Abstract
Introduction
Empagliflozin is a sodium glucose co-transporter 2 inhibitor used to improve glycemic control in adults with type 2 diabetes mellitus (T2DM) by enhancing urinary glucose excretion. Empagliflozin is effective at lowering glycosylated hemoglobin and was recently proven superior to placebo for reduction of cardiovascular disease (CVD) risk. As with any new drug, there are safety considerations that inform its potential use in patients with T2DM.
Areas Covered
Here, we evaluate the safety of empagliflozin and provide an expert opinion as to its current and future role in the treatment of patients with T2DM. A search of the English language literature was performed using PubMed search terms: “empagliflozin”, “sodium glucose cotransporter 2 inhibitors”, and “drug safety”. Articles and bibliographies relevant to the subject were reviewed and additional references known to the authors were included.
Expert Opinion
The evidence for empagliflozin is robust with regard to glycemic efficacy and safety. Low risk of hypoglycemia, absence of weight gain, and demonstrated cardiovascular risk reduction support its consideration as a first line medication in addition to metformin for patients with T2DM and CVD. Ongoing trials will continue to address the safety and efficacy of empagliflozin and expand our clinical knowledge of this medication.
Keywords: type 2 diabetes mellitus, drug safety, empagliflozin, sodium glucose co-transporter 2 inhibitor
1.0 Introduction
Empagliflozin (Jardiance®, Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany), a sodium glucose co-transporter 2 (SGLT2) inhibitor, is one in a novel class of medications used as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (T2DM) by enhancing urinary glucose excretion. SGLT2 inhibition lowers blood glucose through an insulin-independent mechanism, thereby addressing many of the limitations of existing therapies for T2DM such as hypoglycemia and weight gain. SGLT2 inhibitors also exhibit important non-glycemic effects such as lowering blood pressure and body weight and favorably altering fat distribution.[1]
The development of this drug class began with the discovery of phlorizin, a naturally occurring compound found to have glucosuric effects. Mechanistic studies of phlorizin’s inhibition of membrane glucose transport led to the identification of the sodium-glucose transport mechanism. Demonstration that the pharmacological effects of phlorizin resembled primary renal glycosuria, an autosomal dominant disorder of chronic glucosuria due to deficient or dysfunctional SGLT2, sparked further interest in its potential for clinical use. However, development of phlorizin was halted due to prohibitive gastrointestinal side effects and a poor pharmacokinetic and pharmacodynamic profile.[2]
Empagliflozin, a newer SGLT2 inhibitor in current clinical use, has improved potency, longer half-life, and better oral availability compared with phlorizin,[3] and has demonstrated efficacy at lowering glycosylated hemoglobin (HbA1c) as monotherapy or add-on to existing diabetes therapies in multiple phase III, randomized, controlled clinical trials.[4–9] However, as is common in the evaluation of new medications, a number of safety considerations have emerged that may inform the potential use of empagliflozin in patients with T2DM. Here, we review the mechanism of action and clinical application of empagliflozin, evaluate its safety with a focus on Phase III trial data and safety in special populations, and provide an expert opinion as to the role of empagliflozin in the treatment of patients with T2DM within the context of the current clinical landscape.
2.0 Review of empagliflozin
2.1 Mechanism of action
Glucose is freely filtered into the urine at the glomerulus where reabsorption occurs through an efficient system of adenosine triphosphate (ATP) – dependent transporter proteins, the sodium glucose co-transporter (SGLT) proteins 1 and 2. SGLT2 is a high-capacity, low affinity transporter protein that is located on the apical side of the early convoluted segment of the proximal tubule where it is responsible for approximately 90% reabsorption of filtered glucose back into the circulation.[10–12]. It does so by coupling glucose transport to the electrochemical sodium gradient across the luminal cell membrane. [13] The remaining 10% of filtered glucose is reabsorbed by SGLT1, also localized to the proximal tubule. Hence, the expression and function of SGLT1 and SGLT2 are insulin-independent determinants of the renal tubular threshold for glycosuria. [14]
In type 2 diabetes, the expression of SGLT2 is paradoxically upregulated, leading to higher than normal amount of renal tubular glucose reabsorption and thereby exacerbating systemic hyperglycemia and its complications. [15] Empagliflozin, a SGLT2 inhibitor, increases urinary excretion of glucose by markedly reducing the renal tubular threshold for glycosuria. This leads to excretion of 60 to 100 g/day of glucose, improving glucose control with low risk of hypoglycemia, and results in loss of 240 to 400 kCal/day into the urine with associated weight reduction. In addition, a decrease in blood pressure is seen due to osmotic diuresis of glucose and natriuresis of co-transported sodium.[1] Because the SGLT2 protein is primarily expressed within the kidneys, the potential off-target effect of SGLT2 inhibition are minimal.
