Abstract
Transcranial magnetic stimulation (TMS) is an established neurophysiological tool to examine the integrity of the fast-conducting corticomotor pathways in a wide range of diseases associated with motor dysfunction. This includes but is not limited to patients with multiple sclerosis, amyotrophic lateral sclerosis, stroke, movement disorders, disorders affecting the spinal cord, facial and other cranial nerves. These guidelines cover practical aspects of TMS in a clinical setting. We first discuss the technical and physiological aspects of TMS that are relevant for the diagnostic use of TMS. We then lay out the general principles that apply to a standardized clinical examination of the fast-conducting corticomotor pathways with single-pulse TMS. This is followed by a detailed description of how to examine corticomotor conduction to the hand, leg, trunk and facial muscles in patients. Additional sections cover safety issues, the triple stimulation technique, and neuropediatric aspects of TMS.
Keywords: Transcranial magnetic stimulation, Clinical neurophysiology, Corticomotor conduction, Motor-evoked potentials
1. Introduction
Transcranial magnetic stimulation (TMS) was introduced in 1985 by Barker et al. as a non-invasive pain-free method to stimulate the human cortex (Barker et al., 1985). In their seminal communication, Barker et al. demonstrated that a single TMS pulse applied over the primary motor cortex (M1) elicits responses in those muscles that receive corticomotor input from the stimulated motor cortical area (Barker et al., 1985). Because these transcranially evoked motor responses (MEPs) can be readily recorded with surface electrodes, TMS quickly emerged as a routine method in clinical neurophysiology to assess the functional integrity of corticospinal and corticobulbar motor pathways in a wide range of neurological disorders (Rossini and Rossi, 2007).
This review describes how TMS can be used diagnostically to detect an impairment of central motor conduction in corticospinal or corticobulbar pathways. The main goal is to provide guidelines that assist neurophysiologists and technicians in their daily clinical work. The reported guidelines represent a trade-off between methodological sophistication and clinical feasibility. Methodological issues are only covered to the degree as they are relevant to the clinical TMS examination. Since the specific diagnostic value of TMS in various neurological disorders has been reviewed by another IFCN committee in 2008 (Chen et al., 2008), this report only covers general aspects of diagnostic TMS without referring to specific disorders.
2. Technical principles of TMS
2.1. Electromagnetic induction as the underlying principle of TMS
TMS makes use of electromagnetic induction to activate cortical neurons (Barker et al., 1985). A stimulation device consist of a transducing coil which is attached to a high-voltage (400 V– 3 kV), high-current (4 kA–20 kA) discharge system (Jalinous, 1991). For TMS, the stimulation device is discharged producing a strong time-varying magnetic field at right angles to the stimulation coil. The induced magnetic field reaches peak strengths of 1–2.5 Tesla and is very short lasting (⩽1 ms).
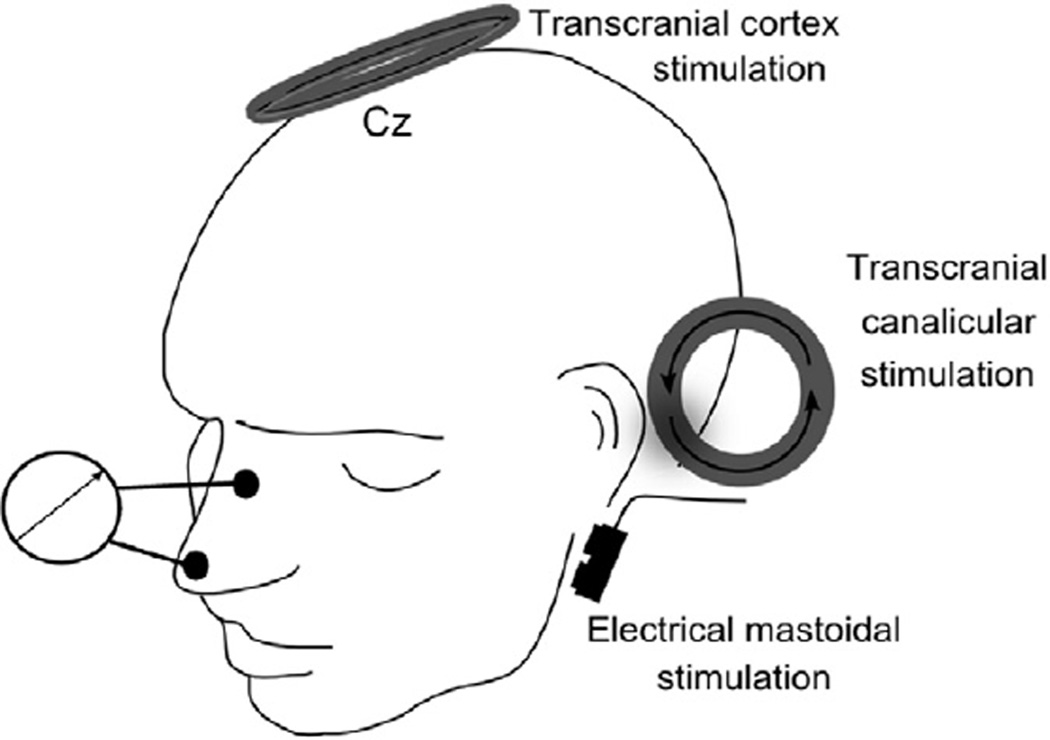
If the stimulation coil is placed tangentially on the head, the magnetic field penetrates the scalp and skull with minimal attenuation and induces a secondary eddy current in conductive intra-cranial tissue. The electrical field in the tissue is oriented perpendicular to the magnetic field and has an opposite direction relative to the electrical current in the stimulation coil. In a medium with homogeneous conductivity, the induced electrical current will run in parallel to the plane of the TMS coil. Because the human brain is not homogeneous and the induced currents and their paths in intracranial tissue are distorted by regional differences in tissue conductivity, the exact current distributions can only be predicted by extensive modeling taking into account the electrical properties of the tissue in vicinity of the coil (Yang et al., 2006). Intracranial differences in electrical conductivity are relevant to the diagnostic use of TMS. For example, the spread of TMS-induced eddy currents in the cerebrospinal fluid after cortical or spinal stimulation can be exploited for effective excitation of the intracranial segment of the facial nerve or the intraforaminal segment of the spinal nerves. Nerve excitation is more likely to occur at the exit point of the nerves to either the facial canal or the spinal foramina. The threshold is lower at this location because of a focal increase in the induced electric field within the foramina.
2.2. How does TMS electrically excite neurons in the stimulated cortex?
To effectively stimulate cortical neurons, the current flow in the tissue has to produce an outward directed trans-membrane current (ion flow) in cortical axons that is sufficiently strong to depolarize the membrane potential, thereby triggering an action potential. The induced electric field and the resultant current flow in the cortex are proportional to the rate of change (i.e., the temporal derivative) of the induced electromagnetic field. In addition, the spatial relationship between the induced tissue current and the stimulated axons (i.e., the spatial derivative) determines the efficacy of TMS to induce action potentials in cortical neurons. Effective membrane depolarization will preferentially occur at sites where the temporal and spatial derivatives of the induced electrical field are maximal. Axonal bendings have been highlighted as an important spatial feature that determines the susceptibility of cortical neurons to TMS (Maccabee et al., 1993): the electric current induced by TMS results in an outward directed transmembrane depolarization in susceptible axons within the field. The most likely to be activated are those that change their orientation relative to the induced electric field.
The action potentials induced by TMS in cortical axons spread trans-synaptically to other neurons, resulting in a propagation of neuronal activation to connected cortical and subcortical areas (Groppa et al., 2012). This volley of excitation travels along the corticospinal tract and peripheral motor nerve resulting in muscle responses that can be recorded as MEPs.
It is important to bear in mind that at the neuronal level, magnetic stimulation excites nerves through the same mechanism as conventional electrical stimulation (Barker et al., 1985). Hence, the term “magnetic stimulation” is misleading because the magnetic field itself does not stimulate the cortical neurons. The rapidly changing magnetic field only serves as a means to induce an electrical current in the neural tissue. It is the induced electrical current that depolarizes cortical axons and triggers action potentials at suprathreshold stimulus intensities. The best coupling of the electromagnetic field to the cortical tissue is achieved by placing the flat surface of the coil tangentially on the scalp over the M1. Since TMS requires no direct electrical contact with the skin, the skin does not need to be prepared prior to TMS. However, marking the optimal site of stimulation on the skin is useful to facilitate the correct positioning of the coil.
TMS can effectively stimulate cortical neurons without producing a high electrical current in the skin and subcutaneous tissue. This explains why TMS, in contrast to transcranial electrical stimulation (TES), does not induce pain and therefore is usually well tolerated by the patients. TES is an alternative stimulation technique which excites cortical neurons transcranially by means of a rapidly rising electrical current pulse applied through surface electrodes attached to the scalp (Merton and Morton, 1980). TMS and TES differ in terms of the direction of the electric fields produced in the brain (Saypol et al., 1991). Electrical fields produced during TMS are parallel to the head surface and are insensitive to the skull conductivity. In contrast, electrical fields produced by TES have components both parallel and perpendicular to the head surface and the induced electrical fields highly depend on the conductivity of the skull. TES tends to directly activate cells probably at the proximal axons level and evoke mainly early descending volleys (i.e., D-waves, see below for further details), while TMS mainly generates later volleys via transsynaptic excitation of the corticospnal neurons (i.e., I-waves). Since D-waves are considerably more resistant to the suppressive effects of anaesthetics on neural excitation, the efficacy of TES is much less affected by anaesthesia than TMS. TES is therefore the method of choice for monitoring conduction of the corticospinal tract in anesthetized patients, for instance during spinal cord surgery (Szelenyi et al., 2005). On the other hand, TES has never been widely used diagnostically in patients who are awake, because it causes considerable pain.
2.3. Propagation of magnetic field
The depth penetration of TMS is limited because the induced electromagnetic field attenuates exponentially with increasing distance from the coil. That means that the intensity required for effective stimulation of M1 increases with the distance between the stimulating coil and the cortical target area. Therefore, the coil should be directly placed on the scalp with the horizontal plane of the coil parallel to the subject’s head which minimizes the distance between coil and cortex. Tissue penetration increases with stimulus intensity and coil diameter. But even if a coil with a large diameter is used and TMS is applied at maximal stimulus intensity, TMS will be unable to excite neural structures that are located deep in the cerebral hemispheres such as the medial part of the temporal lobes, thalamus and basal ganglia. In contrast, TMS of the primary motor hand area (M1-HAND) is already effective at relative low stimulus intensities because this area is large and located at the hemispherical surface. Higher intensities are usually necessary for TMS of the primary motor leg area (M1-LEG) because this part of the M1 is buried in the interhemispheric fissure resulting in a greater distance between the coil and the cortex.
2.4. Stimulus wave form: monophasic versus biphasic stimulation
Two different waveforms referred to as “monophasic” or “biphasic” pulses are commonly used for clinical TMS. The wave form has a significant impact on the stimulation characteristics and thus, needs to be considered when TMS is applied diagnostically. For every waveform, the integral of the induced electric field over the duration of the magnetic pulse is zero. In other words, the induced current flow in one direction is always counter-balanced by the same amount of current flow in the opposite direction.
The monophasic current pulse consists of a strong initial current flow which induces a magnetic field of 1–2.5 Tesla peaking approximately 50 µs after pulse onset. The initial part of the monophasic pulse is balanced by a critically dampened return current. The return current is small in amplitude and lasts several hundreds of milliseconds. The initial rapidly changing current flow is the only one physiologically effective, whereas the dampened reverse current produces no neuronal stimulation in the brain. Therefore, the term “monophasic”, despite of being convenient, is incorrect. The pulse consists of more than one phase, but it is only a single phase (i.e., the first phase of the stimulus) which produces a current flow in the stimulated brain which is strong enough to elicit action potentials.
The biphasic pulse configuration resembles a cosine waveform. Here, the direction-specific stimulation effect is less pronounced, but still present. The direction of the current is reversed twice during a biphasic pulse: an initial current rise is followed by a current reversal below zero and a subsequent rise back–zero). In contrast to the monophasic current wave form, each phase of the biphasic stimulus induces physiologically significant tissue current which flows in the same or opposite direction as the initial rising phase of the biphasic pulse. While all current components of the biphasic pulse contribute to electrical cortex stimulation, the second (reversal) phase has the largest amplitude and the longest duration. The reversal phase does not only induces a stronger tissue current than the initial rising phase, the opposite current direction also causes a stimulation of different neural elements in the cortex as opposed to the initial rising phase. This implies that monophasic and biphasic TMS pulses preferentially excite partly different sets of cortical axons when using the same coil orientation: for monophasic TMS, it is the initial phase that is most relevant for exciting cortical axons, whereas the second (reversal) phase is physiologically most effective for biphasic TMS.
2.5. Coil design: round coil versus figure-of-eight coil
Magnetic coils have different shapes. Either a round coil or a figure-of-eight coil can be used for diagnostic TMS. Large round coils (also named circular coils) with an outer diameter of ⩾10 cm are more powerful than figure-of-eight shaped coils. The former have a good depth penetration and induce electrical current in a large volume of brain tissue; all brain regions underlying the annulus of the coil are equally likely to be stimulated, resulting in a non-focal stimulation. Due to the circular geometry, the induced current loops in the tissue are circular and have an opposite direction relative to the electrical current in the coil. The loops with the strongest current will be below the annulus of the coil. The induced electrical field attenuates towards the geometrical center of the coil. Therefore, the annulus of the coil and not the center has to be placed over the target cortex to achieve effective stimulation.
More focal stimulation is possible with a figure-of-eight-shaped coil (Cohen et al., 1991). The figure-of-eight coil (also named double or butterfly coil) consist of two circular coils which are placed side by side and wired in a way that the current in each circular coil has an opposite direction (Jalinous, 1991). This coil produces the largest current density in the tissue under the intersection of the two round components with the largest component of the electric field being oriented in parallel to the wires in the center of the coil (Cohen et al., 1991). Therefore, the center of the figure-of-eight coil should be placed over the target region to induce effective cortical stimulation. Because the induced tissue current is two to three times higher in the central coil region than at the outside edges, a figure-of-eight coil allows for a relatively selective stimulation of a distinct cortical region at low or moderate intensities of stimulation. The focality of the figure-of-eight coil is more appropriate for research application or for specific clinical situations in which mapping is required.
The design of the stimulating coil has additional practical implications. For the diagnostic use of TMS, the direction of the induced tissue current represents a point of paramount practical importance, because the M1 is best stimulated by currents penetrating it when flowing in the brain in a posterior-to-anterior direction. When using a circular coil, the examiner has to take into account which coil surface touches the skin to induce the optimal current direction in the brain. For TMS with a figure-of-eight coil, it is the orientation of the coil handle that determines the current direction.
When using a round coil, the current direction in the coil is defined by the coil surface which touches the skin. If the examiner wants to reverse the current direction in a round coil, the coil has to be flipped over so that the upper surface of the coil now makes contact with the skin. The orientation of the coil handle has no relevance when TMS is given through a large round coil.
When TMS is carried out with a figure-of-eight coil, the orientation of the coil handle is highly relevant and needs to be kept constant to ensure constant stimulation conditions. The orientation of the coil corresponds to the current direction in the junction region of the figure-of-eight which represents the part of the coil critical for brain stimulation. In the case of an eight-shaped coil configuration, the current direction in the coil can be reversed by in-plane rotation of the coil on the scalp by 180° without flipping the coil surface. The 180° in-plane rotation does not alter the position of the coil relative to the scalp. The only difference is that the handle of the coil now points in the opposite direction and the current in the junction region is reversed. Some commercial stimulators have a built-in switch to reverse the current in the coil without the need to flip or rotate the stimulating coil. In contrast to TMS with a circular coil, it does not matter which surface of the coil touches the skin while using a figure-of-eight coil.
Which coil design is best suited for diagnostic TMS? It is recommended to use a large round coil for diagnostic TMS. This recommendation is based on several considerations. First, coil positioning over the target region of M1 is easier with the large round coil than with the focal figure-of-eight shaped coil because the non-focal round coil effectively stimulates a larger cortical volume. The large round coil also results in a better depth penetration which is advantageous for TMS of the primary motor leg area. Finally, TMS of the M1 with a non-focal round coil is less susceptible to minor changes in the coil position as opposed to TMS with a more focal figure-of-eight shaped coil.
