 |
285 (45), 284 (100) 255 (100), 227 (10) |
Kaempferol-3-O-pentose |
| 13 | 31.64 | C27H30O15 | 593.1518 | 1.02 | 30 70 |
447 (6), 285 (42), 284 (100) 255 (100), 227 (12) |
Kaempferol-3-O-hexose-deoxyhexose |
| 14 | 39.42 | C15H10O7 | 301.0344 | −3.24 | 30 70 |
301 (100) 271 (100), 255 (51), 179 (6), 151 (18) |
Quercetin |
| 15 | 40.83 | C15H10O6 | 285.0411 | 2.24 | 30 70 |
285 (100) 175 (13), 151 (100), 133 (22) |
Luteolin |
| 16 | 42.05 | C15H10O6 | 285.0399 | −1.97 | 30 70 |
285 (100) 255 (100), 227 (5) |
Kaempferol |
Identification of quercetin derivatives in R. idaeus leaves by HPLC–ESI–QTOF–MS-MS
Compounds 1, 2, 4 and 14 all gave the deprotonated aglycone fragment at m/z 301, as shown in Table I, which indicated that these compounds were originated from quercetin. This aglycone was also confirmed by another MS2 spectrum in which the CE was set to 70 eV to give more information about the aglycone. The characteristic product ions at m/z 271, 255, 179 and 151 led to the aglycone identification as quercetin.
Compound 14 (tR = 39.42 min), produced a [M–H]− at m/z 301, was tentatively established to Quercetin by comparing the mass spectrum data and chromatography with an authentic standard (the mass spectral data are shown in Table I).
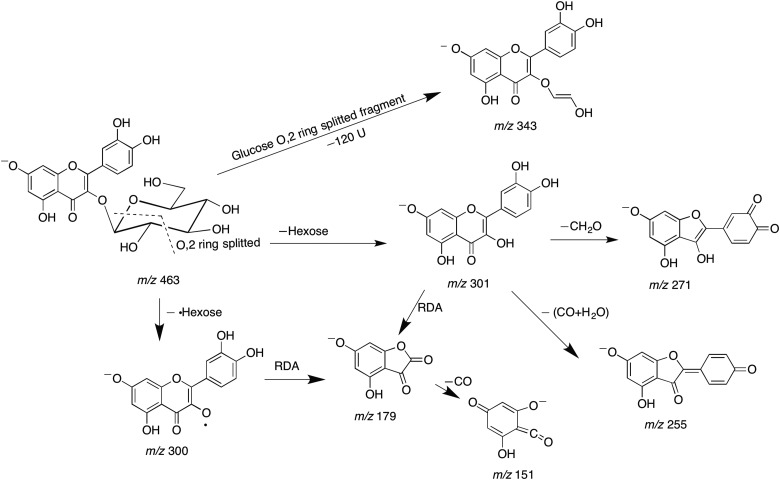
Compound 1 (tR = 22.84 min) produced a deprotonated ion at m/z 463. In analysis of the MS–MS spectrum, two high intensity fragments at m/z 301 [M–H–162]− and 300 [M–H–162]−• were observed. Ions at m/z 301 suggested the loss of a hexose unit. The glycosylation site of 3-OH was determined by the intensity of [M–H–162]−• higher than that of [M–H–162]−. The characteristic product ions at m/z 271, 255, 179 and 151, obtained with CE = 70 eV, led to the aglycone identification as quercetin. The mass spectrum and co-chromatography with an authentic standard tentatively established that Compound 1 was quercetin-3-O-glucoside. A fragmentation scheme of Compound 1 is shown in Figure 3.
Figure 3.
Fragmentation scheme of Compound 1.
Compound 2 (tR = 23.24 min) produced a [M–H]− at m/z 477. In MS2 spectrum, the predominant ion at m/z 301 [M–H–176]− gave the proof for a glucuronyl unit loss. The substituent position of 7-OH was determined by the predominant ion at m/z 301 [M–H–176]− coupled with the weak ion at m/z 300 [M–H–176]−•. The characteristic product ions at m/z 271, 255, 179 and 151, obtained with CE = 70 eV, indicated the aglycone of quercetin. On the basis of the mass spectral data, Compound 2 was tentatively identified as quercetin-7-O-glycuronic acid.