2.1.1 Pharmacodynamics
In a Phase I study of healthy adults designed to evaluate empagliflozin pharmacodynamics, observation of renal tubular reabsorption of glucose over the first 24 hours showed that 40 % of filtered glucose reabsorption was inhibited by empagliflozin 10mg, with higher doses inhibiting 40–60% of glucose reabsorption, reaching a plateau with a 100mg dose.[16] Subsequently, the effects of multiple oral daily doses in patients with T2DM were evaluated in 2 studies. Dose-proportional exposure of empagliflozin over 8 days resulted in urinary glucose excretion ranging from 77.9 g with the 10mg dose to 89.8 g with the 100mg dose.[17] Results were consistent in a study with T2DM patients over a 4-week duration.[18] These results were further supported by a study in 100 Japanese patients with T2DM with once daily doses of empagliflozin 1, 5, 10 and 25mg. After 4 weeks, all empagliflozin groups showed significant increases in daily urinary glucose excretion (40.8 g, 77.1 g, 80.9 g, and 93.0 g, respectively vs. −2.1 g for placebo).[19]
2.1.2 Pharmacokinetics
Pharmacokinetic properties of empagliflozin are similar in healthy subjects and in patients with T2DM. It is an orally active agent reaching peak levels in 1.5 hours with 78% bioavailability.[20] Following absorption, plasma levels decline in a biphasic pattern with rapid distribution and slower elimination phase.[20] No clinically relevant effects on drug exposure after high-fat and high calorie meal were seen on absorption suggesting that empagliflozin may be administered with or without food.[21]
Distribution of empagliflozin is primarily protein bound (86.2%) with 36.8% getting partitioned into red blood cell with a population-based volume of distribution of 73.8L in steady state.[20] Metabolism is via glucuronidation into three glucornide conjugates. No major metabolites are detected in plasma; however each metabolite consists of less than 10% of the total drug in circulation.[20] The terminal half-life of empagliflozin is approximately 12.4 hours with total body clearance of 10.6L per hour. 54.4% of orally absorbed empagliflozin is excreted by the kidney and 41.2% is excreted in feces, mostly as unaltered drug.[20]
2.2 Clinical efficacy
2.2.1 Effects on glycemia
No significant changes in plasma glucose concentration were observed in healthy participants with empagliflozin compared with placebo.[16, 22] However, in a study investigating use of empagliflozin over 4 weeks in 78 participants with T2DM, there were statistically significant reductions in mean fasting plasma glucose from baseline for all 3 empagliflozin doses tested (10 mg, 25 mg, 100 mg) compared with placebo (39 mg/dL, 34 mg/dL, and 30 mg/dL vs. 4 mg/dL, respectively; P<0.05 for each).[18] Empagliflozin has been shown to lower HbA1c in a dose-dependent manner as monotherapy,[4] as an add-on to metformin with or without a sulfonylurea,[5, 6] and as an add-on to pioglitazone[7] or insulin therapy (Table 1).[23] A recent pooled analysis of 10 studies with 6203 participants showed mean changes in HbA1c of −0.62% (95% CI −0.68 to −0.57%) and −0.66% (95% CI −0.76 to −0.57%) for the 10 mg and 25 mg doses, respectively compared with placebo.[24]
Table 1.
Summary of Key Efficacy Outcomes in Phase III Clinical Trials of Empagliflozin (N=4,585)
| Study, Duration | Comparator(s), No. of Patients | Δ HbA1c (95% CI or SE†) (%) | Δ Fasting Plasma Glucose (95% CI) (mmol/l) | Δ Body Weight (95% CI) (kg) | Δ Systolic Blood Pressure (95% CI) (mmHg) |
|---|---|---|---|---|---|
| EMPA REG MONO4 24 weeks |
Placebo, 228 EMPA 10 mg, 224 EMPA 25 mg, 224 Sitagliptin 100mg, 223 |
0.08 (−0.03 to 0.18) −0.66 (−0.76 to −0.56) −0.78 (−0.88 to −0.67) −0.66 (−0.76 to −0.56) |
0.65 (0.44 to 0.87) −1.08 (−1.29 to −0.87) −1.36 (−1.57 to −1.14) −0.38 (−0.60 to −0.17) |
−0.33 (−0.67 to 0.00) −2.26 (−2.60 to −1.92) −2.48 (−2.82 to −2.14) 0.18 (−0.16 to 0.52) |
−0.3 (−1.9 to 1.3) −2.9 (−4.5 to −1.3) −3.7 (−5.3 to −2.1) 0.5 (−1.1 to 2.1) |
| EMPA REG MET5 24 weeks |
Placebo, 207 EMPA 10mg, 217 EMPA 25 mg, 213 (All on metformin) |
−0.13 (0.05) −0.70 (0.05) −0.77 (0.05) |
0.35 (0.10) −1.11 (0.10) −1.24 (0.10) |
−0.45 (0.17) −2.08 (0.17) −2.46 (0.17) |
−0.4 (0.7) −4.5 (0.7) −5.2 (0.7) |
| EMPA REG METSU6 24 weeks |
Placebo, 225 EMPA 10 mg, 225 EMPA 25 mg, 216 (All on metformin and sulfonylurea) |
−0.17 (0.05) −0.82 (0.05) −0.77 (0.05) |
0.31 (0.11) −1.29 (0.11) −1.29 (0.11) |
−0.39 (0.15) −2.16 (0.15) −2.39 (0.16) |
−1.4 (0.7) −4.1 (0.7) −3.5 (0.7) |
| EMPA REG PIO7 24 weeks |
Placebo, 165 EMPA 10 mg, 165 EMPA 25 mg, 168 (All on pioglitazone ± metformin) |
−0.11 (0.07) −0.59 (0.07) −0.72 (0.07) |
0.36 (0.14) −0.94 (0.15) −1.22 (0.14) |
0.34 (0.21) −1.62 (0.21) −1.47 (0.21) |
0.7 (0.9) −3.1 (0.9) −4.0 (0.8) |
| EMPA REG MDI8 52 weeks |
Placebo, 188 EMPA 10 mg, 186 EMPA 25 mg, 189 (All on insulin ± metformin) |
−0.81 (0.08) −1.18 (0.08) −1.27 (0.08) |
−0.63 (0.19) −1.32 (0.19) −1.43 (0.19) |
0.44 (0.36) −1.95 (0.36) −2.04 (0.36) |
−1.2 (0.8) −3.6 (0.8) −2.9 (0.8) |
| EMPA REG H2H SU9 104 weeks |
Glimepiride 1–4 mg, 780 EMPA 25 mg, 769 (All on metformin) |
−0.66 (−0.72 to −0.61) −0.73 (−0.79 to −0.68) |
−0.48 (−0.59 to −0.37) −1.08 (−1.19 to −0.96) |
1.6 (1.4 to 1.8) −3.2 (−3.4 to −3.0) |
2.2 (1.3 to 3.1) −3.6 (−4.5 to −2.8) |
Selected efficacy endpoints reported in patients treated with empagliflozin in six placebo-controlled Phase III clinical studies of empagliflozin monotherapy or combination therapy totaling 4,585 patients.[4–9] EMPA=empagliflozin; HbA1c=glycosylated hemoglobin
Data are adjusted mean changes from baseline from the full analysis set using last observation carried forward.