3. The physiological basis of TMS
3.1. Descending volleys in the corticospinal tract
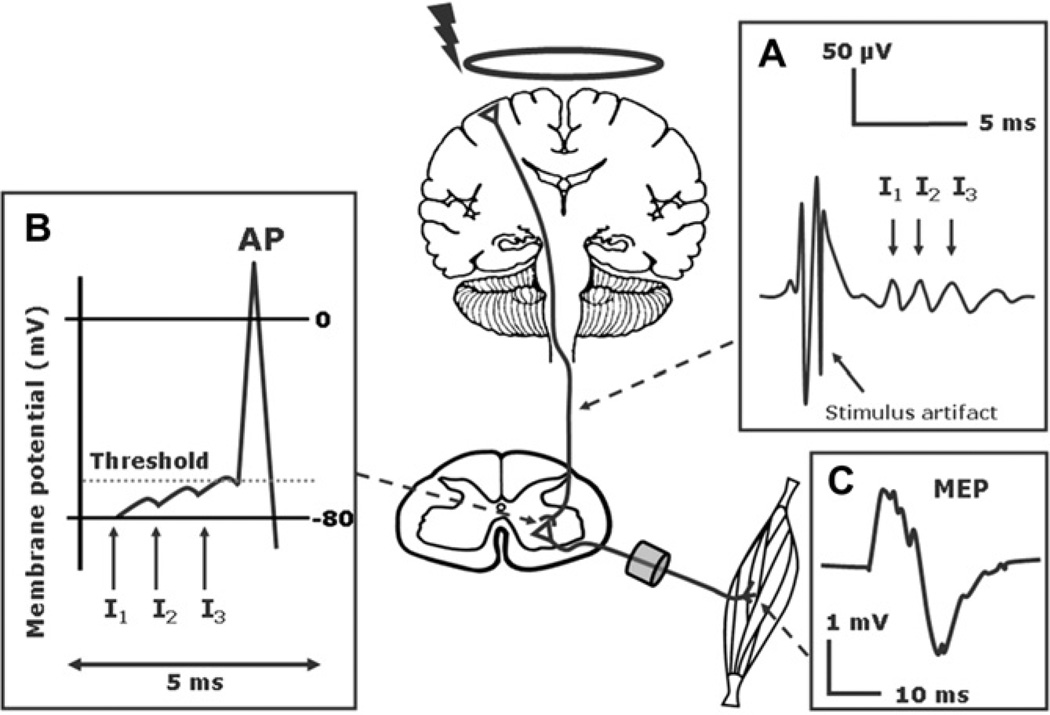
Although it is still unclear which neural elements of the cerebral cortex are the primary target of TMS, epidural recordings in patients with chronically implanted spinal electrodes have provided important insights into how TMS induces corticospinal descending activity (Di Lazzaro et al., 2008). When a single-pulse TMS is given to the human M1 at gradually increasing intensities of stimulation, an increasing number of descending corticospinal volleys can be recorded at the level of the cervical spinal cord. Such descending volleys have previously been recorded in the pyramidal tract after direct stimulation of the cortical surface in animal studies (Amassian et al., 1987; Patton and Amassian, 1954). They are caused by highly synchronized orthodromic action potentials in fast-conducting, large diameter axons originating from corticospinal neurons making direct monosynaptic connection with spinal motoneurons (Fig. 1). Early and late descending volleys can be distinguished by their onset latency. The first volley results from direct excitation of the corticospinal neuron at its axon hillock and has the shortest latency. Because this volley is caused by direct excitation of the corticospinal axon, it has been named D-wave or direct-wave (Di Lazzaro et al., 2004a). Later waves follow at intervals of 1.2–2.0 ms and result from indirect trans-synaptic corticospinal excitation via different sets of intracortical neurons that project onto the pyramidal neurons. These later volleys are named indirect-waves or I-waves and occur at preferential intervals indicating a high degree of synchronization.
Fig. 1.
Neurophysiological basis of the motor evoked potential (MEP). (A) TMS-induced activation of the corticospinal neurons with a predominant contribution by late Indirect waves (I waves). and (B) Temporo-spatial summation at the cortico-motoneuronal synapses (C) Motor evoked potential (modified from: Siebner HR, Ziemann U. Das TMS-Buch, Heidelberg: Springer Medizin Verlag, 2007, with kind permission of Springer Medizin Verlag).
A single TMS pulse with a large round coil at a stimulus intensity that is suprathreshold for evoking MEPs produces a series of descending volleys (multiple I-waves and possibly a D-wave), indicating strong repetitive activation of the fast-conducting corticospinal neurons (Di Lazzaro et al., 2002). However, we know from epidural recordings that the recruitment of the various components of the corticofugal discharge by a single TMS pulse depends on the intensity of stimulation, on the shape of the coil, the pulse configuration, and on the relative threshold of each volley to the direction of the induced current flow in the cortex (Di Lazzaro et al., 2003a, 2004b). These stimulation variables need to be standardized to allow for a comparison within and across subjects.
3.2. Transsynaptic excitation of spinal motoneurons
Each descending corticospinal volley induces glutamate release in cortico-motoneuronal synapses leading to a depolarization at the postsynaptic cell membrane. The glutamate release triggered by each volley sums up temporally and spatially. If the volleys are strong enough to exceed the firing threshold, they trigger an action potential in the spinal motoneurons. These action potentials propagate along the peripheral motor axons inducing a motor response that can be recorded as the MEP (Fig. 1).
The strength of the descending cortical input and the local excitability of the spinal motoneurons determine the efficacy of transsynaptic cortico-motoneuronal excitation. The descending excitatory drive depends on external (e.g., the intensity of the TMS pulse) and internal (e.g., the maturation and integrity of corticospinal connectivity) factors. Manipulations that increase the efficacy of cortico-motoneuronal excitation facilitate the amplitude and shorten the latency of the MEP. For instance, increasing the stimulus intensity will induce a stronger descending excitatory drive resulting in a faster temporo-spatial summation at the cortico-motoneuronal synapses. The excitability of the motor neuron pool is controlled by complex excitatory and inhibitory circuits. Increasing the excitability of the cortical and spinal motor neuron pool, for instance by voluntary contraction of the target muscle, will also facilitate TMS-induced cortico-motoneuronal excitation, as more motoneurons will be recruited to discharge. Indeed, some motoneurons may discharge multiple times.
TMS-induced corticomuscular excitation is subject to more temporal dispersion than the compound muscle action potentials (CMAPs) obtained with supramaximal electrical stimulation of peripheral nerves. This results in a substantially larger phase cancelation of TMS-induced corticomotor excitation and accounts for some of the differential characteristics between the MEPs and the CMAPs. The MEP evoked by single-pulse TMS of M1 has a smaller peak-to-peak amplitude, longer duration and a more polyphasic shape than the CMAP. Also, with consecutive recordings, the MEPs are less consistent in shape than the CMAP because the amount of temporal dispersion varies from trial to trial due to intrinsic fluctuations of excitability in the corticomotor system.
3.3. Defining the threshold for corticomotor excitation
The first step in the clinical use of TMS is to determine the cortical motor threshold (CMT) for the target muscles. The CMT is of basic relevance for the clinical application of TMS, because the stimulus intensity for the further examination is adjusted in each patient to individual CMT. It is not straightforward to estimate the CMT due to the natural fluctuations in excitability of the pyramidal cells and spinal motoneurons (Adrian and Moruzzi, 1939). The CMT reflects the integrated excitability of the corticomotor projection, including the excitability at the spinal level. In clinical practice, the CMT is determined with the target muscle being completely relaxed. This threshold is commonly referred to as resting motor threshold. The CMT can also be determined during a slight tonic contraction of the target muscle with approximately 20% of the maximal strength and is then called active motor threshold. Different procedures for determining the CMT have been described and can be used depending on clinical setting and available technical support.
3.3.1. Relative frequency method
The first general procedure to estimate the CMT was proposed by a committee of the International Federation of Clinical Neurophysiology (IFCN) in 1994 (Rossini et al., 1994). According to Rossini et al. (1994), the CMT is defined as the lowest stimulus intensity (given as percentage of MSO) that is required to induce a MEP in 5 out of 10 trials (Rossini et al., 1994). In case of a relaxed target muscle, a positive MEP response has been defined as an MEP with ⩾50 µV peak-to-peak amplitude because it is difficult to distinguish MEPs with a peak-to-peak amplitude of less than 50 µV from noise (Rossini et al., 1994). If the patient performs a tonic contraction of the target muscle, a cut-off value of 200 µV is usually applied in order to avoid that voluntary electromyographic activity is wrongly classified as MEP.
At present, most laboratories use a relative frequency method based on the Rossini criterion to determine the CMT. However, this CMT criterion has been criticized because it neglects the probabilistic nature of the CMT and thus, is subject to variations and biases (Awiszus, 2003; Tranulis et al., 2006). Further, the guideline published in 1994 (Rossini et al., 1994) does not specify a clear algorithm in order to find the stimulus intensity which meets the relative frequency criterion (i.e., that a single TMS pulse evokes a MEP in 5 out of 10 trials).
To close this gap, we describe a standardized algorithm which is based on a slightly modified “relative frequency” criterion. The CMT is defined as the lowest stimulus intensity at which TMS evokes a MEP in at least 5 out of 10 trials. TMS should start with a subthreshold intensity of stimulation. For instance, one may start with a stimulus intensity of 35% of MSO with the coil placed over the optimal site of stimulation (see Section 6.1). First, stimulus intensity is gradually increased in steps of 5% MSO until TMS consistently evokes MEPs with peak-to-peak amplitude of more than 50 µV in each trial. Thereafter, stimulus intensity is gradually lowered in steps of 1% MSO until less than 5 positive responses out of 10 trials are recorded. This stimulus intensity plus 1 is then defined as CMT.
The relative frequency method is rather time consuming requiring a relative high number of stimuli. For instance, Tranulis et al. (2006) reported that they needed on average 75 stimuli to determine the CMT when using the procedure that applied the Rossini criterion (Tranulis et al., 2006). To reduce the number of stimuli needed for CMT estimation, one might consider to use 6 instead of 10 trials per stimulus intensity level when applying the relative frequency method. In this case, CMT would be defined as the lowest intensity that elicits a clearly discernible MEP in at least 3 out of 6 consecutive trials. However, this modified “relative frequency” criterion has not yet been validated.
3.3.2. Adaptive method
While the procedure described above is based on the relative frequency of evoked MEPs at a given stimulus intensity (rather than the probability to evoke an MEP at a given stimulus intensity), adaptive methods that estimate the probability to evoke an MEP have been introduced. These methods take into account the probabilistic nature of CMT and estimate the probability of evoking a MEP with TMS at a given stimulus intensity. A highly efficient adaptive method to determine the CMT is based on Parameter Estimation by Sequential Testing (PEST) and Maximum Likelihood regression (Awiszus, 2003; Mishory et al., 2004). This adaptive method uses an S-shaped metric function to model the relationship between the TMS intensity and the probability of eliciting an MEP. At each trial, the model predicts a TMS intensity that yields a 50% probability of evoking an MEP. This intensity is then selected as the intensity for the next TMS pulse. The computer program which is necessary to run the maximum-likelihood threshold-tracking algorithm was made freely available by Awiszus and Borckardt (Motor Threshold Assessment Tool, version 2.0: http://www.clinicalresearcher.org/software).
The adaptive stair-case procedure starts with defining an upper and lower boundary. The most conservative approach is to use 0% of MSO as lower boundary and 100% as upper boundary. Alternatively, one might select the boundaries based on the known threshold distribution obtained in a normative data set of healthy subjects (e.g., the 2.5% and 97.5% percentiles). In case the individual resting CMT is known from a previous investigation, one might select a more rigid lower and upper boundary, for instance ±20% of the previously estimated resting CMT. Based on the boundaries, the PC program selects the intensity of the TMS pulse. The information whether or not a MEP was evoked at that intensity is fed back to the PC program which estimates the stimulus intensity of the next TMS stimulus based on the maximum-likelihood threshold-tracking algorithm (Awiszus, 2003). When using the accuracy limits for CMT estimation as suggested by the safety guidelines of Rossi et al. (2009), 14 or 17 stimuli are needed for reliable CMT estimation with or without specific a priori assumptions, respectively (Awiszus, 2011).
Recently, Bayesian regression was used for PEST which enabled two modifications. Using a Bayesian adaptive method, it is possible to incorporate prior CMT knowledge and to determine a systematic and theoretically valid stopping criterion based on the estimation of CMT precision (Qi et al., 2011). After each trial, the likelihood that the MEP is generated by the model is combined with the prior probability distribution of CMT probability to generate a posterior probability distribution of CMT. Using this Bayesian adaptive method, seven TMS pulses were sufficient on average to determine the CMT using a common prior based on a priori CMT distribution at the group level. Three TMS pulses were enough for CMT estimation when subject-specific priors were used. However, the validity to apply a stop criterion has been questioned (Awiszus, 2011), at least if CMT estimation should adhere to the accuracy limits for CMT estimation as suggested by the published safety guidelines (Rossi et al., 2009).
3.3.3. The two-threshold method
Mills and Nithi proposed a procedure to estimate CMT which is based on measuring two thresholds, an inferior (lower) threshold and a superior (upper) threshold (Mills and Nithi, 1997). The lower threshold corresponds to the highest stimulus intensity at which no motor response is evoked in 10 consecutive trials. This lower threshold corresponds to the intensity at which the probability to evoke an MEP is zero. The upper threshold is defined as the lowest stimulus intensity at which MEP responses are evoked in all 10 consecutive trials. This upper threshold corresponds to the intensity at which the probability to evoke an MEP is 1. The arithmetic mean of the lower and upper threshold is defined as CMT (Mills and Nithi, 1997).
A disadvantage of the two-threshold procedure is that the estimation of the CMT is relatively time consuming. However, the total number of stimuli can be reduced by applying less than 10 stimuli at some intensity levels. For instance, while searching the lower threshold, the stimulus intensity can be decreased after the first positive response without completing the 10 stimuli train. When adopting such a procedure, 48 stimuli are needed on average to reach a threshold estimate (Awiszus and Feistner, 2007).
3.3.4. Supervised parametric estimation
An alternative method uses supervised parametric estimation of the CMT (Tranulis et al., 2006). This method is based on the observation that the probability of observing a motor response increases monotonically with increasing stimulus intensity and can be described by a sigmoid probability function. The motor response probability is recorded at various intensity levels. The CMT is then estimated by fitting a sigmoid curve through the experimental data points using a least mean squares method.
3.3.5. Which method should be used for threshold estimation?
Each of the methods described above have a validated scientific background and can be used in a clinical setting because they provide a CMT estimation that is sufficiently accurate for diagnostic purposes (Tranulis et al., 2006). For all methods, it is essential that the algorithm for CMT determination is clearly described and standardized. Yet, there is clear evidence that adaptive methods based on threshold-tracking algorithms provide the most accurate CMT estimation with the same number of stimuli or faster CMT estimation with less stimuli (Awiszus, 2011; Qi et al., 2011). Therefore, the use of adaptive stair-case procedures is preferable to the other methods used for CMT estimation, if clinically feasible. A practical limitation of the adaptive methods is that the outcome has to be fed back to the PC after each trial to inform the algorithm about the occurrence of a MEP. This requires ideally that a second examiner is present during threshold estimation to protocol the occurrence of MEPs after each trial.
3.3.6. Clinical use of cortical motor threshold determination
It needs to be pointed out that the CMT is not a static measure but is subject to state-dependent fluctuations which account for some intra-individual variations in CMT when performing repeated CMT measurements in the same individual. The threshold depends on many variables, including the technical setup, individual posture (i.e., lying or seated), pharmacological influence, age and the target muscle. The CMT is lowest for hand muscles because of their large cortical representation and the relative superficial location of the M1-HAND with respect to the scalp. The CMT is not fixed but can dynamically change over time, for instance during the sleep-wake cycles or with CNS maturation and aging.
The diagnostic use of the CMT is limited because the CMT shows substantial intra-individual and inter-individual variability, even in healthy adults (Kloppel et al., 2008; Tranulis et al., 2006). A marked inter-hemispheric asymmetry in resting CMT of homologous target muscles may indicate a unilateral damage of the corticomotor pathway, resulting in a relative increase in resting CMT. Clear statistical criteria to define a right-left difference in CMT need to be established based on the distribution of interhemispheric CMT differences in healthy individuals (see Section 8).
Even if CMT values are of limited diagnostic importance, it is recommended to integrate these in the overall neurophysiological assessment. The estimated motor thresholds should be listed in the final report, together with the method used for assessing CMT. The CMT might be increased in a wide range of disease states, if the disease leads to an increase in the threshold for excitation at the cortical or spinal level. Another clinically relevant mechanism that reversibly increases the cortical motor threshold is medication. Especially drugs that block the sodium channel in cortical neurons can result in marked increases in cortical motor threshold. This is particularly relevant in patients with epilepsy that receive sodium channel blockers as part of their pharmacological treatment (Ziemann, 2004). Furthermore a side CMT difference can be clinically relevant (for i.e., in stroke) and reflect the outcome. (Traversa et al., 1998). Therefore, it is imperative to enquire about potential confounding or co-existing issues when interpreting the CMT in a clinical setting.
3.4. Electrophysiological characteristics of the motor evoked potential (MEP)
The clinical interpretation of the TMS examination is based on measurements of the size (amplitude or area) and corticomotor latency of the MEP. In this section, we describe the characteristics of the MEP amplitude that are relevant to the clinical use of TMS.
In healthy subjects the MEP amplitude increases monotonically with increasing stimulus intensity. The stimulus–response relationship can be modeled by a cumulative Gaussian and described by a sigmoid curve. The sigmoid curve starts as a flat line which deviates from zero at the stimulus intensity which corresponds to the CMT. The second part of the sigmoid curve is characterized by a linear increase in MEP amplitude with increasing stimulus intensity. The slope of this linear increase reflects the gain in MEP amplitude with increasing stimulus intensity. At higher stimulus intensities, the ascending line turns again into a flat line (i.e., plateau). The flat portion of the sigmoid curve shows no further increase in MEP amplitude despite of an increase in stimulus intensity. The sigmoid stimulus–response function is different for each muscle, and it also differs in the same muscle depending on whether the target muscle is at rest or preactivated. To be able to fully describe the stimulus–response characteristics in a given muscle, one has to record MEPs over a wide range of intensity levels both, at rest and during contraction. These data could then be used to model the stimulus–response function by fitting a sigmoid curve. However, such detailed assessment of the stimulus–response properties is not feasible in a clinical setting and has not yet been used for diagnostic purposes.
Since the primary goal of the clinical examination is to detect a disease-related impairment in corticomotor conduction, TMS should provide a reliable estimation of the maximal corticomotor response and the shortest corticomotor latency. This implies that the stimulus intensity used for diagnostic TMS should be sufficiently high to excite all fast-conducting corticospinal neurons. In addition, the efficacy of TMS can be increased by asking the patient to preactivate the target muscle.
What can be considered an optimal intensity for diagnostic TMS? Optimally, TMS should use a stimulus intensity that marks the transition from the rising slope to the flat portion of the sigmoid stimulus–response curve. At that intensity, TMS evokes MEPs of maximal amplitude but imposes a minimal level of discomfort for the patients. If the target muscle is preactivated, it can be assumed that the transition from the increasing to the flat portion of the stimulus–response function occurs at a stimulus intensity of 140% of resting CMT (corresponding to approximately 170% of active CMT). If the target muscle is at rest, a higher stimulus strength (i.e., 170% of resting CMT) is needed to reach the flat portion of stimulus–response curve.