Compound 4 (tR = 23.82 min) gave the [M–H]− at m/z 609. In analysis of the MS–MS spectrum, two predominant fragments at m/z 301 [M–H–308]− and 300 [M–H–308]−• were observed. Furthermore, m/z 301, corresponding to the loss of 308 Da (162 + 146), indicated that a hexose and a deoxyhexose linked at the same position of the aglycone, or a hexose and a p-coumaroyl group linked at the same position of the aglycone. According to an accurate 146.0582 Da loss, the product ion at m/z 463 was attributed to the neutral loss of a deoxyhexose moiety (C6H10O4, calculated 146.0579 Da, δ 1.99 ppm) rather than a p-coumaroyl group (C9H6O2, calculated 162.0367 Da, δ 146.66 ppm) from [M–H]− at m/z 609. The ion at m/z 463 also proved the deoxyhexose was in a terminal position. The glycosylation site of 3-OH was determined by the intensity of [M–H–308]−• higher than that of [M–H–308]−. Quercetin was confirmed as aglycone by the characteristic fragments (m/z 271, 255, 179 and 151) produced at high CE (70 eV). Thus, Compound 4 was tentatively identified as quercetin-3-O-hexose-deoxyhexose.
Identification of luteolin derivatives in R. idaeus leaves by HPLC–ESI–QTOF–MS-MS
Compounds 7 and 15 produced the deprotonated aglycone fragment at m/z 285, as shown in Table I, suggested that they were originated from luteolin or kaempferol. The aglycone was further confirmed by MS2 spectrum with CE = 70 eV. The characteristic product ions at m/z 199, 175, 151 and 133 determined the aglycone as luteolin.
Compound 15 (tR = 40.83 min), produced a [M–H]− at m/z 285, was tentatively established to luteolin by comparing the mass spectrum data and co-chromatography with an authentic standard (the mass spectral data are shown in Table I).
Compound 7 (tR = 24.86 min) produced a [M–H]− at m/z 447. In MS–MS spectrum, the visibility of two high abundance fragments at m/z 285 [M–H–162]− and 284 [M–H–162]−• indicated the loss of a hexose unit. Meanwhile, the glycosylation site of 3-OH was proved by the intensity of [M–H–162]− higher than that of [M–H–162]−•. Luteolin was confirmed as aglycone by the characteristic fragments (m/z 175, 151 and 133) produced at high CE (70 eV). The mass spectrum and co-chromatography with an authentic standard tentatively established that Compound 7 was luteolin-7-O-glucoside.
Identification of kaempferol derivatives in R. idaeus leaves by HPLC–ESI–QTOF–MS-MS
Compounds 3, 8, 9, 10, 11, 12, 13 and 16 all showed the deprotonated aglycone fragment at m/z 285, as shown in Table I, which indicated that they were originated from luteolin or kaempferol. The aglycone was further confirmed by MS2 spectrum with CE = 70 eV to give more information. The characteristic product ions at m/z 255 and 227 led to the aglycone identification as kaempferol.
Compound 16 (tR = 42.05 min), gave a [M–H]− at m/z 285, was tentatively established to kaempferol by comparing the mass spectrum data and chromatography with an authentic standard (the mass spectral data are shown in Table I).
Compound 3 (tR = 23.61 min) showed a [M–H]− at m/z 609. In the MS–MS spectrum, two predominant fragments at m/z 285 [M–H–324]− and 284 [M–H–324]−• were observed. The loss of 324 Da (162 + 162) indicated that a di-hexose or a hexose and a caffeoyl group linked at the same position of the aglycone. The substituent position of 3-OH was determined by the intensity of [M–H–324]−• higher than that of [M–H–324]−. Kaempferol was confirmed as aglycone by the characteristic fragments (m/z 255 and 227) produced at high CE (70 eV). The mass spectrum and co-chromatography with an authentic standard tentatively established that Compound 3 was Kaempferol-3-O-glucose-glucose.