Standard error (SE) of the adjusted mean given if 95% CI not available.
2.2.1 Effects on other cardiometabolic markers
Empagliflozin treatment is associated with favorable effects on blood pressure with a weighted mean decrease of approximately −4.2 mmHg (95% CI −5.2 to −3.2 mmHg) in systolic blood pressure and −1.9 mmHg (95% CI −2.7 to −1.0 mmHg) in diastolic blood pressure, with consistent results between clinically measured recordings and 24-hour ambulatory blood pressure monitoring (Table 1).[24] Empagliflozin also improves markers of arterial stiffness and vascular resistance in patients with type 2 diabetes through mechanisms possibly related to improved glycemic control, weight loss, volume contraction, or reduced oxidative stress.[25]
Compared with placebo, treatment with empagliflozin has been associated with significant reductions in body weight (Table 1) and indices of total and visceral adiposity. In pooled analyses, both doses decreased body weight by approximately 1.8 kg [24] and significant reductions were seen in waist circumference and the index of central obesity,[26] as well as visceral and abdominal subcutaneous adipose tissue.[9]
All SGLT2 inhibitors (including empagliflozin) have been associated with small increases in low-density lipoprotein (LDL) cholesterol (2.3%, 4.6%, and 6.5% in patients treated with placebo, empagliflozin 10 mg, and empagliflozin 25 mg, respectively per the prescribing information) with concomitant increases in high-density lipoprotein (HDL) cholesterol and reductions in triglyceride levels with no significant changes in LDL/HDL or total cholesterol/HDL ratios.[27] Whether these minor alterations in lipid levels are clinically relevant and how they might augment or offset any potential cardiovascular benefit with empagliflozin is a subject of ongoing investigation.
2.2.2 Effects on cardiovascular outcomes
The effect of empagliflozin on cardiovascular outcomes among patients with type 2 diabetes and prevalent atherosclerotic cardiovascular disease (CVD) was recently evaluated in the EMPA REG OUTCOME trial, a multicenter, randomized, double blind, placebo controlled trial.[28] This study included 7,028 participants with type 2 diabetes and established CVD from 590 centers over 42 countries and tested two doses of empagliflozin (10mg or 25mg) compared with placebo. Over a median follow up of 2.6 years, pooled analyses of the 2 empagliflozin dose groups versus placebo demonstrated a statistically significant 14% reduction in the primary composite outcome of cardiovascular death, nonfatal myocardial infarction or nonfatal stroke (HR 0.86; 95% CI 0.74 to 0.99, P=0.04 for superiority). Furthermore, empagliflozin was also associated with significant risk reduction of the individual outcomes of cardiovascular mortality (HR=0.62; 95% CI 0.49 to 0.77), all-cause mortality (HR=0.68; 95% CI 0.57 to 0.82), and hospitalization for heart failure (HR=0.65; 95% CI 0.50 to 0.85). Whether or not these cardiovascular benefits are unique to empagliflozin or represent a class-effect remains to be seen and is the subject of ongoing investigation. Currently, empagliflozin is the only glucose-lowering agent to have demonstrated CVD risk reduction in a dedicated cardiovascular outcomes trial.
2.3 Safety evaluation
2.3.1 Safety in clinical studies
The physiological effects of empagliflozin and reported adverse drug reactions are summarized in the Figure. Clinical trial data reporting adverse drug reactions from a total of 4,585 patients treated with empagliflozin vs. placebo or standard care in six pooled, randomized, controlled Phase III clinical trials of empagliflozin monotherapy or combination therapy suggest that the drug is generally well tolerated, with a low rate of adverse events leading to study drug discontinuation (3.9% vs. 2.2% vs. 3.9% in placebo, empagliflozin 10 mg, and empagliflozin 25 mg treated groups, respectively, Table 2). There was an increased incidence of genital mycotic infections in the empagliflozin treated groups although events were generally of mild to moderate intensity, rarely led to drug discontinuation, and tended not to recur. Patients with a history of chronic or recurrent genital mycotic infections were more likely to develop these infections when exposed to empagliflozin and women appear to be at greater risk compared with men and uncircumcised men may be at greater risk compared with circumcised men. Because empagliflozin induces glucosuria, it is postulated that higher levels of glucose in the urine promote fungal overgrowth leading to infection. In virtually all cases, these events were reported to subside spontaneously or respond to standard antifungal therapy treatment. There has been no clear incremental risk for bacterial urinary tract infections with empagliflozin treatment compared with placebo (Table 2).[29]
Figure. Physiological effects of empagliflozin and related adverse reactions.