Usually, the MEPs are recorded with surface electrodes over the target muscle. The MEP shape resembles the compound muscle action potentials (CMAPs) evoked by electrical stimulation of peripheral nerves, but there are major differences. These differences are due to the underlying physiology. In contrast to peripheral nerve electrical stimulation, TMS results in a desynchronized excitation of motor units in the target muscle. Several mechanisms contribute to desynchronized muscle excitation, including the temporally dispersed corticospinal volleys induced by TMS, variations in the time to reach action potential threshold in the motoneuron pool and differences in conduction velocity in peripheral motor axons (Fig. 1). The degree of desynchronization, which is usually larger for lower limbs muscles, varies from trial to trial because of intrinsic fluctuations in neural excitability at the cortical and spinal level. The substantial phase cancelation caused by desynchronized muscle activation explains why the MEP evoked by a TMS pulse is smaller in amplitude and more polyphasic than the CMAP evoked by maximal peripheral nerve stimulation. This is even the case at high TMS intensities which induce maximal stimulation of the corticomotor pathway. At high TMS intensities, the maximal stimulation of the fast-conducting corticomotor pathway evokes muscle twitches that match or even exceed the force of muscle twitches evoked by maximal peripheral nerve stimulation (Merton and Morton, 1980). This is due to repetitive discharge of a portion of alpha-motoneurons at higher TMS intensities. However, because of substantial phase cancelation, the corresponding MEP amplitude never matches the amplitude of the maximal CMAP.
Like CMAP measurements after supramaximal peripheral nerve stimulation, clinical MEP measurements are tailored to provide an estimate of the maximal MEP amplitude (or area) and the shortest corticomotor latency of the MEP. This is because the MEP with the largest amplitude and shortest corticomotor latency reflects the optimal conduction from the M1 to the target muscle in a given patient. When using conventional single-pulse TMS, it is therefore recommended to record at least 5–6 consecutive MEPs during tonic contraction of the target muscle and to consider only the MEP with the largest amplitude and shortest latency. Voluntary contraction of the target muscle reduces temporal dispersion of the induced descending volleys, facilitates MEP amplitude and shortens MEP latency relative to MEPs recorded during muscle relaxation, and thus maximizes the evoked motor response.
Generally, the recording of 5–6 consecutive MEPs is considered sufficient to assess corticomotor conduction. However, the number of MEP trials has been defined arbitrarily. A systematic study on how many MEP trials should be recorded from each muscle to optimally estimate the maximal MEP amplitude and the shortest MEP latency is lacking. The rationale behind focusing only on the best of 5–6 consecutive motor responses is that the MEP with the largest amplitude and the shortest latency provides a better estimate of optimal corticomotor conduction in a patient than the statistical distribution of MEP amplitudes and latencies. The estimation of the probabilistic distribution of MEP amplitudes or latencies is more relevant in scientific studies on corticospinal excitability and requires a large number of MEPs to be recorded from each muscle.
In summary, diagnostic TMS requires single-trial analysis of the MEP with the largest amplitude and the shortest latency. An estimation of the mean or median MEP amplitude or latency based on the distribution of all recorded MEPs is not necessary.
When conventional single-pulse TMS is used to elicit the MEP, phase cancelation of the desynchronized action potentials in the muscle is the major source for both, trial-to-trial variability and overall reduction in MEP amplitude as opposed to CMAP amplitude (Magistris et al., 1998). This major limitation can be overcome by using the so-called triple stimulation technique (Magistris et al., 1998) (see Section 13. for detailed description).
3.5. Electrophysiological characteristics of the cortical silent period (CSP)
TMS performed whilst the target muscle is tonically contracted disrupts the ongoing voluntary EMG activity for a period following the MEP (Rossini and Rossi, 2007). This is referred to as the cortical silent period (CSP, Fig. 3). CSP duration increases with TMS intensity. The relationship between stimulus intensity and CSP duration can be fitted to a sigmoid stimulus-duration curve and the rising phase of the sigmoid curve can be described by its linear slope (Kimiskidis et al., 2005; Wilson et al., 1993a). At high stimulus intensities, the CSP can last several hundred milliseconds when recorded in hand muscles of healthy subjects (Cantello et al., 1992). The site of origin of the CSP is largely in the M1 (Inghilleri et al., 1993). Spinal mechanisms contribute to the early part of EMG inhibition, while the later part is caused by a suppression of corticospinal output at the cortical level (Fuhr et al., 1991; Inghilleri et al., 1993). The details regarding the measurement of the CSP are given further below (see point 7.4.).
Fig. 3.
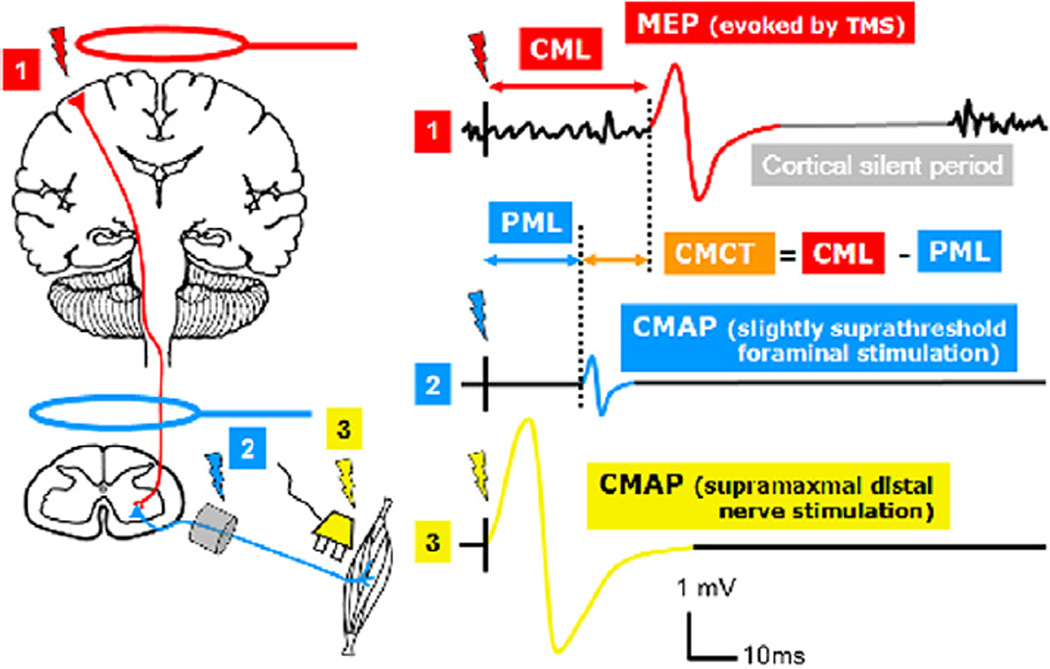
Schematic illustration of the motor responses elicited with TMS of the M1 (1), foraminal electromagnetic stimulation (2), and supramaximal distal nerve stimulation (3). CMAP: Compound motor action potential; MEP: motor evoked potential (note that the MEP is also a CMAP but transcranially evoked), PML: peripheral motor latency (conduction time from neuroforamen to muscle), CML: cortical motor latency (conduction time from cortex to muscle), CMCT: central motor conduction time (calculated as difference between CML and PML). Note that this method of CMCT estimation includes the very proximal peripheral conduction time until the neuroforamen.
The duration of the CSP is compatible with a long-lasting inhibition mediated by GABAB receptors (Connors et al., 1988) and it has been shown to be prolonged following oral administration of the GABA re-uptake inhibitor tiagabine in healthy volunteers (Werhahn et al., 1999) or prolonged intrathecal infusions of the GABAB agonist baclofen in a patient with dystonia (Siebner et al., 1998). This all suggests that the CSP is generated by GABAB-ergic circuits in M1. However, pharmacological experiments in healthy subjects failed to show prolongation of the CSP by the GABAB receptor agonist baclofen (Inghilleri et al., 1996; Ziemann et al., 1996). The doses might have been insufficient to significantly affect GABAB receptor function in the central nervous system. On the other hand, positive modulators of GABAA receptor function, such as the benzodiazepine lorazepam, increase short-duration CSP at low stimulus intensities but shorten long-duration CSP at high stimulus intensities (Kimiskidis et al., 2006). This complex modulation is most likely explained by two effects of GABAA receptor mediated inhibition on the duration of the CSP. GABAA receptor mediated inhibition makes a direct contribution to the CSP in the low-intensity range of the CSP intensity curve, while it suppresses GABAB receptor function via presynaptic GABAA receptors in the high-intensity range (Kimiskidis et al., 2006). Furthermore, various neuromodulating neurotransmitter systems affect CSP duration. In particular dopaminergic drugs may lengthen the CSP (Priori et al., 1994; Ziemann et al., 1996). Finally, motor set, motor task and motor attention (Mathis et al., 1998), visual input (Groppa et al., 2008) or hyperventilation (Priori et al., 1995) may significantly modulate CSP duration and require standardization.
In summary, the CSP is readily obtained by single-pulse TMS and is a measure of motor cortical inhibition, mediated by GABAB receptors, but influenced by a large variety of other physiological factors. Several other paradigms exist to probe various manifestations of intracortical inhibition in M1, but they are based on a conditioning-test approach and require paired-pulse TMS. Only the inhibitory circuits causing the CSP can be probed with single-pulse TMS during a clinical routine examination. Hence, other types of intracortical inhibition are not discussed further in this clinically oriented review.
4. Guidelines for recording the MEP
The technical standards for MEP measurements are identical to the recordings of the CMAP during peripheral motor nerve conduction studies. The EMG signal should be pre-amplified in a way that the MEP is clearly visible on the screen without cutting-off the peaks of the MEP. Usually, an amplification factor of 1000 is sufficient, but a stronger amplification may be necessary for determination of the CMT. The EMG signal is then band-pass filtered and digitized using an analog–digital converter. The cut-off for the high-pass filter should be set at 1 Hz which will help to reduce the duration of the stimulus artifact. The stimulus artifact may pose a problem mainly when magnetic stimulation is performed relatively close to the recording electrodes. The low-pass filter should be set at a frequency well above the maximal frequency spectrum of the EMG signal. It is recommended to set the low-pass filter to ⩾2000 Hz.
The frequency content of the MEP signal determines the minimal sampling rate. The Nyquist theorem says that a function x(t) is completely determined by giving its ordinates at a series of points spaced 1/(2 x B) seconds apart if x(t) contains no frequencies higher than B Hertz. In essence, the theorem shows that a band limited analog signal that has been sampled, for instance the MEP trace, can be perfectly reconstructed from an infinite sequence of samples if the sampling rate exceeds 2 × B samples per second, where B is the highest frequency of the original signal. Therefore, the sampling frequency should be at least two times above the maximal frequency content of the MEP signal. The general practical rule is to use a sampling frequency of 4000–5000 Hz. Many devices have a so called 50 or 60 Hz-Notch-Filter which selectively suppresses signal components at a frequency of 50 or 60 Hz to “filter out” artifacts resulting from electrical devices. This 50 or 60 Hz-Notch-Filter should not be used as the presence of the artifact indicates suboptimal recording conditions. These artifacts can be eliminated by ensuring sufficient grounding and appropriate impedance of the EMG electrodes. Impedance should be below 5 kΩ, but MEP recordings of sufficient quality can often be obtained at slightly higher impedances between 5–10 kΩ.
The recorded MEP should always be clearly visible on the monitor of the recording device. The amplification factor of the screen should be chosen so that the induced MEP is optimally displayed without truncating the positive or negative peaks of the MEP. The optimum screen settings depend on many factors such as the stimulus intensity, the target muscle to be studied and the voluntary preactivation. If the MEP single-trial amplitude is very small, the amplification factor must be increased correspondingly.
As a rule of thumb, the amplification factor of the screen (y-axis) should be 50–100 µV per division unit (e.g., 50 µV/cm) for the measurement of cortical motor threshold and 1–2 mV per division unit during the actual MEP recording (e.g., 1 mV/cm). The temporal resolution of the screen should be chosen in such a way that the TMS artifact, the beginning and the end of the MEP are always visible. When recording MEPs from face or upper limb muscles the temporal magnification of the (x-axis) should be at least 5 ms per division unit and for MEP recordings from the lower extremities at least 10 ms per division unit.
The recorded EMG traces should start at least 50 ms before and include at least 100 ms after the transcranial stimulus. Based on the EMG recordings during the 50 ms period before stimulation, it is possible to infer the level of relaxation or pre-activation in the target muscle at the time of TMS. If the examiner wishes to measure the duration of the CSP, it is recommended that EMG recordings are continued for at least 400 ms after the transcranial stimulus.
5. Safety aspects of diagnostic TMS
An extensive and up-to-date review on TMS safety has been published by the TMS safety study group on behalf of the International Federation of Clinical Neurophysiology (Rossi et al., 2009). The report of the TMS safety study group provides detailed guidelines, covering safety issues of single-pulse and repetitive TMS in healthy individuals and patients. Here we only provide a short summary of the most relevant safety aspects that have to be taken into account when using single-pulse TMS in a diagnostic setting.
Although single-pulse TMS is considered to have no significant risk, a short safety check list should be used to screen patients before they undergo TMS investigations, including, a history of seizures or syncope, brain diseases or medications associated with increase seizure risk, the presence of implanted biomedical devices and pregnancy. To this purpose, an updated version of the questionnaire for the screening of patients before TMS investigations has been recently published (Rossi et al., 2011). With the exception of the implanted devices, all the other condition should be considered only relative contraindication and the risk–benefit of the procedure should be carefully considered before the patients undergo the TMS study.
5.1. Seizure and syncope
Seizures are the most serious possible TMS-related adverse event. Only few cases of TMS induced seizures have been reported so far out of hundreds of thousands examined subjects, and the vast majority of seizures were induced during repetitive TMS (Rossi et al., 2009). The risk to induce a seizure with single-pulse TMS is very low. Overall, less than 5% of the known TMS-related seizures occurred during single-pulse TMS studies: these were subjects with known structural brain pathology or under proactive medication. For example, one patient with a bipolar disorder, taking low doses of lithium and chlorpromazine developed a seizure after five single pulses at 60% of the maximal stimulator output over 3 min (Tharayil et al., 2005). Therefore, the risk/benefit ratio of the TMS investigation has to be individually assessed in patients at risk for seizure occurrence, especially those taking neuroactive drugs or having epileptogenic brain lesions (i.e., tumor, stroke).
Compared to seizure, syncope is more likely to occur during a TMS investigation, but this is a rare event too. No systematic studies addressed the relative incidence of the two phenomena during TMS, but this is a common experience in many labs. Vasodepressor (neurocardiogenic) syncope is a common reaction to anxiety and physical discomfort and it can take place following TMS, as with many other noninvasive or minimally invasive medical procedures. The cardinal feature that distinguishes syncope from seizure is rapid recovery of full consciousness within few seconds and not minutes (Caplan, 2000). The premonitory complaint that “I need to lie down”, or “I need air”, narrowing and blacking out of the visual field, sensations of heat, bradycardia, and loss of peripheral pulses also favor circulatory collapse. Visceral distress, sweating, pallor, nausea, dizziness are frequent symptoms. Gastrointestinal symptoms occur in partial epilepsies as well, but their incidence in seizures provoked by TMS is unknown.
Differential diagnosis between syncope and seizure may be difficult in case of positive phenomena. The former may include phenomena considered typical of seizures: tonic contractions, jerking, vocalizations, oral and motor automatisms, brief head or eye version, incontinence, hallucinations, and injuries from falling. Such episodes can be difficult to distinguish from epileptic events. Upward eye deviation is common in circulatory syncope, but rare in partial seizures unless they progress to generalized convulsions. It is important to remember that patients who develop syncope under TMS often have experienced similar episodes in the past.
5.2. Other adverse effects
Other side effects of single-pulse TMS investigations are mild and, when present, transient. Most of subjects do not complain about discomfort, especially when they are familiarized with the TMS procedure. Some may experience surprise looking at their hand/arm twitching; some may feel a local mild discomfort under the stimulating coil or slight tongue paraesthesias during TMS of high intensity delivered on the midline for activation of lower limb corticospinal neurons. Both, patient and examiner should always wear earplugs during diagnostic TMS to prevent transient auditory threshold changes, which are more likely to occur during repetitive TMS, but are theoretically possible even with single pulse application.
5.3. Safety considerations in particular patient groups
Active brain implants, such as deep brain stimulation systems, epidural/subdural electrode arrays for cortical stimulation, and cochlear implants all contain intracranial electrodes connected to subcutaneous wires in the scalp. TMS near these wires can theoretically induce voltages in the electrode contacts that are substantially higher than the voltages applied during normal operation of the implant. However, clinical experience suggests that single-pulse TMS in patients with implanted electrodes, including those for vagal nerve stimulation, can be performed safely, provided that the coil is not discharging on the skin overlying the electrical device. The same applies for stimulators, infusion pumps or pace-makers. In patients with cochlear implants, the external part of the device has to be removed before the TMS investigation, since they are too close to the site of stimulation.
Single-pulse TMS investigations in pediatrics is a minimal risk procedure (Gilbert et al., 2004). Special care should be paid in children until approximately 18 months of age, because the fontanelles are still open and mechanical injuries due to excessive coil pressure are possible. Children have always to wear earplugs, since the auditory canal is relatively small, resulting in a higher resonance frequency of the external ear. In pregnant women, it seems unlikely that the fetus might be affected by TMS, since the induced electromagnetic field attenuates rapidly with distance, and decays to zero at a distance of 70 cm away from the discharging coil (Karlstrom et al., 2006). However, magnetic nerve root stimulation over the lumbar spine should be avoided. Because of the rapid attenuation of the induced electromagnetic field with increasing distance from the discharging coil, there are no safety hazards in adults carrying metal implants (e.g., prostheses, pumps for drug injection or any other hardware device) at any place in their body outside the head. Despite the above concerns single pulse TMS is considered extremely safe.
