Emerging evidence suggests that significant heterogeneity exists in the cardiometabolic risk associated with excess body fat in obese individuals (1). We investigated the associations of novel imaging markers of adiposity, including visceral adipose tissue (VAT) and abdominal subcutaneous adipose tissue (SAT) by magnetic resonance imaging, lower body subcutaneous adipose tissue (LBAT) by dual-energy x-ray absorptiometry, and liver fat by magnetic resonance spectroscopy, with the risk for cardiovascular disease (CVD) events in a multiethnic cohort of obese adults.
The Dallas Heart Study is a cohort study with methods previously described (1). Participants with body mass index (BMI) values <30 kg/m2, prevalent CVD, and missing imaging data were excluded. The primary outcome was a composite of a first or subsequent CVD event: cardiovascular death; myocardial infarction, hospitalized unstable angina, or coronary revascularization; ischemic stroke, transient ischemic attack, or cerebrovascular revascularization; peripheral arterial revascularization; hospitalization for heart failure; or hospitalization for atrial fibrillation. Multivariate-adjusted marginal Cox proportional hazards modeling using the Wei-Lin-Weissfeld method for recurrent events (2) was used to calculate hazard ratios and 95% confidence intervals for the primary outcome associated with baseline measurements of each adiposity marker.
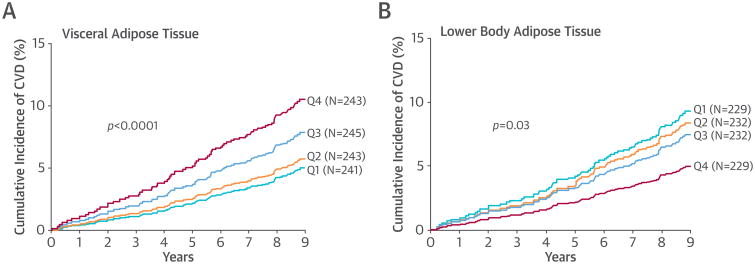
A total of 972 obese participants (mean age 44 years, 62% women, 54% African-Americans) were followed for a median of 9.1 years. Eighty-one individuals had first or subsequent CVD events, resulting in 108 events. The cumulative incidence of CVD increased in a stepwise fashion across sex- and race-specific quartiles of VAT, from 5.3% in quartile 1 to 10.0% in quartile 4 (Figure 1A). In contrast, an opposite association was observed for LBAT: the CVD event rate was 10.0% in quartile 1 and 5.4% in quartile 4 (Figure 1B). BMI, abdominal SAT, and liver fat were not associated with CVD. In multivariate analyses adjusting for age, sex, race, hypercholesterolemia, smoking status, and BMI, VAT remained associated with CVD (hazard ratio per 1 SD: 1.21; 95% confidence interval: 1.03 to 1.43), whereas LBAT was inversely associated with CVD (hazard ratio per 1 SD: 0.56; 95% CI: 0.44 to 0.72). Adjustment for hypertension, biomarkers of inflammation, insulin resistance, and dyslipidemia, and for adipocytokines, did not attenuate these associations. Adjustment for baseline diabetes status modestly attenuated the association of VAT with CVD. Lean mass and physical activity both were inversely associated with CVD and mildly attenuated the relation between VAT and CVD. Substitution of waist circumference for BMI showed generally consistent results; there was no independent association of waist circumference with the development of CVD. No statistically significant interactions by sex or race were found on the relationship between VAT or LBAT and CVD in fully adjusted models.
Figure 1. Associations of Imaging-Based Markers of Adipose Tissue Distribution With CVD Events.
Cumulative incidence of cardiovascular disease (CVD) by visceral (A) and lower body (B) adipose tissue mass.
Here we report substantial heterogeneity in the cardiovascular phenotype of obesity, with increased risk observed with VAT and decreased risk with LBAT. In contrast, BMI, abdominal SAT, and liver fat were not significantly associated with CVD. Our findings suggest that the biological link between fat distribution and CVD may be at least partly independent of traditional CVD risk factors and the influence of inflammation and adipocytokines but that diabetes may have a role in the etiological pathway between VAT and CVD. Findings with lean mass and physical activity are consistent with data that increasing muscle mass and physical activity may counteract the negative effects of VAT through fatty acid catabolism and prevention of insulin resistance (3). The mechanism behind the protective effect of LBAT may relate to its ability to act as a metabolic sink, buffering the influx of dietary lipids and protecting other tissues, including the heart, from lipotoxicity caused by lipid overflow and ectopic fat deposition (4).
Although we focus primarily on the divergent biology of different adipose depots in the development of CVD, our findings also suggest that advanced imaging tools may provide a more accurate phenotypic characterization of obesity than standard anthropometric measurements, allowing better discrimination of CVD risk. They also suggest that therapies aimed at redistribution of fat away from the visceral depot toward the more favorable lower body subcutaneous depot may be more effective for preventing cardiovascular complications in obesity than simply targeting body mass reduction. Whether modified diet, exercise, pharmacologic agents, or bariatric surgery can promote this favorable redistribution of body fat has yet to be determined. Larger studies are needed to determine if adipose depot– specific measurement can improve risk assessment or serve as a treatment target in obese patients.
Acknowledgments
Please note: This work was supported by award T32HL007360 from the National Heart, Lung, and Blood Institute to Dr. Neeland; by grants UL1DE019584 and PL1DK081182 from the National Institutes of Health; and by grant UL1TR000451 from the National Center for Advancing Translational Sciences. Dr. Rohatgi has a research grant from Merck and serves on an advisory board for Aegerion. Dr. McGuire serves on a data and safety monitoring committee for a trial of an obesity drug sponsored by Orexigen; and on the executive committee for a trial of an obesity drug sponsored by Eisai. Dr. Grundy has received consulting income from Janssen Pharmaceuticals, Pfizer, Merck, and Sanofi. Dr. de Lemos has received consulting income from Janssen Pharmaceuticals; and chairs a data and safety monitoring board for Novo Nordisk.
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–9. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li QH, Lagakos SW. Use of the Wei-Lin-Weissfeld method for the analysis of a recurring and a terminating event. Stat Med. 1997;16:925–40. doi: 10.1002/(sici)1097-0258(19970430)16:8<925::aid-sim545>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Yagi S, Kadota M, Aihara K, et al. Association of lower limb muscle mass and energy expenditure with visceral fat mass in healthy men. Diabetol Metab Syndr. 2014;6:27. doi: 10.1186/1758-5996-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–59. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]