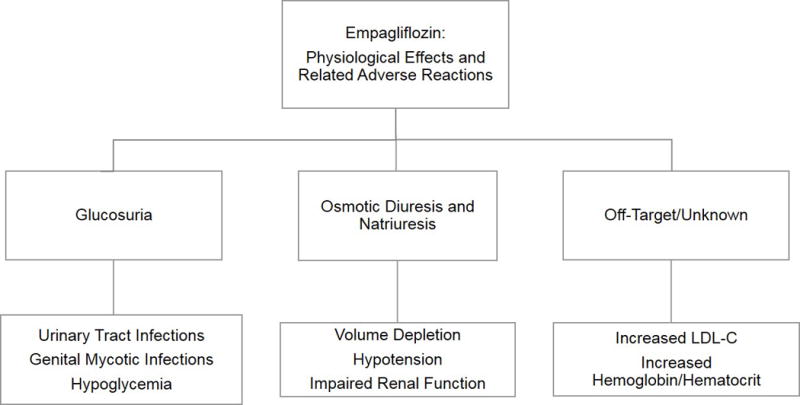
Empagliflozin promotes glucosuria, diuresis, and natriuresis at the level of the proximal tubule in the kidney. Increased levels of urinary glucose may predispose patients to urinary tract or genital infection and may increase the risk for hypoglycemia, especially when used in combination with insulin or sulfonylureas. The diuretic and natriuretic effects may exacerbate volume depletion and increase the risk for hypotension especially among those with renal impairment, the elderly, in patients with low baseline systolic blood pressure, and in patients taking diuretics. Effects with an unknown physiologic mechanism include a small increase in LDL cholesterol levels and increases in hemoglobin and hematocrit, the clinical relevance which is not known.
Table 2.
Summary of Key Safety Outcomes in Pooled Phase III Clinical Trials of Empagliflozin (N=4,585)
| Adverse Event | Placebo Event Rate (%) (n=1,794) |
Empagliflozin (10 mg) Event Rate (%) (n=1,017) |
Empagliflozin (25 mg) Event Rate (%) (n=1,774) |
Comment |
|---|---|---|---|---|
| Urinary Tract Infection* | 11.0 | 10.4 | 11.0 | Males: 4.2% vs. 1.9% vs. 4.0% Females: 17.5% vs. 18.5% vs. 19.0% |
| Genital Mycotic Infection† | 1.4 | 4.2 | 7.8 | Males: 0.8% vs. 2.6% vs. 5.1% Females: 2.1% vs. 6.3% vs. 10.7% |
| Hypoglycemia‡ | 12.5 | 4.5 | 4.7 | Excluding add-on to insulin study |
| Dyslipidemia | 5.6 | 8.0 | 5.4 | Generally small increases in low-density and high-density lipoprotein cholesterol with small decreases in triglycerides |
| Adverse Events Leading to Discontinuation | 3.9 | 2.2 | 3.9 | Deaths: 7 vs. 1 vs. 8 |
Selected adverse reactions reported in patients treated with empagliflozin in six pooled, placebo-controlled Phase III clinical studies of empagliflozin monotherapy or combination therapy totaling 4,585 patients.[4–9]
Based on 67 preferred terms for UTI events
Based on 87 preferred terms for genital infection events
Plasma glucose ≤ 70 mg/dL and/or requiring assistance
Since empagliflozin causes polyuria (increased daily urinary output) through an osmotic diuresis and natriuresis effect, patients may be predisposed to intravascular volume contraction. In a study investigating the efficacy and safety of empagliflozin among patients with T2DM and hypertension, empagliflozin reduced mean systolic blood pressure vs. placebo by 3.4 (95% CI 4.8-2.1) mmHg with the 10 mg dose and by 4.2 (95% CI 5.5-2.8) mmHg with the 25 mg dose using 24-hour ambulatory blood pressure monitoring after 12 weeks of treatment (both P<0.001).[30] Reductions in blood pressure were not associated with increases in pulse rate (suggesting negligible hemodynamic alterations in autonomic tone) and only 1 patient on placebo and 1 patient on 10 mg empagliflozin reported events consistent with volume depletion. Nevertheless, according to the prescribing information, symptomatic hypotension may occur after initiating empagliflozin, especially in patients with renal impairment, the elderly, in patients with low baseline systolic blood pressure, and in patients taking diuretics, so caution is advised in these higher risk subgroups and volume status should be assessed and corrected prior to initiating treatment.[20]
The incidence of hypoglycemia (defined as plasma glucose ≤ 70 mg/dL) is generally low with empagliflozin treatment as compared with placebo (Table 3). The incidence of hypoglycemia has ranged between 0.4% to 2.4% in phase III placebo-controlled clinical trials of empagliflozin monotherapy or in combination with metformin or pioglitazone. Combined use of empagliflozin and insulin or an insulin-secretagogue can increase the risk of hypoglycemia, occasionally prompting a reduction in dose or temporary discontinuation of the insulin or insulin-secretagogue medication. When added to a sulfonylurea or in combination with insulin, rates of hypoglycemia were as high as 41.3% (compared with 37.2% with placebo).[20] Rates of severe hypoglycemia (defined as requiring assistance regardless of the blood glucose level) were between 0% and 0.5% and only observed in studies in which empagliflozin was used as an add-on to insulin and/or sulfonylureas.
Table 3.