6. Step-by-step description of a clinical examination
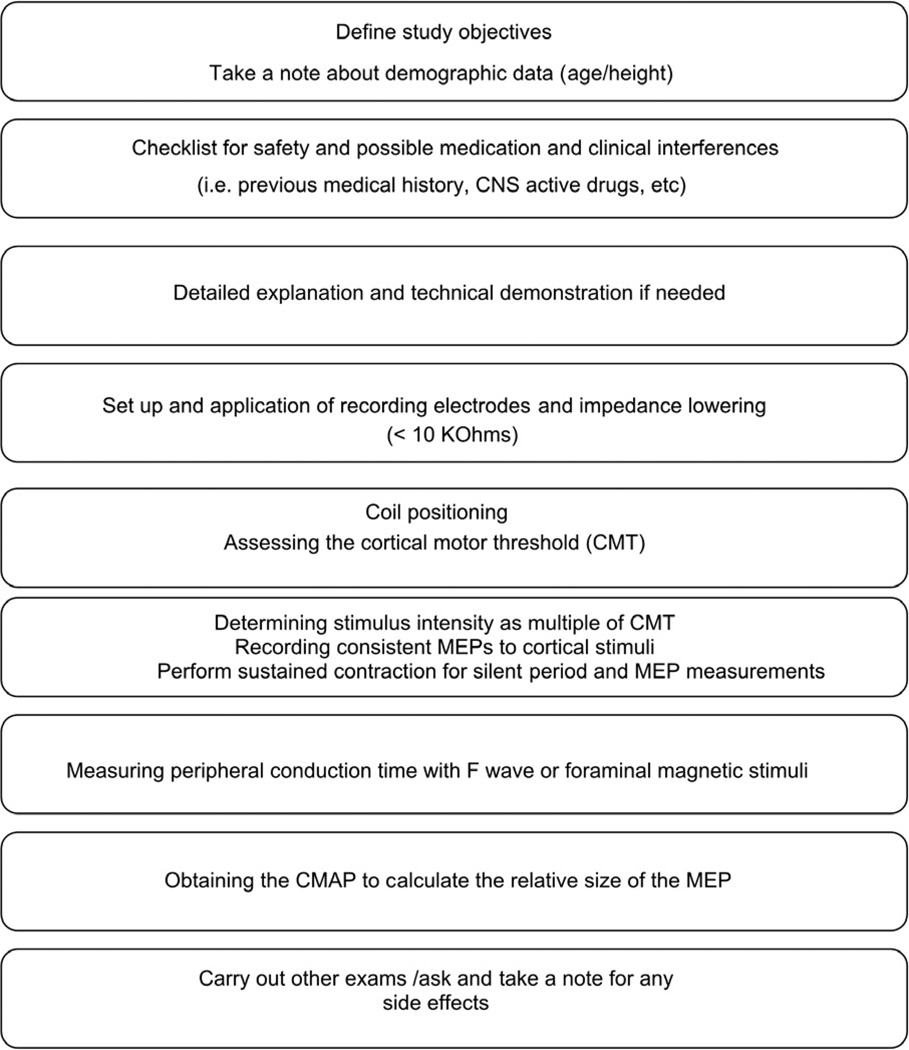
Fig. 2 shows a flow chart to illustrate the steps for a routine TMS examination. The standard clinical examination usually involves bilateral MEP recordings from distal muscles of the hand and leg and may have to be adjusted depending on the clinical question. This also allows definition of excessive side asymmetry for all those clinical conditions affecting only or predominantly one side of the body. The specific procedures for recording MEPs from hand or leg muscles are presented in detail below.
Fig. 2.
Schematic representation of the investigative steps to assess the corticomotor conduction. The order of peripheral and transcranial neurostimulation can be interchanged according to the preferences of the examiner. CMAP: compound motor action potential; MEP: motor evoked potential, CMT: cortical motor threshold.
Patients can be examined while they are seated or lying on a bed or armchair. Although lying may be better for complete relaxation, the sitting position is also comfortable and adequate for all recordings, provided that the seat does not have a large backrest, preventing appropriate handling of the coil. If a patient is examined for the first time, it is advisable to explain to the patient what will happen and to use initially a few stimuli at low intensity (e.g., 20% of MSO) to give the patient the possibility to get used to TMS. It is preferable to ask patients to keep their eyes open to ensure that the subjects remain awake during the examination. It has further been suggested that M1 stimulation is easier when the EEG is less dominated by background alpha activity (Rossini et al., 1991). As in many other neurophysiological procedures the TMS examination should be directed by the patient’s clinical picture including a detailed knowledge of the neurological signs and symptoms. The examiner also needs to know whether the patient is taking medication that might affect the results. It is helpful to have an assistant (technician) participating in the examination. Clinical TMS examinations should be preferably carried out in a medical environment with implemented safety standards and established procedures for eventual emergency interventions in case of TMS-induced seizures.
MEPs are usually recorded with bipolar surface electrodes (e.g., Ag/AgCl cup electrodes) after routine skin preparation and application of electrode gel. As pointed out above, impedance should be less than 5 kΩ, but MEP recordings of sufficient quality can often be obtained at slightly higher impedances between 5 and 10 kΩ. Using a bipolar electrode arrangement, the so-called active electrode is attached to the skin overlying the “motor point” of the target muscle, which is the region with the highest concentration of endplates. Whenever possible, a belly-tendon montage is used with the reference electrode attached to the skin over the muscle tendon. For some muscles (e.g., the orbicularis oris), when a belly-tendon montage is not feasible the reference electrode can be placed 2 cm lateral to the active electrode. The ground electrode is attached over a suitable electrically inactive area between the TMS coil and the surface recording electrodes.
6.1. Transcranial stimulation of the primary motor cortex
Stimulus intensity for TMS should be expressed in relation to the CMT at rest. The resting CMT is firstly determined as mentioned above (see Section 3.3.) and expressed in percentage of the maximal stimulator output. It is convenient to record MEPs from the two sides simultaneously in order to evaluate the small latency difference between sides that is due to the preferential stimulation of one hemisphere. For proper characterization of MEP amplitudes and latency several MEPs should be recorded from homonymous (right and left) target muscles. Care is needed to avoid bending of the cables, which could induce artifacts in the presence of the magnetic field.
There is no universal agreement on the preferred state of the muscle for clinical studies, but a slight tonic contraction is commonly used to increase the efficacy of TMS. The clinical report must state whether the MEPs were recorded with the muscle at rest or during contraction, since the latter affects only the MEP but not the CMAP. If tonic muscle contraction is employed, it is recommended that both contra and ipsilateral muscles are simultaneously contracted. Every laboratory should establish a standard instruction and a monitoring method (e.g., acoustic or visual online feedback of EMG activity) to ensure a standardized procedure across patients. Those patients who can not perform a contraction due to hemiparesis should not be asked to unilaterally preactivate the healthy side as this would artificially induce an asymmetric MEP result.
The first step of the TMS examination is to identify the location on the scalp where TMS elicits the largest single-trial MEP. When using the large round coil with limited focality, it is feasible to use a standard positioning procedure based on external landmarks (Figs. 5–7). This “standard coil position” places the coil over the optimal stimulation site in most of the patients. A clinically feasible way to check whether the standard coil position is optimal for eliciting MEPs in the target muscle is to reduce the stimulus intensity over the standard position to elicit MEPs with an average peak-to-peak amplitude of 0.5–1 mV and then shift the coil 1 cm in the anterior, posterior, medial, and lateral direction while evoking 3 MEPs at each site. If TMS over one of the tested sites clearly evokes larger MEPs as compared to the other site, this site should be used for TMS. Otherwise, the standard coil position should be used for TMS.
Fig. 5.
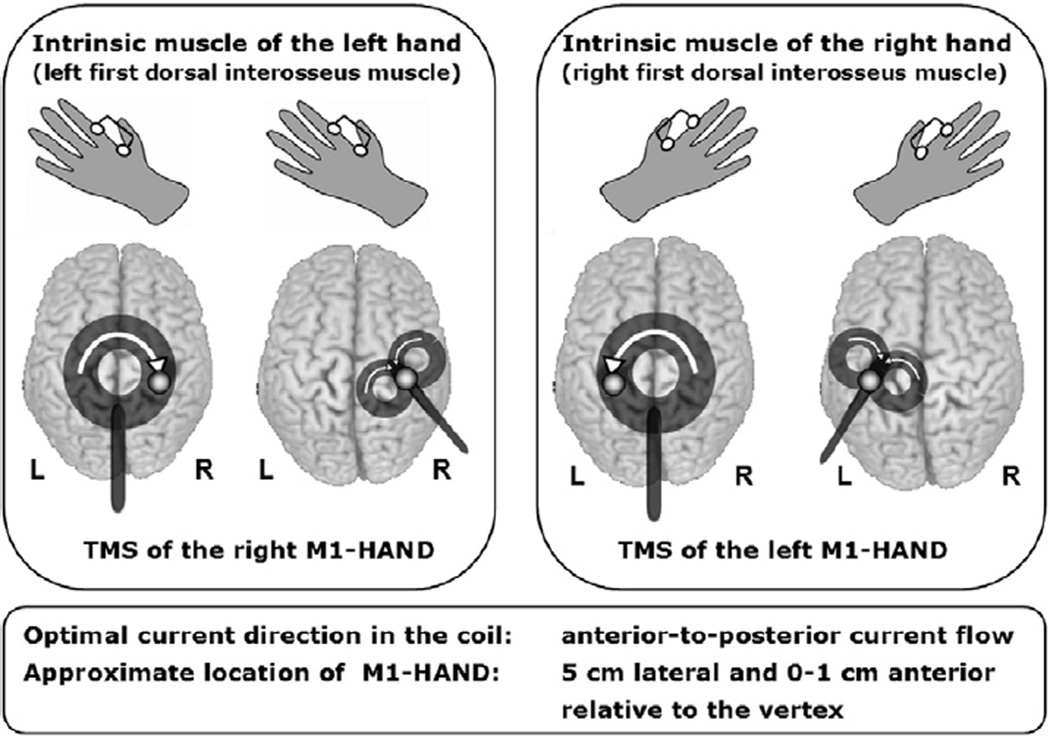
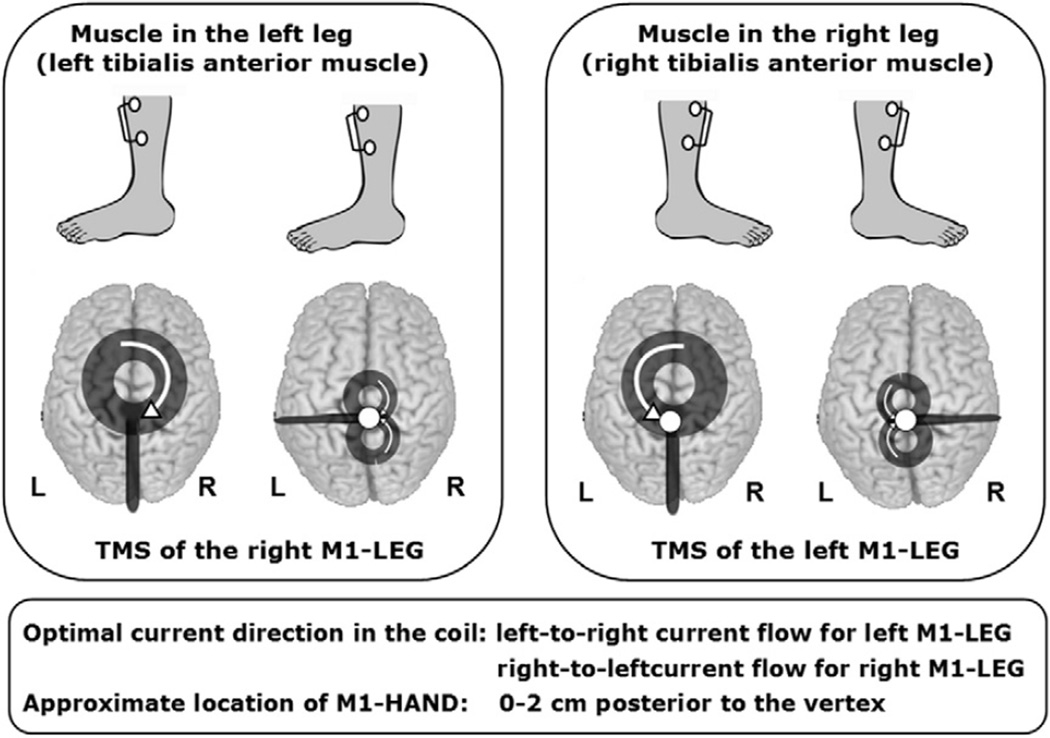
Transcranial stimulation of corticospinal paths to the distal upper extremities, with the round respectively figure-of-eight coil. Point on the coil ring: optimal stimulation spot of the primary motor hand area to the right (A) or left (B), Arrows: technical direction of current flow in the coil. For optimal stimulation of the right motor hand area (derivative of the MEP of an intrinsic muscle of the left hand) should be the direction of current in the coil as seen from above shown clockwise. For the stimulation of the left motor hand area (derivative of the MEP of an intrinsic muscle of the right hand) is made with the round stimulation coil, the technical current flow in the coil is oriented counter-clockwise seen from above. As a starting point in the derivation of hand muscles the coils can be positioned so that the stimulating coil is over the M1, approximately 0–1 cm anterior and about 4–5 cm lateral from the vertex (modified from: (Groppa et al., 2010), with kind permission of Georg Thieme Verlag).
Fig. 7.
Schematic illustration of the facial nerve diagnostic with transcranial M1 stimulation with the round coil; extra-axial canalicular magnetic stimulation as well as supramaximal electrical stimulation at stylomastoid foramen.
Once the coil position for TMS has been defined, the rim of the coil should be marked with a pen on the scalp to enable the examiner to maintain a constant position of the coil. Thereafter, the individual CMT should be estimated at rest (see Section 3.3). Finally, single-pulse stimulation is performed at a pre-determined suprathreshold intensity which should be set to 140% of resting CMT and 5–6 MEPs are recorded from the target muscle during isometric tonic contraction. In most patients, a stimulus intensity of 140% of resting CMT should be sufficient to evoke a maximal motor response. Especially if the MEP amplitude appears to be reduced, it is advisable to apply 5–6 stimuli at a higher stimulus intensity (i.e., 170% of resting CMT) to test whether the MEP amplitude can be increased at higher intensities. Since the primary interest of diagnostic TMS is to estimate the maximal corticomotor response with the shortest corticomotor latency, only the trial with the largest MEP response and the shortest latency is used for analysis. It is not necessary to estimate a characteristic MEP amplitude or MEP latency from all MEP trials.
It is possible to simultaneously elicit MEPs in homologous target muscles of the right and left limb using a large round coil. For instance, a large round coil can evoke MEPs in the left and right hand, if the coil is centered over the vertex with the annulus of the coil overlying the right and left primary motor hand area. It needs to be stressed, however, that the coil needs to be placed differently for optimal stimulation of the two hemispheres as optimal coil positioning can not be satisfied with a single placement. Optimal stimulation is a matter of coil placement and current direction which is different for right and left M1 when stimulating with a round coil centered over the vertex. Therefore, each hemisphere has to be investigated separately and only the MEPs elicited in the contralateral target muscle should be analyzed although MEPs are recorded bilaterally. Please see above for the detailed description of coil placement and current directions (Section 2.3–2.5.).
6.2. Measurements of peripheral motor conduction time
Central motor conduction time (measured in ms) is obtained by subtracting the peripheral nerve component from the total latency obtained by TMS. Measurement of the peripheral component should be performed with the target muscle being fully relaxed. Two main methods are currently used in clinical routine to estimate the conduction time in the peripheral motor nerve (see Fig. 4). One method applies magnetic stimuli over the proximal motor nerve and is referred to as foraminal electromagnetic stimulation (Ugawa et al., 1994). The other method involves the recordings of F-waves to estimate peripheral motor conduction time (Rossini et al., 1994).
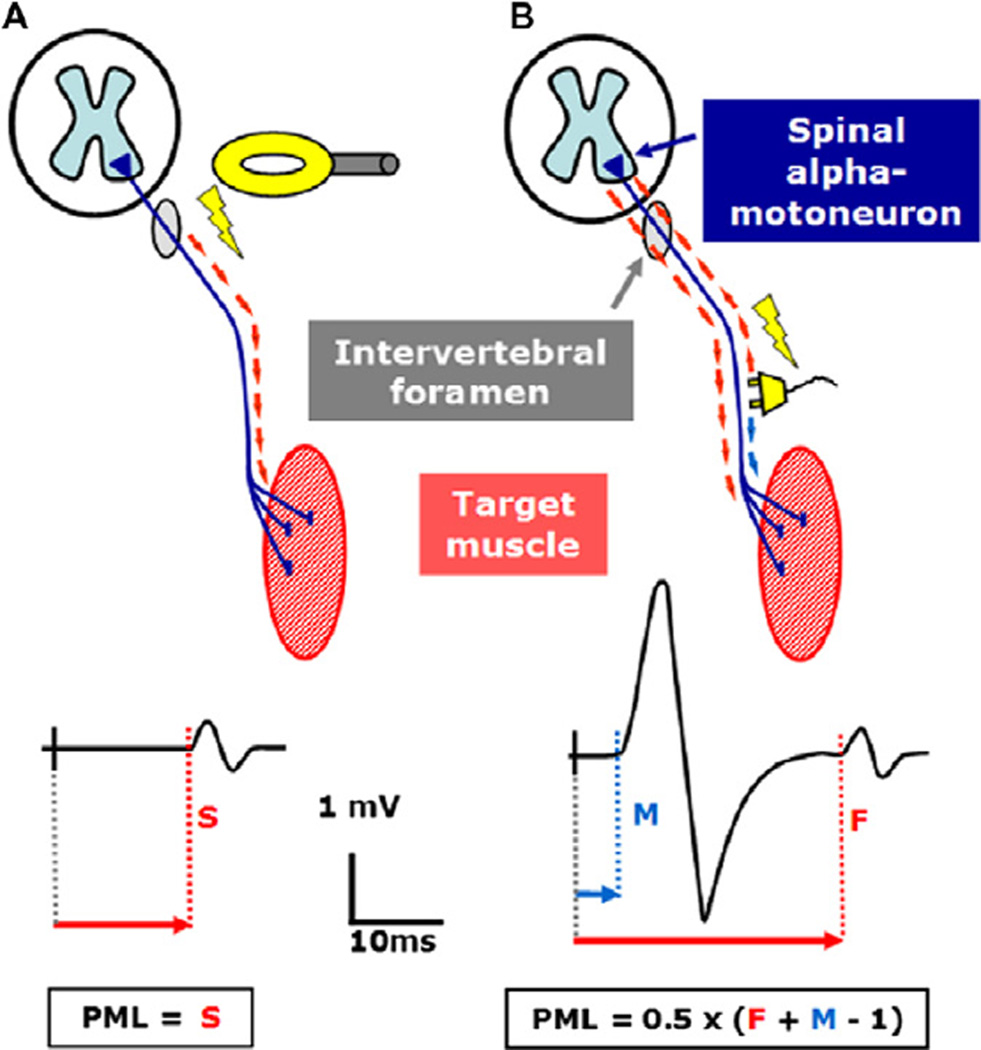
Fig. 4.