Compounds 8 (tR = 25.21 min), 9 (tR = 25.29 min) and 10 (tR = 25.56 min) produced the same deprotonated ion at m/z 461. In MS2 spectrum, the same predominant ions at m/z 285 [M–H–176]− gave the proof of same glucuronyl unit loss. In another MS2 spectrum with CE = 70 eV, the same characteristic product ions at m/z 255 and 227 determined the same aglycone of kaempferol. The glycosylation site at 3-OH of Compound 8 was indicated by the intensity of m/z 284 [M–H–176]−• higher than that of m/z 285 [M–H–176]−. The 7-OH substituent position of Compound 9 was determined by the predominant ion at m/z 285 [M–H–176]− coupled with the weak ion at m/z 284 ([M–H–176]−•). The abundance fragments at m/z 285 [M–H–176]− coupled with the absence of the ion at m/z 284 ([M–H–176]−•) suggested the 4′-OH substituent position of Compound 10. Thus, based on the mass spectral data, Compounds 8, 9 and 10 were tentatively identified as kaempferol-3-O-glycuronic acid, kaempferol-7-O-glycuronic acid and kaempferol-4′-O-glycuronic acid, respectively.
Compounds 11 (tR = 25.99 min) and 12 (tR = 26.49 min) produced the same [M–H]− at m/z 417. In analysis of the MS–MS spectrum, the same observation of predominant ions at m/z 285 [M–H–132]− and 284 [M–H–132]−• proved the same loss of a pentose unit. The same substituent position of 3-OH was determined by the intensity of [M–H–132]−• higher than that of [M–H–132]−. The same aglycone of kaempferol was confirmed by the same characteristic fragments (m/z 255 and 227) produced at high CE (70 eV). According to the mass spectrum data, Compounds 11 and 12 were both tentatively identified as kaempferol-3-O-pentose but with different pentose units. It is difficult to identify the isomers of sugar by mass spectrometry alone (23).
Compound 13 (tR = 31.64 min) gave a [M–H]− at m/z 593. In MS2 spectrum, two intensive fragments at m/z 285 [M–H–308]− and 284 [M–H–308]−• were observed. The loss of 308 Da (162 + 146) suggested that a hexose and a deoxyhexose linked at the same position of the aglycone, or a hexose and a p-coumaroyl group linked at the same position of the aglycone. According to an accurate 146.0570 Da loss, the product ion at m/z 447 was attributed to the neutral loss of a deoxyhexose moiety (C6H10O4, calculated 146.0579 Da, δ −6.22 ppm) rather than a p-coumaroyl group (C9H6O2, calculated 162.0367 Da, δ 138.44 ppm) from [M–H]− at m/z 593. The ion at m/z 447 also proved that the deoxyhexose was in a terminal position. The glycosylation site of 3-OH was determined by the intensity of [M–H–308]−• higher than that of [M–H–308]−. The aglycone of kaempferol was confirmed by the same characteristic fragments (m/z 255 and 227) produced at high CE (70 eV). Thus, Compound 13 was tentatively identified as kaempferol-3-O-hexose-deoxyhexose.
Identification of isorhamnetin derivatives in R. idaeus leaves by HPLC–ESI–QTOF–MS-MS
Compounds 5 and 6 produced the deprotonated aglycone fragment at m/z 315, as shown in Table I, which suggested that they were originated from isorhamnetin. The aglycone was further confirmed by MS2 spectrum with CE = 70 eV to give more information. The characteristic product ions at m/z 300, 271, 255 and 227 led to the aglycone identification as isorhamnetin.
Compound 5 (tR = 24.05 min) produced a [M–H]− at m/z 623. In the MS–MS spectrum, two predominant fragments at m/z 315 [M–H–308]− and 314 [M–H–308]−• were observed. The loss of 308 Da (162 + 146) indicated that a hexose and a deoxyhexose linked at the same position of the aglycone. The substituent position of 3-OH was determined by the intensity of [M–H–308]−• higher than that of [M–H–308]−. Isorhamnetin was confirmed as aglycone by the characteristic fragments (m/z 300, 271, 255 and 227) produced at high CE (70 eV). Compared the mass spectrum and chromatography with an authentic standard, Compound 5 was tentatively established as isorhamnetin-3-O-rutinoside.
Compound 6 (tR = 24.13 min) produced a [M–H]− at m/z 447. In MS2 spectrum, the predominant ion at m/z 315 [M–H–132]− gave the proof for a pentose unit loss. The substituent position of 7-OH was determined by the predominant ion at m/z 315 [M–H–132]− coupled with the weak ion at m/z 314 [M–H–132]−•. The characteristic product ions at m/z 300, 271, 255 and 227, obtained with CE = 70 eV, indicated the aglycone of isorhamnetin. On the basis of the mass spectral data, Compound 6 was tentatively identified as isorhamnetin-7-O-pentose.