Incidence of Overall and Severe Hypoglycemic Events in Phase III Placebo-Controlled Clinical Trials of Empagliflozin*
| Monotherapy (24 weeks) |
Placebo (%) (n=229) |
Empagliflozin (10 mg) (%) (n=224) |
Empagliflozin (25 mg) (%) (n=223) |
|---|---|---|---|
| Overall† | 0.4 | 0.4 | 0.4 |
| Severe‡ | 0 | 0 | 0 |
|
+ Metformin (24 weeks) |
Placebo (%) (n=206) |
Empagliflozin (10 mg) (%) (n=217) |
Empagliflozin (25 mg) (%) (n=214) |
| Overall† | 0.5 | 1.8 | 1.4 |
| Severe‡ | 0 | 0 | 0 |
|
+ Metformin and Sulfonylurea (24 weeks) |
Placebo (%) (n=225) |
Empagliflozin (10 mg) (%) (n=224) |
Empagliflozin (25 mg) (%) (n=217) |
| Overall† | 8.4 | 16.1 | 11.5 |
| Severe‡ | 0 | 0 | 0 |
|
+ Pioglitazone (24 weeks) |
Placebo (%) (n=165) |
Empagliflozin (10 mg) (%) (n=165) |
Empagliflozin (25 mg) (%) (n=168) |
| Overall† | 1.8 | 1.2 | 2.4 |
| Severe‡ | 0 | 0 | 0 |
|
+ Insulin (18 weeks)‖ |
Placebo (%) (n=188) |
Empagliflozin (10 mg) (%) (n=186) |
Empagliflozin (25 mg) (%) (n=189) |
| Overall† | 37.2 | 39.8 | 41.3 |
| Severe‡ | 0.5 | 0.5 | 0.5 |
|
+Metformin vs. Glimepiride/Metformin (104 weeks) |
Glimepiride (%) (n=780) |
N/A |
Empagliflozin (25 mg) (%) (n=765) |
| Overall† | 25 | N/A | 4 |
| Severe‡ | N/A | N/A | N/A |
Hypoglycemic events reported in patients treated with empagliflozin in six pooled, placebo-controlled Phase III clinical studies of empagliflozin monotherapy or combination therapy totaling 4,585 patients.[4–9]
Modified from empagliflozin prescribing information [20]
Plasma glucose ≤ 3.9 mmol/L
Requiring assistance regardless of blood glucose level
Insulin dose could not be adjusted during the initial 18 week treatment period
Multiple studies have seen greater increases in hemoglobin and hematocrit concentrations among empagliflozin treated groups compared with placebo or active controls. For example, in the EMPA REG OUTCOME trial, the mean change from baseline in hemoglobin and hematocrit among those treated with empagliflozin 25 mg was 0.8 ± 1.3 g/dL and 5.0 ± 5.3 %, respectively, compared with −0.1 ± 1.2 g/dL and 0.9 ± 4.7 % with placebo.[28] These changes appear to be a class effect with all SGLT2 inhibitors, although a recent systematic review found that empagliflozin demonstrated the largest increase in hematocrit.[31] Whether these changes are related to alterations in blood volume leading to hemoconcentration or if they represent off-target effects (erythropoietin stimulation?), as well as the clinical relevance of such changes, remains to be determined.
Recently, there has been a growing concern over an increased risk for developing diabetic ketoacidosis related to use of SGLT2 inhibitors (including empagliflozin) in the literature and media. A recent search of the FDA Adverse Event Reporting System (FAERS) database identified 20 case of ketoacidosis in patients treated with SGLT2 inhibitors from March 2013 to June 2014.[32] All patients required emergency room visits or hospitalization and there were no deaths. The median time to onset of symptoms following initiation of drug therapy was 2 weeks (range 1 to 175 days). Importantly, the case presentations were atypical in that blood glucose levels were only mildly elevated (<200 mg/dL) in some reports, raising the issue of point-of-care or home-based testing of urinary ketones and/or plasma beta-hydroxybutyrate for closer monitoring. Furthermore, cases of ketoacidosis occurred in both type 1 and type 2 diabetes patients. The FDA warning was preceded by reports in the literature suggesting a risk of ketoacidosis related to empagliflozin [33] and canagliflozin [34] in patients with type 1 diabetes and use of the drug is currently not approved for this patient population. Factors identified as having possibly triggered the episodes included concurrent illness, reduced oral intake, and reduced insulin dose.[34] It is not currently known whether the risk for developing diabetic ketoacidosis related to SGLT2 inhibitors is greater in patients with type 1 or type 2 diabetes or whether any individual SGLT2 inhibitor imparts excess risk compared with others in the drug class. Further research is being directed toward identifying which patients are at greatest risk for this side effect and toward developing strategies to minimize the risk to patients.
Another recent FDA warning was issued regarding an increased risk for bone fractures associated with decreased bone mineral density and osteoporosis with canagliflozin use, with the risk increasing over time.[35] In a pooled analysis of 8 clinical trials, there was a 30% increased risk for fractures associated with canagliflozin treatment and, after 52 weeks, bone mineral density in the lumbar spine and hip was decreased.[36] SGLT2 inhibitors are thought to increase serum phosphate concentrations with the potential to adversely affect bone metabolism.[37] Although adverse effects on bone turnover have not been reported with empagliflozin or dapagliflozin, given a similar mechanism of action as canagliflozin, the potential for osteoporosis associated with use of these agents should be carefully considered.
2.3.2 Safety in special populations
Pregnancy/Nursing
Empagliflozin is rated a safety category C in pregnancy. There are currently no controlled human studies of the drug in pregnant women. In animal studies, empagliflozin can cross the placenta and result in impaired kidney development and maturation. However, no evidence of teratogenicity was found in doses 48-times and 128-times the maximum human clinical dose in rats and rabbits, respectively.[20] It is recommended that the drug be used during pregnancy only if the potential benefit justifies the potential harm to the fetus and no other alternatives are available. Similarly, empagliflozin is considered possibly unsafe for use in lactation, although it is not known if the drug is excreted in human milk. Since there is potential for serious adverse reactions in nursing infants due to the glucose lowering and volume contraction effects of the drug, it is recommended that empagliflozin is discontinued while nursing.