Schematic illustration of peripheral motor latency (PML) calculation: (A) electric or magnetic stimulation of the proximal spinal nerve at the level of intervertebral foramen and (B) F-wave method. The studied nerve is electrically stimulated at the vicinity of the studied muscle. The orthodromic response (M-Wave) precedes the antidromic response of the spinal motoneurons (F-wave). F corresponds to the shortest F-wave latency and M equals the distal motor latency. One millisecond is subtracted to account for a central delay at the cell body of the motoneuron (modified from: Siebner HR, Ziemann U. Das TMS-Buch, Heidelberg: Springer Medizin Verlag, 2007, with kind permission of Springer Medizin Verlag).
(1) Foraminal electromagnetic stimulation is performed with the same round coil that is used for TMS of the M1. The coil should be centered over the C7/C8 cervical spine, or over the L1/L2 lumbar spine, with the windings of the coil following the orientation of the target root. The round coil is placed flat on the skin and centered over the body midline or 1–2 cm lateral to the cervical spine. The coil position needs to be adjusted in the perpendicular direction in a way that the windings of the coil overlay the horizontal segment of the spinal nerve in the neuroforamen. The technical current direction in the coil should point towards the target muscle. In this position, magnetic stimulation stimulates the spinal nerve while passing through the neuroforamen. This is because the neuroforamen has a lower electrical resistance as the surrounding bone structures and thus the electrical field lines are focused in the neuroforamen causing a sufficient field gradient for peripheral nerve stimulation.
In contrast to TMS of M1, foraminal magnetic stimulation (Ugawa et al., 1994) should use a relatively low stimulus intensity which is just suprathreshold for giving rise to a small response in the target muscle. It is recommended to use a stimulus intensity that is just suprathreshold for motoneuron stimulation. As a rule of thumb, the amplitude of the foraminally induced CMAP should have a single-trial peak-to-peak amplitude of less than 1 mV. If higher intensities of stimulation are used, the point of nerve stimulation will move distally, inducing an error in the estimation of peripheral motor conduction time (Taylor and Gandevia, 2004). Transcutaneous foraminal magnetic stimulation at low intensity also has the advantage that it is better tolerated by the patients than high intensity stimulation because the concomitant induction of muscle contractions of the trunk is less pronounced.
The CMAP evoked by foraminal magnetic stimulation is used to calculate the peripheral motor latency (PML) which corresponds to the peripheral conduction time from the neuroforamen to the muscle (Fig. 4). The PML is defined as the time from stimulation to the first deflection of the CMAP from baseline. This implies that the conduction time along the very proximal section within the spinal canal is assigned to the central motor conduction time (CMCT). The resulting error in CMCT estimation depends on the length of the nerve section in the spinal canal and amounts to 0.5–1.4 ms for foraminal electromagnetic stimulation over the cervical spine and 3.0–4.1 ms for foraminal electromagnetic stimulation over the lumbar spine.
Foraminal electromagnetic stimulation is also used to assess peripheral motor conduction time of cranial nerves. Again, nerve stimulation takes place in the intraforaminal segment when the nerve is passing through the basis of the skull. For instance, the facial nerve can be stimulated in the proximal segment of the facial canal when the round coil is positioned above and behind the ear (see below). In analogy to foraminal electromagnetic stimulation over the spine, the calculated corticobulbar motor conduction time includes the peripheral motor conduction in the proximal facial nerve in the pontocerebellar angle.
(2) F-waves are elicited by supramaximal peripheral nerve stimulation with the cathode pointing proximally. Due to the variability of F-waves, 10–20 consecutive F-waves need to be recorded and the F-wave with the shortest latency is used for calculation of peripheral motor conduction time. The interval between two consecutive peripheral electrical stimuli should be longer than 2 s. If the F-wave method is used, the examiner has to be aware of the different excitability of the alpha motoneuron to cortical and anti-dromic nerve inputs (the latter usually more effective than the former in generating a response). The F-wave method gives a better approximation of the central motor conduction time than intraforaminal magnetic stimulation, because the F-wave probes peripheral motor conduction along the entire peripheral motor axon, including the intraspinal (proximal) nerve segment. A disadvantage of the F-wave method is that it can only be used to estimate PML in distal but not proximal muscles unless a relatively complicated technique of antidromic collision is employed. Moreover, it only gives information on a relatively small sample of alpha-motoneurons and related motor axons.
Many laboratories prefer foraminal electromagnetic stimulation because it is less painful and requires less time than the F-wave method. However, because conduction in the intraspinal part of the peripheral motor axons contribute to the central rather than the peripheral conduction time it can falsely increase CMCT in patients with nerve root lesions. In these cases, the F-wave method is preferable as the proximal conduction deficit will delay the F-wave latency and thus, increase PML without prolonging the CMCT (Molinuevo et al., 1999). Using both methods may help to distinguish between lesions affecting the cauda equine or the lumbosacral spinal cord (Di Lazzaro et al., 2004b).
A third method for the stimulation of the proximal motoneurons is the transcutaneous electrical stimulation. Using a high-voltage stimulator, it is possible to directly stimulate the proximal segment of the intraspinal motor nerve roots (Claus et al., 1992). The cathode is placed over the spinous process of the target segment, while the anode is placed 5–6 cm cranially for cervical stimulation or over the iliac crest contralateral to the target muscle. Although this method is capable of stimulating peripheral motor axons shortly after they exit the spinal cord, it is rarely used by clinically because it is painful.
6.3. Supramaximal distal nerve stimulation
The electrophysiological examination also includes supramaximal peripheral nerve stimulation. The procedure is identical to standard peripheral motor neurography. An electrical square stimulus is given to the distal peripheral nerve supplying the target muscle via bipolar surface electrodes with the cathode pointing distally. The maximal CMAP is recorded by gradually increasing stimulus intensity until the CMAP amplitude shows no further increase. The peal-to-peak amplitude of the maximal peripheral CMAP is measured to be able to calculate the MEP/CMAP ratio.
7. Analysis of the neurophysiological recordings
7.1. Measurement of MEP size
As pointed out above, the overall configuration of the MEP is more polyphasic and has a longer duration than the peripherally evoked CMAP. In certain disorders MEP duration can be very long due to poor synchrony of neuromuscular excitation, as in patients with multiple sclerosis or peripheral demyelinating disease. Since single-trial MEP amplitude and latency show considerable trial-to-trial variability, 5–6 consecutive MEP traces should be recorded per muscle. The single-trial MEP with the largest amplitude is selected for analysis as this MEP best reflects optimal corticomotor conduction. In contrast to scientific TMS studies on corticospinal excitability, it is not necessary to analyze the amplitude of all MEPs and to estimate the amplitude distribution of all recorded MEPs when TMS is used diagnostically.
The amplitude of a single MEP can be measured in different ways. The simplest way to measure the MEP amplitude is to measure the voltage difference from the maximal negative to maximal positive deflection of the largest MEP (referred to as peak-to-peak amplitude). For distal limb muscles, measurement of peak-to-peak amplitude is accurate enough in most cases, provided MEPs have been appropriately recorded. MEP amplitude is more difficult to determine in proximal arm or leg muscles because the configuration is usually more polyphasic with less clear positive or negative peaks. MEP area may be preferential in some situations but this does not provide information on the synchrony or jitter of the response. The largest single-trial peak-to-peak amplitude (or MEP area) out of the 5–6 trials is used to define the size of the MEP, not the mean value of all recorded MEPs.
The MEP amplitude can be altered by central or peripheral disease of the corticomotor pathway. Moreover, there is a normal reduction in MEP amplitude relative to the maximal peripheral CMAP due to temporal dispersion of neural excitation along the corticomotor pathway. Therefore, the cortically evoked MEP amplitude should always be related to the maximal CMAP amplitude that is obtained in the same muscle by supramaximal distal nerve stimulation. The ratio between the maximal (transcranially evoked) MEP amplitude and the maximal (distally evoked) CMAP should be used as the MEP/CMAP ratio to reflect the central mechanisms contributing to the MEP amplitude. An additional advantage of using the MEP/CMAP ratio is that it reduces inter-subject variability caused by inter-individual differences in peripheral MEP amplitude.
7.2. Measurement of MEP latency
The latency of the MEP reflects the corticomotor conduction time and should be measured from the initial deflection, regardless of its polarity, providing that it is consistent. Initial positive deflections may indicate that the recording electrode is picking up a volume conducted signal from a muscle different from the targeted one. For each muscle, the trial with the shortest MEP latency is selected out of the 5–6 trials. The MEP latency is only measured in the trial with the shortest latency. The duration of the MEP is usually not considered, although increased dispersion suggests abnormal spread of conduction time in the corticospinal tract.
7.3. Estimation of central motor conduction time
The calculation of central motor conduction time (CMCT) for both foraminal electromagnetic stimulation and the F-wave method is explained in Fig. 4. Both methods are currently used in clinical practice. The CMCT is calculated by subtracting the shortest PML which provides a measure of conduction time along the peripheral motor axon from the shortest corticomotor latency (CML) which corresponds to the fastest corticomotor conduction time. When foraminal electromagnetic stimulation is used peripheral motor conduction time corresponds to the PML of the foraminally evoked CMAP. When using the F-wave method, peripheral motor conduction time is calculated according to the formula:
In this formula, F corresponds to the shortest F-wave latency and M equals the distal motor latency. One millisecond is subtracted to account for a central delay at the cell body of the motoneuron. When foraminal electromagnetic stimulation is used peripheral motor conduction time corresponds to the PML of the foraminally evoked CMAP.
7.4. Measurement of the cortical silent period
The CSP is commonly defined as the time elapsing from the onset of the MEP to the resumption of sustained EMG activity (Fig. 3). This implies that the CSP can only be measured when MEP recordings are performed during tonic voluntary activation of the target muscle. Since the CSP can last up to 200–300 ms, care should be taken that the time window of EMG recording is sufficiently long, covering at least 400 ms after the TMS stimulus.
Note that the recording of 5–6 trials using a single intensity level only enables a coarse evaluation of the CSP properties. A larger number of trials (e.g., 20–30 trials) collected would enable a more precise estimation of the characteristic CSP duration based on the underlying statistical distribution. Since CSP is only measured at a single intensity level, the diagnostic measurements provide insufficient information about the relationship between stimulus intensity and CSP duration (Kimiskidis et al., 2005). For instance, it is possible that the CSP duration is only abnormal at lower stimulus intensities but normal at the stimulus intensity used for diagnostic TMS. In other words, the clinical examination which focuses primarily on estimating the maximal MEP amplitude and the shortest corticomotor latency is not tuned towards an optimal estimation of the CSP duration. The clinical CSP measurements are a by-product that might indicate abnormal intracortical inhibition in M1. This information is not provided by the MEP amplitude or latency.
Given these limitations, two strategies can be adopted to estimate the CSP duration. Both strategies use all CSP recordings (5– 6 trials per muscle) to estimate the characteristic CSP duration. The first method calculates the mean CSP duration (or median) based on trial-by-trial measurements of the CSP duration. In a single trial, the CSP is measured as the time elapsing from the onset of the MEP until the recurrence of voluntary tonic EMG activity. Determining the end of the CSP is sometimes difficult as voluntary EMG activity does not recover abruptly but gradually. Further, the TMS pulse may induce two periods of EMG silence interrupted by a short bout of EMG activity. In this case, it is difficult to decide whether the end of the first or second period of EMG silence marks the end of the CSP. Therefore it is important to specify the criteria for defining the end of the CSP on a single trial basis. The following criteria can help to resolve ambiguities in clinical practice: If the EMG activity reaches or exceeds the pre-TMS baseline level and lasts for at least 50 ms, reoccurring EMG activity marks the end of the CSP. In this case, the occurrence of the first visible EMG activity marks the end of the CSP.
An alternative method is to first single trial rectify and then average the collected 5–6 MEP/CSP traces. The rectified and averaged trace provides a good visualization of the voluntary EMG activity level at baseline (i.e., prior to the TMS pulse). Therefore, the end of the CSP can be defined by the reoccurrence of voluntary EMG activity relative to the tonic baseline EMG level. For instance, the end of the CSP may be defined as the time point at which the reoccurring (rectified and averaged) EMG activity exceeds 25% of tonic EMG activity that was measured in the time window preceding the TMS stimulus (e.g., 1–100 ms before TMS). Several groups have successfully applied automatic algorithms to determine the end of CSP based on the pre-stimulus EMG activity level (Daskalakis et al., 2003; Garvey et al., 2001a; King et al., 2006). These methods can be easily integrated in clinical practice and might help to increase the reliability of CSP estimation, both at a single trial basis or when using rectified and averaged traces of multiple trials.
8. Interpretation of the electrophysiological measurements
For clinical examination, every department using TMS should have a table of normative values for CMCT, resting CMT, MEP/ CMAP ratio and CSP if needed, in both absolute values and right-to-left differences. Normative values should be available for each muscle tested and should be divided for decades of age, sex and height. This normative data can be used to define a cut-off value that separates normal and abnormal measurements. The electrophysiological measurements can be judged as being abnormal when a given value is 2.5 or more conservatively 3 SD different from the mean of the normative data acquired in the control group. While often difficult, it is crucial to build a set of control data that will match the specific population to study. Sensitivity and specificity of measurements may suffer if this is not done. A right-left comparison of TMS-related variables is also recommended. This is particularly helpful to detect subtle abnormalities on one side. Again, building-up normative data sets for right-left differences of clinically relevant neurophysiological measures helps to define the border between normal and pathological asymmetries in the neurophysiological recordings.
8.1. Interpretation of cortical motor threshold
In a normal population, although relatively stable between sessions in a given individual, resting CMT varies between subjects with a nearly Gaussian distribution and no correlation with sex or age in adults (Wassermann, 2002). The reasons for this inter-subject variability in resting CMT are not fully understood, but a relevant genetic determination exists (Wassermann, 2002). Sodium channel blocking drugs increase the resting CMT (Siniatchkin et al., 2006) and therefore, genetic polymorphisms of ion channels may contribute significantly to the individual CMT. Excitability of excitatory glutamatergic synapses, which connect the cortico-cortical fibers with the corticospinal neurons, also influences CMT (Di Lazzaro et al., 2003b). A more trivial factor relates to the interindividual thickness of the convexity skull bones which impacts on the distance separating the stimulating coil from the excitable elements (Stokes et al., 2007). Another contributor is the number and density of cortico-cortical axons and corticospinal neurons to a given target muscles. This is highest for hand muscles (Chen et al., 1998). CMT does not correlate with phosphene (visual percept induced by cortical stimulation) threshold over the visual cortex (Di Lazzaro et al., 2003b). Therefore, CMT must not be interpreted as a general excitability marker for cortical areas other than M1.
In patients, CMT will typically increase if a significant portion of the corticospinal tract is damaged, such as in cerebral stroke (McConnell et al., 2001), advanced motoneuron disease (Chen et al., 1998), or spinal cord injury. In contrast, CMT will typically decrease in situations of a hyperexcitable corticospinal system, such as in early stages of ALS (Desiato et al., 2002), or in untreated patients with idiopathic generalized epilepsy (Groppa et al., 2008; Reutens et al., 1993). When interpreting CMT data, the patient’s medication should be taken into account because some centrally active drugs can alter CMT (Siniatchkin et al., 2006; Ziemann, 2004). While absolute CMT shows large inter-subject variability, the variability of interhemispheric differences in CMT is less marked (Cicinelli et al., 1997a). Therefore, a right-to-left comparison of CMT may increase sensitivity of detecting subtle alteration, e.g., in case of hemispheric lesions such as in stroke (Traversa et al., 1997).
8.2. Interpretation of MEP amplitude
The MEP amplitude elicited at 140% of resting CMT reflects a more complex and indirect measure of TMS-induced activation of the corticospinal neurons with a predominant contribution by late I-waves. In a clinical setting, the most accurate strategy to interpret the MEP amplitude is to express it relative to the maximal CMAP in the same muscle elicited by supramaximal peripheral nerve stimulation, and to compare inter-side differences. The advantage of this approach is that it allows to approximate the maximum percentage of the total motoneuron pool activated by a single stimulus to the cortex (note that these values are higher for distal than proximal arm or leg muscles).
The clinical interpretation of the MEP amplitude is complicated by the fact that temporal dispersion of corticospinal conduction has a strong influence on the recorded MEP amplitude as phase cancelation reduces due to asynchronous excitation of the motor units the amplitude of the MEP. This explains why the ratio between MEP and peripherally evoked CMAP amplitude is almost always well below 1 and shows substantial inter-subject variability (Magistris et al., 1998). In hand muscles, only a MEP/CMAP ratio <15% is considered abnormal (Hess et al., 1987). If the MEP/CMAP ratio in a given muscle is more than 2.5 or 3 standard deviation (SD) below the mean of the normative data this typically indicates loss of cortico-motoneuronal cells, e.g., due to cerebral stroke or compressive myelopathy, or neurodegeneration, such as motoneuron disease. In case of a reduced MEP/CMAP ratio, it is recommended to increase the TMS intensity (e.g., from 140% to 170% of resting CMT) to see whether a higher stimulus intensity increases (normalizes) the MEP/CMAP ratio.
It is important to note that increased chronodispersion of corticospinal conduction, e.g., due to demyelinating disorders such as multiple sclerosis will typically also cause a decrease in MEP/CMAP ratio because of prominent phase cancelation. Finally, centrally active drugs can also lead to significant depression of the MEP/CMAP ratio and, therefore, current medication should be interrogated (Paulus et al., 2008; Ziemann, 2004). The problem of phase cancelation can be overcome by using the triple stimulation technique (TST) which is described in detail below. As for resting CMT, inter-subject variability of inter-hemispheric differences in MEP amplitude is less than inter-subject variability of absolute MEP amplitude (Cicinelli et al., 1997a). Therefore, a right-to-left comparison of MEP amplitudes in corresponding muscles may help to detect subtle abnormalities in MEP amplitude, for instance after a unilateral lesion in stroke (Traversa et al., 1997).