Discussion
In this study, a qualitative characterization of flavonoids was performed on ESI negative ionization mode. Accurate mass data were acquired in the full scan analysis, and product ion mass were acquired in the IDA method. A Waters HSS T3 column was employed to provide increased chromatographic separation. In addition, formic acid was introduced into the mobile phase (0.1%) to alleviate the peak tailing and produce better shapes. For the purpose of optimizing signals and obtaining maximal structural information from the ions of interest, the MS–MS experiments were performed at different CE values. Lower energy (CE = 30 eV) was used to observe the losses of substituent groups, and higher energy (CE = 70 eV) was applied to obtain information about the basic skeletons.
Conclusion
In this investigation, an HPLC–ESI–QTOF–MS-MS method has been successfully established for rapid separation and identification of flavonoids in the methanol extract of raspberry leaves. Based on accurate mass measurements and Collision Induced Dissociation (CID) techniques, 16 flavonoids, including 4 quercetin derivatives, 2 luteolin derivatives, 8 kaempferol derivatives and 2 isorhamnetin derivatives were identified or tentatively identified in the methanol extract of raspberry leaves. Compounds 3 and 4, compounds 6 and 7 and compounds 15 and 16 were isomers with different aglycones and different saccharides. Compounds 8, 9 and 10 were isomers with the same aglycone and the same saccharide, but different substituent positions. Compounds 11 and 12 were isomers with the same aglycone but different saccharides. Compounds 2, 8, 9 and 10 possessed the same substituent saccharide of glycuronic acid. To our knowledge, most of them were reported in R. idaeus for the first time.
This study contributed to promote the use of raspberry leaves in phytotherapeutics due to the biologically active compounds that were identified. The HPLC–ESI–QTOF–MS-MS method presented here has been demonstrated to be an effective tool for the analysis of the components in a complex plant extract.
Funding
This research was financially supported by the grants from the National Natural Science Foundation of China (31200259) and the Scientific Research Starting Foundation for High Level Talents of Shihezi University of China (RCZX2011011).
Conflicts of Interest statement. The authors declare that they have no conflicts of interest.
References
- 1.Gudej J.; Kaempferol and quercetin glycosides from Rubus idaeus L. leaves; Acta Poloniae Pharmaceutica, (2003); 60: 313–315. [PubMed] [Google Scholar]
- 2.Gudej J., Tomczyk M.; Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. species; Archives of Pharmacal Research, (2004); 27: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 3.Mullen W., Yokota T., Lean M.E.J., Crozier A.; Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC–MSn; Phytochemistry, (2003); 64: 617–624. [DOI] [PubMed] [Google Scholar]
- 4.Zheng X., Shi P., Cheng Y., Qu H.; Rapid analysis of a Chinese herbal prescription by liquid chromatography–time-of-flight tandem mass spectrometry; Journal of Chromatography A, (2008); 1206: 140–146. [DOI] [PubMed] [Google Scholar]
- 5.Fabre N., Rustan I., de Hoffmann E., Quetin-Leclercq J.; Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry; Journal of the American Society for Mass Spectrometry, (2001); 12: 707–715. [DOI] [PubMed] [Google Scholar]
- 6.Li C., Liu Y., Abdulla R., Aisa H.A., Suo Y.; Characterization and identification of chemical components in extract of Neopicrorhiza scrphulariiflora roots by liquid chromatography–electrospray ionization quadrupole time-of-flight tandem mass spectrometry; Analytical Methods, (2014); 6: 3634–3643. [Google Scholar]
- 7.Vukics V., Ringer T., Kery A., Bonn G.K., Guttman A.; Analysis of heartsease (Viola tricolor L.) flavonoid glycosides by micro-liquid chromatography coupled to multistage mass spectrometry; Journal of Chromatography A, (2008); 1206: 11–20. [DOI] [PubMed] [Google Scholar]