Elderly
The geriatric population represents another special population of interest with regard to treatment with empagliflozin. Because elderly patients often have impaired autonomic function, may be taking diuretics, and are often prescribed multiple medications with significant potential for drug-drug interactions, adverse events related to empagliflozin treatment may be amplified in this group. Furthermore, empagliflozin is expected to have diminished efficacy among elderly patients due to concomitant kidney impairment common in the elderly. Although only 6% of patients included in randomized clinical trials were over age 75, the risk of volume depletion-related adverse reactions and risk for urinary tract infections were increased in this age group compared with younger patients. Therefore, caution is advised when prescribing empagliflozin in the elderly and close monitoring for adverse effects is necessary.
Chronic kidney disease
Chronic kidney disease (CKD) is a well-known complication of T2DM and up to 40% of patients with T2DM have CKD.[3] Because SGLT2 inhibitors must be filtered at the glomerulus and reach the proximal tubule in the nephron to exert their inhibitory effects, they require a certain degree of glomerular filtration for efficacy.[38]. The efficacy and safety of empagliflozin was evaluated in a study of patients with T2DM and CKD stage 2 (n=290) and stage 3 (n=374) compared with placebo.[39] In general, adverse events were similar between placebo and empagliflozin treated groups. Small decreases in estimated glomerular filtration rate were reported with empagliflozin treatment, with larger changes on average among those with worse baseline renal function, but generally resolved up to 3 weeks after treatment cessation. The glucose lowering benefit of empagliflozin compared with placebo was decreased, and the risks of declining kidney function, volume depletion, and urinary tract infection were increased, with worsening kidney impairment (CKD stage >3). It is also important to recognize that the efficacy and safety of empagliflozin has not been adequately tested in patients with more severe kidney impairment (CKD stages 4 and 5), or in patients receiving dialysis. In the aforementioned study, a small group (n=30) patients with CKD stage 4 were randomized to receive empagliflozin vs. placebo but empagliflozin treatment did not reduce glycosylated hemoglobin in this group and the incidence of adverse events was higher compared with placebo. Thus empagliflozin is not likely to be effective in patients with more advanced CKD and its use should be avoided in this patient population, with the product labeling reflecting this contraindication. Furthermore, if a patient treated with empagliflozin goes on to develop CKD stage 4 or higher, empagliflozin should be discontinued and alternative therapies should be considered.
2.3.3 Comparison with safety of other drugs for diabetes
The insulin-independent action of empagliflozin makes it an attractive option compared with insulin and insulin-secretagogues due to lower risk for hypoglycemia. This hypothesis was directly addressed when the efficacy and safety of empagliflozin treatment compared with glimepiride (a sulfonylurea) was investigated in the EMPA-REG H2H SU [9] trial among patients with inadequate T2DM control with metformin alone. In this head-to-head study, the adverse event rates were similar between the two treatment arms (overall/severe: 86%/9% with empagliflozin vs. 86%/9% with glimepiride), but the rates of confirmed hypoglycemia (plasma glucose ≤ 70 mg/dL or requiring assistance) at week 104 were reported in only 2% of patients treated with empagliflozin vs. 24% reported in those treated with glimepiride.
Furthermore, the beneficial effects of empagliflozin on blood pressure, body weight, and HDL-cholesterol may result in improved cardiovascular and metabolic health, in contrast to several other anti-hyperglycemic medications such as rosiglitazone, pioglitazone, sulfonylureas, and saxagliptin which have been associated with an increased risk for atherosclerotic CVD [40, 41] and/or heart failure.[42, 43] Although longer-term data are lacking, the results of the EMPA REG OUTCOME trial demonstrating reduced CVD risk with empagliflozin, supports its consideration as a first-line anti-hyperglycemic medication for T2DM patients with established CVD.
3.0 Conclusion
Empagliflozin lowers glycosylated hemoglobin as monotherapy or add-on to existing anti-hyperglycemic therapies among patients with T2DM, with consistent observations of associated weight reduction and blood pressure lowering. More recently, empagliflozin was proven superior to placebo for reduction of major adverse cardiovascular risk among patients with T2DM and established CVD. The safety and tolerability of empagliflozin has been demonstrated in multiple phase III clinical trials, with the most common adverse reactions (≥5%) being urinary tract and genital mycotic infections associated with very low rates of study drug discontinuation. Along with the other SGLT2 inhibitors, empagliflozin is not yet approved for the treatment of type 1 diabetes, partly owing to concern for risk of diabetic ketoacidosis. Due to its mechanism of action, caution is advised among patients with renal impairment, the elderly, and those with low blood pressure or taking diuretics.
4.0 Expert opinion
The accumulated evidence across the class of SGLT2 inhibitors in general, and for empagliflozin specifically, are encouraging with regard to glycemic efficacy, overall safety and tolerability, and for empagliflozin-incremental CVD risk reduction. Given their low risk of hypoglycemia and absence of weight gain-adverse effects of glucose management that have complicated treatment of T2DM for decades-the SGLT2 inhibitors compliment the GLP1 receptor antagonists and the DPP4 inhibitors in that regard. Moreover, given the insulin-independent mechanism of action, the SGLT2 inhibitors can be used in combination with all other available classes of medications for T2DM, and can be continued even in the context of complete ß-cell failure. Extending the latter concept, these medications have potential utility in type 1 diabetes, and despite the absence of rigorous trial data or a product indication or claim for type 1 diabetes, providers are increasingly using the SGLT2 inhibitors for patients with type 1 diabetes. While some trials are underway to explore the safety and efficacy of such use, the signal for diabetic ketoacidosis risk must be taken seriously, and it is especially important to educate patients and front-line providers about the normal or relatively low plasma glucose levels that have been observed in the few cases of ketoacidosis associated with SGLT2 inhibitors reported to date. Providers should counsel their patients about the risks for severe genital infections and osteoporosis and consider alternative therapies in patients at higher risk for developing such adverse effects.