8.3. Interpretation of central motor conduction time
The most parsimonious reason for a pathological lengthening of CMCT are disorders that cause a demyelination of the fastest-conducting cortico-motoneuronal fibers, in particular multiple sclerosis (Hess et al., 1986; Currà et al., 2002). However, another frequent cause of CMCT lengthening is axonal destruction or degeneration of fastest-conducting cortico-motoneuronal fibers, such as in stroke, motoneuron disease or compressive myelopathy. Axonal loss in the corticospinal tract may lengthen CMCT by a few milliseconds simply due to impaired temporal and spatial summation of excitatory postsynaptic potentials from weakened corticomotoneuronal input at spinal motoneurons. If CMCT is not tested by the F-wave method, an apparent CMCT prolongation can also be found in conditions with reduced conduction velocity in the intraspinal segment of the proximal nerve such as chronic inflammatory demyelinating radiculoneuropathy (Ormerod et al., 1990; Pineda et al., 2007).
8.4. Interpretation of contralateral cortical silent period
There are substantial inter-individual differences in CSP duration, even in healthy subjects. This renders difficulties to establish a normative data set that is clinically useful. Fortunately, the left-to-right asymmetry of the CSP is rather small in healthy subjects, rarely exceeding 20%. Therefore, it is more meaningful to focus on relative right-to-left differences in estimated CSP duration rather than the absolute amplitude of the MEP recorded under muscular contraction.
The CSP may be shortened or even abolished in conditions of damaged inhibitory interneurons in M1. This was observed in particular in stroke patients with circumscribed motor cortical ischemic infarcts (Schnitzler and Benecke, 1994). In contrast, stroke patients with infarcts outside the M1 often have a lengthened CSP, likely due to a pathologically reduced excitatory afferent drive of M1 from the affected cortical areas (Schnitzler and Benecke, 1994) or deficits in motor attention (Classen et al., 1997; von Giesen et al., 1994).The CSP may be abnormal in many other pathological conditions which have been reviewed elsewhere (Cantello, 2002). However, the diagnostic use of measuring the CSP in individual patients is limited.
9. Examination of corticomotor conduction to hand and forearm muscles
The corticomotor projections to the hand are the easiest to study with TMS because the large cortical motor representation and location on the hemispheric surface (Fig. 5). In most adults the scalp site overlaying the M1-HAND is approximately one-third of the distance from the vertex to the pre-auricular crease (usually 5–6 cm lateral from the vertex along the inter-aural line and 0–1 cm rostral to the inter-aural line (Wilson et al., 1993b).
9.1. Stimulation
For diagnostic TMS, it is recommended to use a large circular coil centered on the vertex. The motor hand area can be easily stimulated with a circular coil, centered over the vertex and one edge of the coil tilted slightly towards the hemisphere to be stimulated. When using a monophasic pulse waveform, current flow in the part of the coil over the M1-HAND to be stimulated should run antero-posterior (Fig. 5). This induces a corresponding postero-anterior current in the underlying brain.
If TMS is performed with a focal figure-of-eight coil, the juncture of the two wings is centered over the M1-HAND 5 cm lateral and 0–1 cm anterior to the vertex. Fig. 5 illustrates the appropriate coil positions for the round and figure-of-eight coil for the right and left M1-HAND, respectively. Both coil designs stimulate the M1-HAND readily when the coil handle is held in the parasagittal plane. However, it is preferable to align the handle of the figure-of-eight coil at approximately 45° to the parasagittal plane with the coil handle pointing postero-laterally to induce a tissue current that runs perpendicular to the motor strip in the precentral sulcus (Fig. 5).
9.2. Recordings
MEPs can readily be recorded from intrinsic hand muscles, whereas the anatomical arrangement of extrinsic hand muscles in the forearm can make it difficult to record a MEP from the target muscle in isolation, resulting in polyphasic waveforms. In clinical practice, the most frequently examined arm muscles are the small hand muscles such as the first dorsal interosseous (FDI), abductor pollicis brevis (APB) and abductor digiti minimi (ADM) muscle. The FDI muscle is a good choice as it is small, localized and identifiable by palpation. Common target muscles for the forearm are the flexor carpi radialis (FCR) and extensor carpi radialis (ECR) muscle, but again expect polyphasic responses. For more proximal muscles such as biceps, motor threshold will be elevated and MEPs will be smaller and less well defined due to the greater spatial distribution and smaller central representation. The cortical representation of a proximal muscle such as deltoid is ~1.5–2 cm medial to that of a hand area. MEPs are elicited at lower stimulus intensities from distal as opposed to proximal limb muscles, and MEP recordings from proximal muscles may be more difficult because of less synchrony of the corticomotoneuronal discharge.
9.3. Interpretation
The CMCT is the most relevant electrophysiological measure regarding corticospinal integrity. Table 1 gives an overview of the published data on CMCT to the upper limb muscles in healthy individuals. The normal values depend on the different methods used to estimate peripheral motor conduction time (see Section 7.3.). The values are given in Table 1. Another relevant neurophysiological measure is the MEP amplitude which should always be related to the maximum amplitude of the target muscle CMAP (see previous Section 8.2).
Table 1.
Normative data for the upper limb examination.
| Target muscle | Method | CML | PML | CMCT | References |
|---|---|---|---|---|---|
| First dorsal interossus | Cervical MS | 20.6–21.2 ± 1.8* | 14.0–14.9 ± 1.4* | 5.8–6.5 ± 1.1* | Kloten et al. (1992) |
| Cervical MS | No data | 13.2 ± 1.5 | No data | Britton et al. (1990) | |
| F-wave method | No data | 14.5 ±1.4 | No data | Britton et al. (1990) | |
| Abductor digiti minimi | Cervical MS | 18.8 ±1.2 (f) | 11.8 ± 1.0 | 7.0 ± 0.8 | Chu (1989) |
| Cervical MS | 19.7 ± 1.0 (m) | 12.7 ± 1.1 | 7.1 ± 1.1 | Chu (1989)) | |
| Cervical MS | 14.0 ± 1.5 | 6.0 ± 0.9 | Claus (1990) | ||
| Cervical MS | 20.5 ±1.2 | 7.0 ± 0.9 | Furby et al. (1992) (21–54 years) | ||
| F-wave method | 6.1 ± 1.0 | Furby et al. (1992) (21–54 years) | |||
| F-wave method | 7.2 ± 1.2 | Cicinelli et al. (1997a) | |||
| F-wave method | 5.8 ± 0.8 | Claus (1990) | |||
| Abductor pollicis brevis | Cervical MS | 21.8 ± 1.8 | 14.4 ± 1.4 | 7.2 ± 1.8 | Tabaraud et al. (1989) |
| Cervical MS | 21.4 ±1.5 | 14.8 ± 1.2 | 6.6 ±1.4 | Ludolph et al. (1989) | |
| Cervical MS | 20.2 ±1.6 | 7.9 ± 2.1 | Eisen and Shtybel (1990) | ||
| F-wave method | 5.66 + 0.84 | Rossini et al. (1992) (16–35 years) | |||
| F-wave method | 5.45 + 0.72 | Rossini et al. (1992) (51–86 years) | |||
| Biceps brachii | Cervical MS | 10.8–11.4 ± 1.3* | 6.3–6.8 ± 1.1* | 4.5–4.6 ± 1.0* | Kloten et al. (1992) |
| Cervical MS | 12.5 ± 1.2 | 7.1 ± 1.1 | Furby et al. (1992) (21–54 years) | ||
| Cervical MS | 9.4 ± 1.7 | 6.0 ±1.2 | Eisen and Shtybel (1990) |
Cervical MS – cervical foraminal magnetic stimulation; CML – Corticomotor latency; PML – Peripheral motor latency; CMCT – Central motor conduction time.
Increase with age (age range: 19–60 years; (f) females; (m) males).
Age and sex have negligible effects on the MEP measures. While the cortical motor threshold show a slight increase and the normalized MEP amplitudes a decrease with increasing age, the CMCT shows no significant age effect provided that stimulus intensity is sufficiently high.
10. Examination of corticomotor conduction to leg muscles
Although generally possible, evoking MEPs in leg muscles is more difficult than in hand muscles and stimulus intensity needs to be relatively high. The factors rendering it more difficult to obtain MEPs in leg muscles include the deeper location of the M1-LEG in the interhemispheric fissure, less intense monosynaptic cortico-spinal projections from M1-LEG to the spinal motor neurons (Porter and Lemon, 1993), and a possible difference in the direction of the axons so that they are more difficult to stimulate transsynaptically. Finally, the long central conduction distance for leg muscles translates into less optimal summation of the descending volley associated with more temporal dispersion.
10.1. Stimulation
Anatomically the M1-LEG is located close to the midline approximately 1–2 cm posterior to the vertex in the depth of the interhemispheric fissure although this may vary considerably between subjects (Fig. 6). Because the M1-LEG is more difficult to target with TMS, the use of a circular coil is recommended for the standard clinical examination. Some laboratories use a specially designed double-cone coil to examine M1-LEG, but a standard circular coil is sufficiently strong to stimulate the corticospinal projections to the anterior tibial muscle. Using a monophasic pulse waveform, the corticospinal output neurons in the M1-LEG are stimulated most effectively if the induced current in the tissue runs in the coronal plane. For stimulation of the left M1-LEG, the optimal current direction in the coil should have a left-to-right orientation, inducing a right-to-left oriented current in the tissue (Fig. 6). Conversely, when stimulating the right M1-LEG, the current in the coil should flow from right to left, inducing a left-to-right oriented current in the tissue (Fig. 6). In some healthy individuals (<10%), it might be difficult to elicit a reliable MEP in the anterior tibialis muscle at maximal stimulator output when using a standard round coil and a monophasic pulse. In this instance, the use of a double cone coil with angulated wings might be advantageous. This coil is more efficient although it induces a current in the sagittal direction which is not optimal for M1-LEG stimulation. If only a standard round coil is available, TMS should be given at maximal stimulator output during a slight tonic activation of the target muscle.
Fig. 6.
Transcranial stimulation of corticospinal paths to the distal lower extremities with the circular and figure-of-eight coil respectively. Point on the coil ring: optimal stimulation spot of the primary motor leg area right (A) or left (B), Arrows: technical direction of current flow in the coil. For stimulation of right hemisphere leg area (derivation of MEP from a leg or foot muscle of the left leg), the current direction proceeds is in the coil portion, which rests on the leg area, from right to left. For stimulation of the left hemisphere leg area (derivation of MEP from a leg or foot muscle of the left leg), the current direction proceeds in the coil portion, which rests on the leg area, from left to right. The coil center is about 5–6 cm in front of the vertex and the coil whorls of the posterior coil portion lie about 1–2 cm behind the vertex over the primary motor leg area on the skull (modified from: (Groppa et al., 2010), with kind permission of Georg Thieme Verlag).
10.2. Recordings
The principles for MEP recordings from the upper limb can be applied for the analysis of leg MEPs (see Sections 8.2 and 9.2). The anterior tibial muscle is a good target muscle it has a more pronounced representation than most other leg muscles (Petersen et al., 2003). The tibialis anterior MEPs can be obtained at a relatively low threshold and the MEP has a larger amplitudes compared to other leg muscles (Petersen et al., 2003). Contraction-induced facilitation may sometimes be needed to obtain reliable MEP recordings (Valls-Sole et al., 1994).
The peripheral component required to calculate the CMCT is obtained by supramaximal electrical stimulation of the peroneal nerve at the head of the fibula (with the cathode pointing proximally) to obtain an F-wave (not as easy as for hand muscles) or by foraminal electromagnetic stimulation with the circular coil over the lumbar spine with the target muscle at rest. Patients should slightly bend the trunk forward to facilitate foraminal electromagnetic stimulation over the lumbosacral region. The stimulation coil is tangentially placed flat on the skin over the lower lumbar spine, with the windings of the coil following the orientation of the target root. The circular coil is usually centered over the body midline or 1–2 cm laterally towards the side of the target muscle. The coil can be moved vertically up and down to determine the most effective level for stimulation. Current direction in the coil should point towards the target muscle so that the spinal nerve is stimulated while passing through the neuroforamen.
10.3. Interpretation
The CMCT is the most relevant electrophysiological measure regarding corticospinal integrity. Table 2 summarizes the published data on CMCT to lower limb muscles that have been estimated in healthy individuals. The normal values depend on the different methods used to estimate peripheral motor conduction time (see Section 7.3.). The data listed in Table 2 illustrates the normal variation in CMCT, but the values cannot replace the requirement that each laboratory needs to establish its own normative data with respect to the upper normal limit of CMCT and maximal side-to-side differences. The body height has to be considered when defining the normal physiological range because CMCT increases linearly with increase in body height.
Table 2.
Normative data for the lower limb examination. Overview of published normative data on central motor conduction time (CMCT) for the lower limbs using M. tibialis anterior as target muscle. In all studies a standard circular coil and a monophasic pulse configuration was used for TMS of the M1-LEG. Data is given as mean ± SD: standard deviation.
| Reference | Group description number (age range) | Central motor conduction time (lower limb) |
||
|---|---|---|---|---|
| Mean±SD (ms) | Upper normal limit (ms) | Side-to-side difference (ms) | ||
| F-wave method | ||||
| Claus et al. (1991) | n = 45 (18–71 years) | 9.7 ±2.7 | 16.5 | 4.5 |
| Furby et al. (1992) | n = 50 (21–54 years) | 9.9 ± 1.8 | 1 | |
| Rossini et al. (1992) | n = 25 (16–35 years) | 12.7 + 1.20 | 1 | |
| n = 40 (51–86 years) | 12.9 + 1.69 | 2 | ||
| Lumbar (foraminal) electromagnetic stimulation | ||||
| Chu (1989) | n = 52 (17–35 years) | 14.8 ± 1.1 | 17.6 | No data |
| Kloten et al. (1992) | n = 18 (19–29 years) | 13.4 ±1.9 | 18.2 | 1.6 |
| n = 21 (30–59 years) | 16.1 ± 1.9 | 18.5 | 2.2 | |
| n = 18 (>60 years) | 13.4 ±1.9 | 20.9 | 2.0 | |
| Furby et al. (1992) | n = 50 (21–54 years) | 13.8 ± 1.5 | 0.9 | |
| Electrical high-voltage stimulation | ||||
| Claus (1990) | n = 54 (19–59 years) | 12.5 ± 1.7 | 16.7 | 3.6 |
11. Examination of corticomotor conduction to the facial muscles
A large section of the facial nerve is located intracranially and peripheral electrical stimulation can only excite its extracranial part. TMS significantly extends the neurophysiological possibilities. TMS can be used to demonstrate a peripheral dysfunction in the extramedullary, intracranial portion of the facial nerve and to assess corticomotor conduction to facial muscles (Fig. 7). In conjunction with supramaximal peripheral nerve stimulation, the TMS examination can identify the anatomical level of damage and also provides information regarding etiology and prognosis. Importantly, TMS permits an immediate differentiation between an isolated central and an incomplete peripheral facial paresis affecting predominantly the branches supplying the lower face muscles.
11.1. Stimulation
11.1.1. Extracranial stimulation
Initially a supramaximal, peripheral electrical stimulus is applied to the facial nerve at the stylomastoid foramen (Fig. 7). The cathode (active electrode) is placed at the stylomastoid fossa, just anterior and below the mastoid process. The anode is positioned to minimize stimulus artifact and inadvertent masseter stimulation. Stimulus intensity is gradually increased until no more increase in CMAP amplitude is observed. The target muscle should be completely relaxed. The supramaximal CMAP is used to calculate the peripheral motor latency and to estimate the excitability of the peripheral axons of the facial nerve.
It has two advantages to start with supramaximal peripheral stimulation of the facial nerve. In case of severe peripheral axonal damage, the peripherally induced supramaximal CMAP will be reduced in amplitude or absent. This might lead to a situation where it would be impossible to elicit a response with TMS. Second, the simultaneous CMAP recording from the non-stimulated contralateral muscle will indicate if there is substantial volume conduction across the face. In this instance, the recording electrodes should be replaced until such responses are no longer recordable.
11.1.2. Intracranial extra-axial stimulation
The next step involves extra-axial electromagnetic stimulation of the facial nerve. The magnetic coil should be placed flat over the ipsilateral parieto-occipital region with the lower part of the coil over the mastoid process (Fig. 7). The facial target muscle should be relaxed. When using a flat circular coil and a monophasic pulse configuration, the current induced in the coil (viewed from above) should have a clockwise orientation for right-sided stimulation. Conversely, the current in the coil should have a counter-clockwise direction (viewed from above) for stimulation of the left facial nerve.
The exact site of facial nerve excitation by magnetic stimulation remains controversial, but it possible lies within the facial canal. This segment of the facial nerve is most susceptible to the induced electrical current because there is a marked change in the electrical conductivity of the surrounding tissue when the facial nerve enters the petrous bone (Schmid et al., 1992).
In healthy subjects, one can usually elicit a maximal motor response with stimulation at relatively low stimulus intensity below 40% of maximal stimulator output. If the stimulus intensity is too high, electromagnetic stimulation may spread peripherally, resulting in excitation of the peripheral facial nerve distal to the stylomastoid foramen. In line with a canalicular site of nerve excitation, ipsilateral parieto-occipital magnetic stimulation causes an ipsilateral CMAP with a latency of around 5 ms which is referred to as canaliculomotor latency.