- 8.Li C., Liu Y., Abdulla R., Aisa H.A., Suo Y.; Determination of phenylethanoid glycosides in Lagotis brevituba Maxim. by high-performance liquid chromatography–electrospray ionization tandem mass spectrometry; Analytical Letters, (2014); 47: 1862–1873. [Google Scholar]
- 9.Vukics V., Guttman A.; Structural characterization of flavonoid glycosides by multi-stage mass spectrometry; Mass Spectrometry Reviews, (2010); 29: 1–16. [DOI] [PubMed] [Google Scholar]
- 10.Abu-Reidah I.M., Arráez-Román D., Quirantes-Piné R., Fernández-Arroyo S., Segura-Carretero A., Fernández-Gutiérrez A.; HPLC–ESI–Q-TOF–MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract; Food Research International, (2012); 46: 108–117. [Google Scholar]
- 11.Castillo-Munoz N., Gomez-Alonso S., Garcia-Romero E., Gomez M.V., Velders A.H., Hermosin-Gutierrez I.; Flavonol 3-O-glycosides series of Vitis vinifera Cv. Petit Verdot red wine grapes; Journal of Agricultural and Food Chemistry, (2009); 57: 209–219. [DOI] [PubMed] [Google Scholar]
- 12.Geng P., Sun J., Zhang R., He J., Abliz Z.; An investigation of the fragmentation differences of isomeric flavonol-O-glycosides under different collision-induced dissociation based mass spectrometry; Rapid Communications in Mass Spectrometry, (2009); 23: 1519–1524. [DOI] [PubMed] [Google Scholar]
- 13.Gouveia S., Castilho P.C.; Characterisation of phenolic acid derivatives and flavonoids from different morphological parts of Helichrysum obconicum by a RP-HPLC–DAD-(−)–ESI-MSn method; Food Chemistry, (2011); 129: 333–344. [DOI] [PubMed] [Google Scholar]
- 14.Gu D., Yang Y., Abdulla R., Aisa H.A.; Characterization and identification of chemical compositions in the extract of Artemisia rupestris L. by liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry; Rapid Communications in Mass Spectrometry, (2012); 26: 83–100. [DOI] [PubMed] [Google Scholar]
- 15.Hughes R.J., Croley T.R., Metcalfe C.D., March R.E.; A tandem mass spectrometric study of selected characteristic flavonoids; International Journal of Mass Spectrometry, (2001); 210: 371–385. [Google Scholar]
- 16.Hvattum E., Ekeberg D.; Study of the collision-induced radical cleavage of flavonoid glycosides using negative electrospray ionization tandem quadrupole mass spectrometry; Journal of Mass Spectrometry, (2003); 38: 43–49. [DOI] [PubMed] [Google Scholar]
- 17.Kajdzanoska M., Gjamovski V., Stefova M.; HPLC–DAD–ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia; Macedonian Journal of Chemistry and Chemical Engineering, (2010); 29: 181–194. [Google Scholar]
- 18.Sanchez-Rabaneda F., Jauregui O., Casals I., Andres-Lacueva C., Izquierdo-Pulido M., Lamuela-Raventos R.M.; Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao); Journal of Mass Spectrometry, (2003); 38: 35–42. [DOI] [PubMed] [Google Scholar]
- 19.Schwaiger S., Seger C., Wiesbauer B., Schneider P., Ellmerer E.P., Sturm S. et al. ; Development of an HPLC–PAD–MS assay for the identification and quantification of major phenolic edelweiss (Leontopodium alpium Cass.) constituents; Phytochemical Analysis, (2006); 17: 291–298. [DOI] [PubMed] [Google Scholar]
- 20.Singh A.P., Wilson T., Luthria D., Freeman M.R., Scott R.M., Bilenker D. et al. ; LC–MS-MS characterisation of curry leaf flavonols and antioxidant activity; Food Chemistry, (2011); 127: 80–85. [Google Scholar]
- 21.Fang L., Cao J., Duan L., Tang Y., Zhao Y.; Protein tyrosine phosphatase 1B (PTP1B) and α-glucosidase inhibitory activities of Schisandra chinensis (Turcz.) Baill; Journal of Functional Foods, (2014); 9: 264–270. [Google Scholar]
- 22.Nguyen P.H., Sharma G., Dao T.T., Uddin M.N., Kang K.W., Ndinteh D.T. et al. ; New prenylated isoflavonoids as protein tyrosine phosphatase 1B (PTP1B) inhibitors from Erythrina addisoniae; Bioorganic Medicinal Chemistry, (2012); 20: 6459–6464. [DOI] [PubMed] [Google Scholar]
- 23.Kite G.C., Veitch N.C.; Identification of common glycosyl groups of flavonoid O-glycosides by serial mass spectrometry of sodiated species; Rapid Communications in Mass Spectrometry, (2011); 25: 2579–2590. [DOI] [PubMed] [Google Scholar]