The statistically significant improvement for the primary cardiovascular outcome in the EMPA REG OUTCOME trial represents the first ever positive cardiovascular outcomes trial for any anti-hyperglycemic medication. Most notably, the primary outcome result was driven primarily by a reduction in cardiovascular death and was accompanied by a reduction of similar magnitude and significance in the risk for hospitalization for heart failure. The mechanistic underpinnings of these observations remain unclear. Perhaps this represents an effect on the renin-angiotensin-aldosterone system by changes to glomerular hemodynamics attributable to solute delivery to the macula densa.[44] Alternatively, with SGLT1 (but not SGLT2) expressed in cardiac myocytes, inhibition of cardiac SGLT1 could favorably affect substrate utilization for glucose over free fatty acids with potential for more efficient cardiac energetics and perhaps concomitant anti-arrhythmic effects. However, all hypotheses are completely speculative at this point and require further study.
From a broad clinical perspective, the combined dataset available for empagliflozin punctuated by the recent EMPA-REG OUTCOME results support its consideration to become a first line medication in addition to metformin for T2DM with established CVD. The most evident hurdle to such a strategy will be the expense associated with this and other new proprietary medications for T2DM, compared with less expensive generic agents such as metformin. Whether this approach should be extended to patients with clustered cardiovascular risk factors but without manifest cardiovascular disease, or even extended to include all patients with T2DM, will be a matter of great debate and further investigation moving forward. All SGLT2 inhibitors in current clinical use have shown glycemic efficacy; however, until the ongoing cardiovascular outcomes trials assessing dapagliflozin and canagliflozin are completed (estimated 2017–2019; https://clinicaltrials.gov, accessed November 6, 2015), we may only speculate that the cardiovascular benefit observed with empagliflozin might be a class effect. However, using the best available evidence presently available and until further data are available with other SGLT2 inhibitors, we can only confidently conclude that empagliflozin favorably affects CVD risk.
Ongoing trials will also address the safety and efficacy of canagliflozin and dapagliflozin among patients with CKD, and more data are needed in this regard for empagliflozin. In addition, future research should specifically evaluate the safety and efficacy of SGLT2 inhibitors in patients with heart failure, ideally among patients with both reduced and preserved ejection fraction, and possibly both with and without prevalent T2DM. Another potential focus of research will be to assess in empagliflozin in lower cardiovascular risk populations, such as those with prediabetes. Lastly, whether dual inhibition of SGLT1 and SGLT2 would improve upon efficacy and/or safety remains to be determined, and is the focus of the sotagliflozin development program presently limited to Phase III evaluation in patients with type 1 diabetes.[45]
5.0 Drug Summary Box.
|
| |
| Drug name (generic) | Empagliflozin |
|
| |
| Phase | IV |
|
| |
| Indication | Indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus |
|
| |
| Pharmacology description/method of action | Inhibits the renal sodium glucose co-transporter 2 isoform thereby enhancing urinary glucose excretion |
|
| |
| Route of administration | Oral |
|
| |
| Chemical structure | D-Glucitol,1,5-anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-,(1S)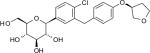
|
|
| |
| Pivotal trials | EMPA-REG MONO (NCT01177813) [4] EMPA-REG MET (NCT01159600) [5] EMPA-REG METSU (NCT01159600) [6] EMPA-REG PIO (NCT01210001) [7] EMPA-REG MDI (NCT01306214) [8] EMPA-REG H2H SU (NCT01167881) [9] |
|
| |
Acknowledgments
Funding
Dr. Neeland is supported by grant K23DK106520-01 from the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health and as a Dedman Family Scholar in Clinical Care at UT Southwestern.
Abbreviations
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- HbA1c
glycosylated hemoglobin
- SGLT2
sodium glucose co-transporter 2
- T2DM
type 2 diabetes mellitus
Footnotes
Relationship with Industry
Dr. Neeland and Dr. Salahuddin have nothing to disclose; Dr. McGuire reports research support and consultancy honoraria from Boehringer Ingelheim, Lilly USA, Janssen Research and Development LLC, Sanofi Aventis Groupe, Genentech, Inc., Merck Sharp and Dohme Corp., Daiichi Sankyo, Inc., Novo Nordisk, GlaxoSmithKline, Takeda Pharmaceuticals North America, Bristol-Myers Squibb, AstraZeneca, Orexigen, Lexicon, Eisai, Regeneron, Pfizer, and Genfit.