11.1.3. Cortico-nuclear stimulation
TMS is applied over the contralateral face representation in the M1 (M1-FACE) to induce MEPs in facial muscles. As for TMS of M1-HAND or M1-LEG, TMS of M1-FACE can be performed with a circular or figure-of-eight coil (Cruccu et al., 1997). The scalp area where TMS yields a motor response in a given facial muscle is rather limited compared to the surface maps of intrinsic hand muscles. The cortical motor threshold is also higher for TMS of M1-FACE than M1-HAND. Therefore it may be sometimes difficult to obtain reliable MEPs with TMS of the cortical face representation even in normal subjects, especially at rest. This is the reason why the recording of facial MEPs should be performed during voluntary contraction of the target muscle (Rosler et al., 1994). When TMS of the M1-FACE is performed with a circular magnetic coil, the coil is positioned tangentially on the scalp with the coil centered 2 cm laterally on a line from the vertex to the external auditory meatus (Fig. 7). The coil is then moved stepwise (0.5 cm) anterior, posterior, medial and laterally until TMS produces the best motor response. If the TMS pulse has a monophasic configuration, the current in the part of the circular coil overlying the M1-FACE should flow from anterior to posterior. For stimulation with a circular coil, this implies that the current in the coil should flow in a clockwise direction (viewed from above) for TMS of the right M1-FACE, and in a counter-clockwise direction (viewed from above) for TMS of the left M1-FACE.
11.2. Recordings
The motor responses elicited in the facial muscles are measured as CMAP with surface electrodes with the active electrode placed over the muscle of interest and the reference electrode approximately 2 cm apart on the ipsilateral side of the face. Although EMG recordings can be made from several facial muscles, it is advisable to use always the same facial muscle as target muscle for studying corticofacial motor conduction. This will help to establish an optimally standardized procedure and a sufficiently large normative data set. Recordings of MEPs should always be performed bilaterally to allow for side-to-side comparison. It has to be kept in mind that the central conduction time to facial muscles is usually derived from motor responses obtained at rest to intra-cranial and extracranial peripheral stimulation and during a slight tonic contraction to cortical stimulation.
The nasal muscle, orbicularis oris muscle (lateral part), levator labii superioris muscle, and buccinator muscle are all suitable target muscles, and the MEPs from these have a clear negative onset which is important to determine the MEP latency. If the superior orbicularis oris muscle is used as target muscle, the active surface recording electrode is placed 1 cm lateral from the corner of the mouth and the reference electrode 2 cm laterally. The active surface electrode for recordings from the levator labii superioris muscle is attached to the lower third of the nasolabial fold with the reference electrode being placed over the superior part of the nasolabial fold. For recordings from the nasal muscle, the active electrode is placed on the ala of the nose and the inactive electrode on the tip of the nose to minimize volume-conducted activity from other facial muscles. The motor responses from the buccinators muscle can be readily recorded with specially devised surface electrodes attached to the inner surface of the cheek. For side-to-side comparison, it is important that the active and reference electrodes are strictly mirrored to avoid artifactual recorded asymmetries. The ground electrode can be attached to the forehead or above the zygomatic arch. In order to monitor a concomitant activation of the motor trigeminal branch, in some cases, additional recordings from the masseter muscle might be helpful.
The frontalis muscle, orbicularis oculi muscle and mentalis muscle should be avoided as target muscles. The frontalis muscle is too close to the stimulating coil which often causes substantial artefacts in the recording. Motor responses evoked in the orbicularis oculi muscle by TMS of M1-FACE are often confounded by the R1 component of the blink reflex. The mentalis muscle is located too close to the face midline and has fibers that cross the midline.
11.3. Interpretation
The cortical motor projections to facial muscles are stimulated at three different levels: at the cortical level, within the internal acoustic meatus and peripherally just distal to the stylomastoid foramen. This enables the clinician to assess conduction in the corticofacial, extra-axial to intracranial and extracranial segments of the facial nerve. The CMCT can be calculated by subtracting the corticomotor latency (CML) from the canaliculomotor latency (Ca-ML). Note that the CMCT also includes the peripheral conduction of the proximal nerve segment until the internal acoustic meatus. The transosseal conduction time (TOCT) equals the canaliculomotor latency (Ca-ML) minus the distal motor latency (DML).
The MEP latency after cortical TMS is around 10 ms. The MEPs are small and more polyphasic than CMAPs to supramaximal peripheral stimulation of the facial nerve. Therefore, the MEP amplitude is not used for interpretation of the cortical evoked facial MEPs. Facial MEPs may be difficult to interpret due to artifacts from uncrossed ipsilateral MEPs, by blink and other facial reflex contamination, by simultaneous peripheral stimulation of the ipsilateral facial nerve, and possibly by responses from other neural structures such as muscles innervated by the trigeminal nerve (Paradiso et al., 2005). Please consider Table 3 for normative data.
Table 3.
Normative data for the facial muscles examination.
| References | n | Target muscle | DML (ms) | TOCT (ms) | CML (ms) | Ca-ML (ms) | CMCT (ms) |
|---|---|---|---|---|---|---|---|
| Rosler et al. (1989) | 12 | Nasal muscle | 3.7 ± 0.3 | 1.2 ±0.2 | 10.0 ± 1.0 | 4.9 ± 0.4 | 5.1 ± 0.6 |
| Riepe and Ludolph (1993) | 25 | Orbicularis oris muscle | No data | No data | 11.0 ± 0.8 | 4.5 ± 0.4 | No data |
| Urban et al. (1997) | 43 | Buccinator muscle | 2.9 ± 0.3 | 1.3 ±0.3 | 10.3 ± 1.1 | 4.3 ± 0.5 | 5.8 ± 1.0 |
Data is given as mean ± SD: standard deviation. DML (distal motor latency), corticomotor latency (CML) from the canaliculomotor latency (Ca-ML), and the transosseal conduction time (TOCT) are presented.
12. Diagnostic TMS of other non-routine muscles of the trunk
12.1. Respiratory muscles
In humans, TMS of the M1 can be used to activate phrenic motor nuclei in the cervical spinal cord via rapidly conducting mono- or oligosynaptic pathways from the M1 to the inspiratory motoneurons (Lissens, 1994). MEPs should be recorded with surface electrodes, with the active electrode at the xiphoid process, the reference electrode on the lower border of the rib cage at the midclavicular line, and the ground electrode over the sternum. This recording method is to be preferred as it has been shown to be the best in the studies of phrenic nerve conduction. Using a figure-of-eight coil, the coil center should be placed 3 cm lateral to the midline and 2 cm anterior to the vertex for stimulation of the cortical representation of the right and left hemidiaphragm, respectively (Maskill et al., 1991). Using a double cone coil, the best place for stimulation is around the vertex (Sharshar et al., 2004). TMS is more effective to evoke a motor response during a deep inspiration. Eliciting a MEP with the diaphragm relaxed is difficult but would be useful to evaluate patients under mechanical ventilation (Sharshar et al., 2003). For measurement of peripheral conduction time, the proximal nerves are stimulated in the neuroforamen at the cervical (C4) level and the central motor conduction time is calculated in the usual way.
TMS of the diaphragm has potential for evaluating respiratory function, especially in patients with lesions of the higher cervical cord or brainstem, but presently large scale studies are lacking (Lissens and Vanderstraeten, 1996). Since the trial-to-trial variability of latency and CMCT of MEPs recorded from the diaphragm is small and reliable, these measures are used for the diagnosis and follow-up of neurological and respiratory disorders affecting the central motor conduction properties of the diaphragm. In contrast, MEP amplitudes show considerable variability and have little clinical application.
12.2. Laryngeal muscles
TMS can be used to assess corticomotor conduction to the laryngeal muscles (Rödel et al., 2004; Sims et al., 1996). Due to bilateral descending projections form the M1 to the laryngeal motoneurons, unilateral TMS of the M1 elicits bilateral MEPs in laryngeal muscles. The laryngeal M1 representation is not clearly segregated from the face representation of M1. Indeed, the sites where TMS can elicit laryngeal responses largely overlaps with the M1-FACE (Rödel et al., 2004). The optimal stimulation site for eliciting MEPs in contralateral laryngeal muscles was reported to be approximately 8 cm lateral and 1 cm anterior to the vertex (Khedr and Aref, 2002). The optimal site for stimulating the vocalis muscle has been fond to be located more laterally (approximately 10 cm lateral from the vertex along the interaural line) than the cricothyroid muscle (approximately 7.5 cm lateral from the vertex along the interaural line) (Rödel et al., 2004). Accordingly, reliable MEP responses can be elicited in the cricothyroid muscle with a large circular coil centered over the vertex (Ertekin et al., 2001). TMS of laryngeal muscles may provide diagnostically useful information in patients with laryngeal dysfunction, for instance in patients with vocal-fold motion restriction, yet large scale clinical studies are lacking.
The recording of laryngeal MEPs requires the use of needle electrodes because the larynx is covered by thick layers of extralaryngeal muscles. If surface electrodes were used to record laryngeal MEPs, TMS evoked motor activity in these superficial muscles would obscure the laryngeal MEP. Needle electrodes can be inserted in the target muscle under endoscopic control transorally or transcutaneously. The needle electrode might dislocate during the examination due to swallowing or neck movements. Correct position of the needle electrodes has to be continuously monitored and the position might have to be adjusted during the examination. This invasive procedure may cause inconvenience because of pain and salivation. Using needle electrodes, MEPs can be recorded bilaterally from the cricothyroid muscle (supplied by the superior laryngeal nerve) as well as the vocalis muscle (supplied by the recurrent laryngeal nerve) (Ertekin et al., 2001; Rödel et al., 2004).
Since laryngeal MEPs can only be recorded with needle electrodes, only the corticomotor latency can be used to assess corticomotor conduction to the laryngeal muscles. The MEP amplitude is not considered. The ipsilateral and controlateral MEPs recorded from the cricothyroid muscle show bilateral symmetrical corticomotor latencies. Using a figure-of-eight coil, (Rödel et al., 2004) elicited MEPs with a mean corticomotor latency of 10.8 ± 1.7 ms and 10.1 ± 1.5 ms in the ipsi- and contralateral cricothyroid muscle. For the vocalis muscle, corticomotor latency shows a right–left difference which is due to the different length of the left and right recurrent laryngeal nerve. The corticomotor latency of the MEPs recorded from the left vocal muscle shows a physiological delay compared to the MEP of the right vocal muscle. This is due to a longer peripheral conduction time because the left recurrent laryngeal nerve passes below the aortic arch. Unilateral TMS with a focal figure-of-eight coil elicited MEPs with mean latencies of 10.7 ± 1.7 ms (ipsilateral TMS) and 10.8 ± 1.8 ms (contralateral TMS) in the right vocalis muscle (Rödel et al., 2004). For the left vocalis muscle, mean corticomotor latencies were 11.7 ± 2.4 ms (ipsilateral TMS) and 11.1 ± 1.8 ms (contralateral TMS) (Rödel et al., 2004).
12.3. Pharyngeal muscles
MEPs can also be elicited with a round coil over the vertex in the cricopharyngeal muscle of the upper oesophageal sphincter. These MEPs show corticomotor latencies (10.7 ± 0.5 ms) that are similar to those of larynx muscles (Ertekin et al., 2001). Alternatively, the transcranially evoked EMG responses can be recorded from the oro-pharynx and upper oesophagus using two pairs of bipolar platinum ring electrodes built into a intraluminal catheter (Aziz et al., 1994). By applying single-pulse TMS to M1 whilst recording the MEPs from the oro-pharynx and upper oesophagus, the size, location and excitability of the M1 representation of swallowing muscles can be studied in healthy individuals and patients with dysphagia (Fraser et al., 2002; Michou and Hamdy, 2009; Hamdy et al., 1998). Healthy subjects show a somatotopic arrangement of the various swallowing muscles with marked inter-hemispheric asymmetry independent of handedness (Aziz et al., 1996; Hamdy et al., 1996). This hemispheric asymmetry appears to be functionally relevant for instance with respect to the occurrence of dysphagia and the course of recovery from dysphagia after stroke (Hamdy and Rothwell, 1998; Hamdy et al., 1996, 1997).
12.4. Paraspinal muscles
TMS activates spinal motoneurons supplying paraspinal muscles via rapidly conducting monosynaptic pathways from the M1 to the spinal cord. The evaluation of central motor conduction time for paraspinal muscles is potentially useful because it may help to determine the exact level of a spinal lesion of the corticospinal tract (Urban and Vogt, 1994). MEPs have been recorded with surface electrodes, with the active electrode located 1–3 cm lateral and the reference electrode 3–9 cm lateral from the body midline with the ground electrode is normally positioned over the neck at the midline. This recording method is problematic as evoked responses can be contaminated by other superficial back muscles such as the trapezius and rhomboids or by contiguous paraspinal muscles. For these reasons needle recordings are preferred. The needle electrode should be inserted at least 3 cm deep into the paraspinal muscles, 1–2 cm lateral to the spinous processes.
Using a figure-of-eight coil, TMS is applied at 2–4 cm lateral to the midline at 2 cm anterior to the vertex. Alternatively, a circular coil over the vertex can be used. Muscle preactivation by slight contraction of the back muscles is essential to obtain a response. Because the peripheral component to the paraspinal muscles cannot be obtained the latency of the MEP is a mixture of central and peripheral conduction and will not be of diagnostic value if there is a proximal peripheral disorder (radiculopathy, demyelinating neuropathy).
12.5. Pelvic muscles and sphincters
Urination, defecation and sexual functions depend on the anatomical and functional integrity of central and peripheral nervous pathways to the pelvic floor and the sacral region. TMS of M1 and of sacral roots can provide information about these neural functions, although clinical utility is presently sparse (Brostrom et al., 2003; Opsomer et al., 1989). MEPs have been recorded from different muscles of the sacral region: striated urethral sphincter, external anal sphincter, and bulbocavernous and puborectalis muscles. In all these muscles recording can be done with either concentric needles or surface electrodes. Surface recordings of the external anal sphincter can be performed within the anal canal, using electrodes mounted on a glove, a sponge, a catheter, a plug, or electrodes placed at the anal verge. Needle recording can be done with a needle inserted in external anal sphincter. This kind of recordings has to be performed at several depths, because latency depends on the depth of insertion. Surface recordings of the striated urethral sphincter can be performed with a sponge with two electrodes placed in the vagina or using an intra-urethral ring electrode mounted on a Foley catheter.
Insertion in the striated urethral sphincter is made through the anterior vaginal wall under direct visual inspection. The puborectalis muscle is targeted transvaginally and the needle insertion is guided by digital palpation of the muscle. To record from bulbocavernous muscle the penile bulb is palpated and a needle is inserted in the muscle overlying it or a surface electrode is placed just over it (Brostrom, 2003; Pelliccioni et al., 1997). In all cases, needle recordings are preferable as MEPs obtained from surface electrodes are often contaminated by motor responses from neighboring hip or proximal leg muscles.
For cortical stimulation, a circular or a double-cone coil over the vertex can be used. The double-cone coil is preferred because it can stimulate deep within the interhemispheric fissure. Sacral foraminal stimulation can be applied over the sacral roots to estimate peripheral motor conduction time and to calculate CMCT. Stimulus artifact can be reduced using an intrarectal ground electrode.
13. Triple-stimulation technique
The principle of the triple-stimulation technique (TST) is illustrated in Fig. 8. The TST takes advantage of a collision method to synchronize the transcranially induced discharges of spinal motor neurons (Magistris et al., 1998; Roth and Magistris, 1989). The main advantage of TST over conventional TMS lies in a more synchronized TMS-induced excitation of the target muscle. This prevents phase cancelation caused by temporal dispersion of cortico-muscular excitation when using conventional TMS.
Fig. 8.
Schematic representation of the triple pulse stimulation (TST). (A) Experimental setup with three stimulation devices that are triggered at a defined temporal pattern by a synchronization device. A transcranial magnetic stimulation of the M1-Hand (1) is followed by an electric supramaximal stimulation of the distal peripheral nerve (2) and supramaximal stimulation at the Erb’s point at the proximal nerve pathway (3). (B) Schematic representation of the stimulation of different segments of the pathway in normal subjects. (C) Altered propagation of action potentials in peripheral nerves and the motor response induced by TST in the presence of a partial corticospinal block. (D) Peripheral stimulation with initial stimulation over the Erb’s point instead of the TMS of the contralateral hand area. Amplitude (AMP) of MEP from the intrinsic hand muscles. UMN = upper (corticospinal) motoneuron, LMN = lower (peripheral) motoneuron. AMPTST Amplitude after TST (modified from: Siebner HR, Ziemann U. Das TMS-Buch, Heidelberg: Springer Medizin Verlag, 2007, with kind permission of Springer Medizin Verlag).
Since TST “resynchronizes” corticomotor excitation at the level of the peripheral motor axon, the MEP amplitudes recorded with TST closely match that of the maximal electrically evoked CMAP so that the MEP/CMAP ratio in normal subjects equals 1. The TST is also sensitive to TMS induced double discharges of spinal motoneurons. TST can be implemented as a routine procedure to assess corticomotor conduction to distal limb muscles (see below). In patients, the use of TST has been shown to result in a substantial increase in diagnostic sensitivity compared to conventional single-pulse TMS, especially in patients with a subtle dysfunction of corticospinal conduction (Magistris et al., 1999). Therefore, TST is considered to be the diagnostic method of choice and should be used when ever possible.
In addition to a transcranial magnetic stimulator, TST requires two electrical peripheral nerve stimulators, and a timing device to control the timing of stimulation at three different sites (Fig. 8A). The procedures for recording the evoked motor responses is identical to the standard TMS examination (see Section 4). TST requires coordinated transcranial stimulation of the M1 and supramaximal electrical stimulation of the distal and proximal segments of the peripheral motor axons. The TST protocol (referred to as TST-test) consists of three consecutive stimuli (Fig. 8B). The first stimulus is the transcranial stimulus. The second is a supramaximal electrical stimulus given over the distal part of the peripheral nerve innervating the target muscle. This stimulus is timed in such a way that the antidromical action potentials collide with the orthodromic action potentials evoked by TMS. A third supramaximal electrical stimulation is then applied to the proximal part of the nerve (e.g. Erb’s point stimulation for MEP recordings from hand muscles), which will collide with the antidromic inputs remaining (if any) after distal nerve stimulation.