References
- 1.Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21:31–8. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- 3.White JR., Jr Empagliflozin, an SGLT2 inhibitor for the treatment of type 2 diabetes mellitus: a review of the evidence. Ann Pharmacother. 2015;49:582–98. doi: 10.1177/1060028015573564. [DOI] [PubMed] [Google Scholar]
- 4.Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–19. doi: 10.1016/S2213-8587(13)70084-6. **This is the first phase 3 clinical trial to demonstrate the efficacy of empagliflozin monotherapy on reducing glycosylated hemoglobin compared with placebo. [DOI] [PubMed] [Google Scholar]
- 5.Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–9. doi: 10.2337/dc13-2105. **This is the first phase 3 clinical trial to demonstrate the efficacy of empagliflozin vs. placebo as add-on to metformin background therapy on reducing glycosylated hemoglobin, body weight, and blood pressure. [DOI] [PubMed] [Google Scholar]
- 6.Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–58. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–23. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 9.Ridderstrale M, Andersen KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700. doi: 10.1016/S2213-8587(14)70120-2. *This trial showed that empagliflozin was non-inferior to glimepiride as add-on to metformin therapy for glycemic control and resulted in less hypoglycemic adverse events compared with glimepiride. [DOI] [PubMed] [Google Scholar]
- 10.Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22:104–12. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–94. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 12.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 13.Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994;74:993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5–14. doi: 10.1111/j.1463-1326.2011.01511.x. [DOI] [PubMed] [Google Scholar]
- 15.Rahmoune H, Thompson PW, Ward JM, et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54:3427–34. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- 16.Seman L, M S, Nehmiz G, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev. 2013;2:152–61. doi: 10.1002/cpdd.16. [DOI] [PubMed] [Google Scholar]
- 17.Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4:331–45. doi: 10.1007/s13300-013-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:613–21. doi: 10.1111/dom.12073. [DOI] [PubMed] [Google Scholar]
- 19.Kanada S, Koiwai K, Taniguchi A, et al. Pharmacokinetics, pharmacodynamics, safety and tolerability of 4 weeks’ treatment with empagliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;4:613–7. doi: 10.1111/jdi.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Empagliflozin Prescribing Information. Available from: http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Jardiance/jardiance.pdf. Accessed August 24, 2015.
- 21.Seman L, M S, N G, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev. 2013;2:152–61. doi: 10.1002/cpdd.16. [DOI] [PubMed] [Google Scholar]
- 22.Sarashina A, Koiwai K, Seman LJ, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet. 2013;28:213–9. doi: 10.2133/dmpk.dmpk-12-rg-082. [DOI] [PubMed] [Google Scholar]
- 23.Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–23. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 24.Liakos A, Karagiannis T, Athanasiadou E, et al. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:984–93. doi: 10.1111/dom.12307. [DOI] [PubMed] [Google Scholar]
- 25.Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–93. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neeland IJ, McGuire DK, Chilton B, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabtes mellitus. Diab Vasc Dis Res. 2015 doi: 10.1177/1479164115616901. [In Press]. *This study comprehensively evaluated the effects of empagliflozin treatment on body weight and indices of total body fat and visceral adiposity across 3,300 participants with type 2 diabetes in five clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:457–66. doi: 10.1111/dom.12244. [DOI] [PubMed] [Google Scholar]
- 28.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 doi: 10.1056/NEJMoa1504720. [Epub ahead of print]. **This is the first cardiovascular outcomes trial of an anti-hyperglycemic medication to demonstrate efficacy in reducing cardiovascular disease risk including 14% reduction in the primary composite outcome of cardiovascular death, nonfatal myocardial infarction or nonfatal stroke and significant risk reduction of the individual outcomes of cardiovascular mortality, all-cause mortality, and hospitalization for heart failure. [DOI] [PubMed] [Google Scholar]
- 29.Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–31. doi: 10.1210/er.2010-0029. [DOI] [PubMed] [Google Scholar]
- 30.Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–8. doi: 10.2337/dc14-1096. *This trial demonstrated clinically meaningful reductions in 24-hour ambulatory blood pressure with empagliflozin treatment compared with placebo in patients with type 2 diabetes and hypertension. [DOI] [PubMed] [Google Scholar]
- 31.Baker WL, Smyth LR, Riche DM, et al. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8:262–75.e9. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 32.FDA Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. Accessed August 24, 2015. **This safety announcement from the FDA warned that SGLT2 inhibitors may increase the risk for diabetic ketoacidosis, eventually leading to a revised labeling of SGLT2 inhibitors to include this risk.
- 33.Perkins BA, Cherney DZ, Partridge H, et al. Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care. 2014;37:1480–3. doi: 10.2337/dc13-2338. [DOI] [PubMed] [Google Scholar]
- 34.Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors May Predispose to Ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849–52. doi: 10.1210/jc.2015-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Invokana and Invokamet (canagliflozin): Drug Safety Communication - New Information on Bone Fracture Risk and Decreased Bone Mineral Density. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm461876.htm. Accessed December 10, 2015.
- 36.Kwon H. CANA: clinical efficacy and safety. Endocrinology and Metabolic Drugs Advisory Committee Meeting. 2013 Available from http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM336234.pdf. Accessed December 10, 2015.
- 37.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. The Lancet Diabetes Endocrinol. 2015;3:8–10. doi: 10.1016/S2213-8587(14)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res. 2015;12:78–89. doi: 10.1177/1479164114561992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–84. doi: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 40.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170:1191–201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 41.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–8. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 42.Varas-Lorenzo C, Margulis AV, Pladevall M, et al. The risk of heart failure associated with the use of noninsulin blood glucose-lowering drugs: systematic review and meta-analysis of published observational studies. BMC Cardiovasc Disord. 2014;14:129. doi: 10.1186/1471-2261-14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–88. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 44.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–97. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 45.Lapuerta P, Zambrowicz B, Strumph P, Sands A. Development of sotagliflozin, a dual sodium-dependent glucose transporter 1/2 inhibitor. Diab Vasc Dis Res. 2015;12:101–10. doi: 10.1177/1479164114563304. *This review focuses on the the development of sotagliflozin, a dual inhibitor of SGLT1 and SGLT2, and whether it would improve upon the efficacy and/or safety in patients with type 1 diabetes. [DOI] [PubMed] [Google Scholar]