The inter-stimulus intervals are chosen to ensure that all the action potentials induced by the TMS are captured by peripheral nerve collision. This needs to be individualized according to the following guidelines. The first stimulus interval (stimulus interval I) separating the TMS stimulus and peripheral electrical stimulus is calculated by subtracting the peripheral motor latency from the shortest corticomotor latency (rounded up to the nearest millisecond). The second stimulus interval (stimulus interval II) between the distal and proximal peripheral electrical stimulus can be calculated by subtracting the distal motor latency (evoked by peripheral electrical stimulation) from the proximal motor latency (evoked by proximal electrical stimulation). Patients should perform a slight tonic contraction, if possible, of the target muscle to facilitate TMS-induced corticospinal excitation. A pre-innervation of the target muscle should also be carried out during the peripheral control TST to ensure uniformity.
Impaired corticospinal conduction will result in a decrease in MEP amplitude (Fig. 8C). If the TMS pulse fails to discharge a spinal motor neuron due to corticospinal damage, the antidromic distal nerve excitation produced by the second stimulus will now collide with the orthodromic proximal nerve excitation induced by the third stimulus, and thus cancel each other out. It follows that the third stimulus will no longer induce a maximal stimulation of the target muscle, and the MEP amplitude will decrease, indicating a deficit in corticospinal excitation.
The TST is still affected by desynchronization that occurs during the excitation propagation along the peripheral nerves. This is due to different conduction velocities of individual peripheral motor axons. These differences in conduction velocity cause temporal dispersion while the action potentials travel orthodromically along the peripheral nerve. The resulting desynchronization is small in normal subjects, with little phase cancelation of the induced electrical activity in the motor units. However, in patients with peripheral neuropathy, desynchronization along the peripheral motor axon may be substantial, and reduce the MEP amplitude due to phase cancelation. In addition, musculoneural re-excitation may occur in intramuscular nerve endings and induce antidromically ascending nerve action potentials that will interfere with the collisions of the TST.
To circumvent this, a modified TST protocol is employed as control measurement, in which the first transcranial stimulus is replaced by a proximal peripheral stimulus (Fig. 8D). The second distal nerve stimulus and the third proximal nerve stimulus are administered as in the actual TST-test protocol. For the control TST, the stimulus interval I and stimulus interval II are identical. The stimulus interval corresponds to the difference of CMAP latency after proximal stimulation and CMAP latency after distal stimulation (rounded to the nearest millisecond).
Since TST-control and TST-test are subjected to the same degree to peripheral desynchronization and musculoneural coupling, the influence of peripheral desynchronization and re-excitation can be controlled for by calculating the ratio between the CMAP induced by the TST-test and the one induced by the TST-control. The critical electrophysiological parameter is thus the amplitude ratio of the test and control responses as the TST amplitude ratio reflects the number of corticospinal neurons brought to discharge by TMS, expressed as a percentage of total number of neurons innervating the target muscle. In healthy subjects, the ratio is close to 100%. If the ratio is substantially below 100%, it is recommended to gradually increase the TMS intensity to see whether an increase in MEP/ CMAP ratio can be induced at higher intensities. Like the conventional MEP measurement, the TST can not differentiate between a structural lesion of the axons or a functional conduction block with the axons still being intact (neuropraxia).
Especially the supramaximal proximal nerve stimulation is uncomfortable for most patients but usually reasonable. It is recommended to start with the TST control measurement, since this is more unpleasant than the TST-test measurement. The examination time per patient is usually well under 30 min. If the used EMG device has two built-in electrical stimulators, it is possible to control the desired stimulus sequence as well as start and stop the EMG recording through software program. The software can also calculate the intervals between the three stimulations and adapt the stimulation timing for the test-TST and control-TST. This simplifies and shortens the analysis. The TST is suitable only for distal muscles (intrinsic hand and foot muscles and leg muscles) with sufficient long distance between distal and proximal nerve stimulation.
14. Neuropediatric aspects
The clinical use of single-pulse TMS in children has been proven feasible in more than 30 published studies (Quintana, 2005). Most of the children consider the examination painless and would repeat the investigation (Garvey et al., 2001b), yet it may be difficult to assure compliance especially in children younger than the age of six. The examination should be kept as short as possible. It may be helpful that one of the parents is present during the TMS examination and that the examiner first demonstrates the effects of TMS on himself/herself or another person. The instructions to relax or to preactivate the target muscle should be adapted to the child’s mental development and interests to facilitate optimal compliance. For instance, it might help that the examiner directs the attention of the child to the auditory or visual feedback of the EMG activity produced in the target muscle. If verbal communication is possible, relaxation and contraction of the target muscle might be embedded in a game-like context in which the child is asked to imitate an adult.
Risks and adverse events of using TMS in children are similar to those in adults. Children must always wear earplugs to protect their ears from TMS induced noise. They are particularly sensitive to excessive acustic stimulation because the auditory canal is relatively small in children, resulting in a higher resonance frequency of the external ear. The potential for seizure induction is theoretically greater because children have a lower seizure threshold and the stimulus intensity required for TMS is higher. Despite these concerns to date there have been no reports of TMS causing seizures in infants and children. This holds true for children who may be considered at risk, newborns and even preterms as well as children with epilepsy (Eyre et al., 1991). Therefore all available data so far indicate use of TMS in children safe.
In newborn and infants small electrodes are used tapered to the size of the small hand muscles. The first dorsal interosseus muscle has been shown to be reliable for MEP recording because it can be activated by clenching small soft objects. EMG recording is performed according to guidelines for adulthood. Starting with too low stimulation intensities prolongs examination time. In a clinical context, TMS is usually performed only in children older than 1 year of age, although reproducible MEPs can be obtained even in neonates and preterm children. However, in this very young age group, it is often impossible to standardize preactivation of the target muscle.
In children, TMS has emerged as a valuable instrument to study the functional maturation of the corticospinal motor pathway which is completed at the age of 13 (Garvey and Gilbert, 2004; Nezu et al., 1997). Most TMS-evoked MEP measures show age-related changes in typically developing children (Garvey and Mall, 2008). For instance, CMCT is 2–3 times higher in the first years of life and only approaches adult levels around age of 3–5 years although the length of the corticospinal tract may be 3–4 times longer in adults compared to newborns. The prolonged conduction times in the first years of life mainly reflect immature myelination. Given the marked developmental changes of the corticospinal tract during childhood, it is critical to take into account the patient’s age when judging the MEP recordings. A deviation from the normal developmental trajectory can be observed in a number of childhood-onset neurological disorders (Garvey and Mall, 2008).
HIGHLIGHTS.
This guideline paper provides an up-date on the clinical use of transcranial magnetic stimulation (TMS).
The clinically relevant technical and physiological principles of TMS are outlined.
A detailed description how to examine corticomotor conduction to the hand, leg, trunk and facial muscles is presented.
Abbreviations
- ADM
abductor digiti minimi
- APB
abductor pollicis brevis
- Ca-ML
canaliculomotor latency
- CMAP
compound muscle action potentials
- CMCT
central muscle conduction time
- CML
corticomotor latency
- CMT
cortical motor threshold
- CSP
cortical silent period
- D-wave
direct wave
- DML
distal motor latency
- ECR
extensor carpi radialis
- EMG
electromyography
- I-wave
indirect wave
- IFCN
international federation of clinical neurophysiology
- FCR
flexor carpi radialis
- FDI
first dorsal interosseous
- LLR
long latency reflex
- M1
primary motor cortex
- M response
middle latency response
- MEP
motor evoked potential
- MRI
magnetic resonance imaging
- MSO
maximal stimulator output
- PEST
parameter estimation by sequential testing
- PML
peripheral motor latency
- SD
standard deviation
- TES
transcranial electric stimulation
- TMS
transcranial magnetic stimulation
- TOCT
transosseal conduction time
- TST
triple-stimulation technique
References
- Adrian ED, Moruzzi G. Impulses in the pyramidal tract. J Physiol. 1939;97:153–199. doi: 10.1113/jphysiol.1939.sp003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- Awiszus F. TMS and threshold hunting. Suppl Clin Neurophysiol. 2003;56:13–23. doi: 10.1016/s1567-424x(09)70205-3. [DOI] [PubMed] [Google Scholar]
- Awiszus F. Fast estimation of transcranial magnetic stimulation motor threshold: is it safe? Brain Stimul. 2011;4:58–59. doi: 10.1016/j.brs.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Awiszus F, Feistner H. Kortikale Reizschwelle. Das TMS-Buch. 2007:149–158. [Google Scholar]
- Aziz Q, Rothwell JC, Barlow J, Hobson A, Alani S, Bancewicz J, et al. Esophageal myoelectric responses to magnetic stimulation of the human cortex and the extracranial vagus nerve. Am J Physiol. 1994;267:G827–G835. doi: 10.1152/ajpgi.1994.267.5.G827. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Rothwell JC, Hamdy S, Barlow J, Thompson DG. The topographic representation of esophageal motor function on the human cerebral cortex. Gastroenterology. 1996;111:855–862. doi: 10.1016/s0016-5085(96)70053-7. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Britton TC, Meyer BU, Herdmann J, Benecke R. Clinical use of the magnetic stimulator in the investigation of peripheral conduction time. Muscle Nerve. 1990;13:396–406. doi: 10.1002/mus.880130506. [DOI] [PubMed] [Google Scholar]
- Brostrom S. Motor evoked potentials from the pelvic floor. Neurourol Urodyn. 2003;22:620–637. doi: 10.1002/nau.10151. [DOI] [PubMed] [Google Scholar]
- Brostrom S, Jennum P, Lose G. Motor evoked potentials from the striated urethral sphincter and puborectal muscle: reproducibility of latencies. Clin Neurophysiol. 2003;114:1891–1895. doi: 10.1016/s1388-2457(03)00199-8. [DOI] [PubMed] [Google Scholar]
- Cantello R. Applications of transcranial magnetic stimulation in movement disorders. J Clin Neurophysiol. 2002;19:272–293. doi: 10.1097/00004691-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Civardi C, Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology. 1992;42:1951–1959. doi: 10.1212/wnl.42.10.1951. [DOI] [PubMed] [Google Scholar]
- Caplan L. Epileptic seizures. In: Lüders H, Noachtar S, editors. Epileptic seizures: pathophysiology and clinical semiology. Churchill Livingstone; 2000. p. 757. [chapter 75] [Google Scholar]
- Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG. Mechanisms of cortical reorganization in lower-limb amputees. J Neurosci. 1998;18:3443–3450. doi: 10.1523/JNEUROSCI.18-09-03443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Chu NS. Motor evoked potentials with magnetic stimulation: correlations with height. Electroencephalogr Clin Neurophysiol. 1989;74:481–485. doi: 10.1016/0168-5597(89)90039-7. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Bassi A, Scivoletto G, Rossini PM. Interhemispheric differences of hand muscle representation in human motor cortex. Muscle Nerve. 1997a;20:535–542. doi: 10.1002/(sici)1097-4598(199705)20:5<535::aid-mus1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim YS, Kessler KR, et al. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic. Brain. 1997;120(Pt 4):605–619. doi: 10.1093/brain/120.4.605. [DOI] [PubMed] [Google Scholar]
- Claus D. Central motor conduction: method and normal results. Muscle Nerve Suppl. 1990;13:1125–1132. doi: 10.1002/mus.880131207. [DOI] [PubMed] [Google Scholar]
- Claus D, Weis M, Jahnke U, Plewe A, Brunholzl C. Corticospinal conduction studied with magnetic double stimulation in the intact human. J Neurol Sci. 1992;111:180–188. doi: 10.1016/0022-510x(92)90066-t. [DOI] [PubMed] [Google Scholar]
- Claus D, Weis M, Spitzer A. Motor potentials evoked in tibialis anterior by single and paired cervical stimuli in man. Neurosci Lett. 1991;125:198–200. doi: 10.1016/0304-3940(91)90027-q. [DOI] [PubMed] [Google Scholar]
- Cohen L, Bandinelli S, Topka H, Fuhr P, Roth B, Hallett M. Topographic maps of human motor cortex in normal and pathological conditions: mirror movements, amputations and spinal cord injuries. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:36. [PubMed] [Google Scholar]
- Connors BW, Malenka RC, Silva LR. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988;406:443–468. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruccu G, Frisardi G, Pauletti G, Romaniello A, Manfredi M. Excitability of the central masticatory pathways in patients with painful temporomandibular disorders. Pain. 1997;73:447–454. doi: 10.1016/S0304-3959(97)00139-5. [DOI] [PubMed] [Google Scholar]
- Currà A, Modugno N, Inghilleri M, Manfredi M, Hallett M, Berardelli A. Transcranial magnetic stimulation techniques in clinical investigation. Neurology. 2002;59:1851–1859. doi: 10.1212/01.wnl.0000038744.30298.d4. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Molnar GF, Christensen BK, Sailer A, Fitzgerald PB, Chen R. An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clin Neurophysiol. 2003;114:938–944. doi: 10.1016/s1388-2457(03)00038-5. [DOI] [PubMed] [Google Scholar]
- Desiato MT, Bernardi G, Hagi HA, Boffa L, Caramia MD. Transcranial magnetic stimulation of motor pathways directed to muscles supplied by cranial nerves in amyotrophic lateral sclerosis. Clin Neurophysiol. 2002;113:132–140. doi: 10.1016/s1388-2457(01)00724-6. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Mazzone P, Insola A, Ranieri F, et al. Corticospinal volleys evoked by transcranial stimulation of the brain in conscious humans. Neurol Res. 2003a;25:143–150. doi: 10.1179/016164103101201292. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004a;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Insola A, Mazzone P, et al. Descending volleys evoked by transcranial magnetic stimulation of the brain in conscious humans: effects of coil shape. Clin Neurophysiol. 2002;113:114–119. doi: 10.1016/s1388-2457(01)00696-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Pilato F, Zito G, et al. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol. 2003b;547:485–496. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Saturno E, Dileone M, Tonali PA. Role of motor evoked potentials in diagnosis of cauda equina and lumbosacral cord lesions. Neurology. 2004b;63:2266–2271. doi: 10.1212/01.wnl.0000147296.97980.ca. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U, Lemon RN. State of the art: physiology of transcranial motor cortex stimulation. Brain Stimul. 2008;1:345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Eisen A, Shtybel W. AAEM minimonograph# 35: clinical experience with transcranial magnetic stimulation. Muscle Nerve Suppl. 1990;13:995–1011. doi: 10.1002/mus.880131102. [DOI] [PubMed] [Google Scholar]
- Ertekin C, Turman B, Tarlaci S, Celik M, Aydogdu I, Secil Y, et al. Cricopharyngeal sphincter muscle responses to transcranial magnetic stimulation in normal subjects and in patients with dysphagia. Clin Neurophysiol. 2001;112:86–94. doi: 10.1016/s1388-2457(00)00504-6. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Ramesh V. Constancy of central conduction delays during development in man: investigation of motor and somatosensory pathways. J Physiol. 1991;434:441–452. doi: 10.1113/jphysiol.1991.sp018479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser C, Power M, Hamdy S, Rothwell J, Hobday D, Hollander I, et al. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002;34:831–840. doi: 10.1016/s0896-6273(02)00705-5. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Furby A, Bourriez JL, Jacquesson JM, Mounier-Vehier F, Guieu JD. Motor evoked potentials to magnetic stimulation: technical considerations and normative data from 50 subjects. J Neurol. 1992;239:152–156. doi: 10.1007/BF00833916. [DOI] [PubMed] [Google Scholar]
- Garvey M, Ziemann U, Becker D, Barker C, Bartko J. New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clin Neurophysiol. 2001a;112:1451–1460. doi: 10.1016/s1388-2457(01)00581-8. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Gilbert DL. Transcranial magnetic stimulation in children. Eur J Paediatr Neurol. 2004;8:7–19. doi: 10.1016/j.ejpn.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Mall V. Transcranial magnetic stimulation in children. Clin Neurophysiol. 2008;119:973–984. doi: 10.1016/j.clinph.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MA, Kaczynski KJ, Becker DA, Bartko JJ. Subjective reactions of children to single-pulse transcranial magnetic stimulation. J Child Neurol. 2001b;16:891–894. doi: 10.1177/088307380101601205. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Garvey MA, Bansal AS, Lipps T, Zhang J, Wassermann EM. Should transcranial magnetic stimulation research in children be considered minimal risk? Clin Neurophysiol. 2004;115:1730–1739. doi: 10.1016/j.clinph.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Groppa S, Peller M, Siebner HR. Functional assessment of corticospinal conduction with transcranial magnetic stimulation: basic principles. Klinische Neurophysiol. 2010;41:12–22. [Google Scholar]
- Groppa S, Schlaak BH, Munchau A, Werner-Petroll N, Dunnweber J, Baumer T, et al. The human dorsal premotor cortex facilitates the excitability of ipsilateral primary motor cortex via a short latency cortico-cortical route. Hum Brain Mapp. 2012;33:419–430. doi: 10.1002/hbm.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S, Siebner H, Kurth C, Stephani U, Siniatchkin M. Abnormal response of motor cortex to photic stimulation in idiopathic generalized epilepsy. Epilepsia. 2008;49:2022–2029. doi: 10.1111/j.1528-1167.2008.01709.x. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Crone R, Hughes D, Tallis RC, et al. Explaining oropharyngeal dysphagia after unilateral hemispheric stroke. The Lancet. 1997;350:686–692. doi: 10.1016/S0140-6736(97)02068-0. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Hobson A, Thompson DG. Sensorimotor modulation of human cortical swallowing pathways. J Physiol. 1998;506(Pt 3):857–866. doi: 10.1111/j.1469-7793.1998.857bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG, et al. The cortical topography of human swallowing musculature in health and disease. Nat Med. 1996;2:1217–1224. doi: 10.1038/nm1196-1217. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC. Gut feelings about recovery after stroke: the organization and reorganization of human swallowing motor cortex. Trends Neurosci. 1998;21:278–282. doi: 10.1016/s0166-2236(97)01212-5. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Measurement of central motor conduction in multiple sclerosis by magnetic brain stimulation. Lancet. 1986;2:355–358. doi: 10.1016/s0140-6736(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NMF, Schriefer TN. Magnetic brain stimulation: central motor conduction studies in multiple sclerosis. Ann Neurol. 1987;22:744–752. doi: 10.1002/ana.410220611. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- Jalinous R. Technical and practical aspects of magnetic nerve stimulation. J Clin Neurophysiol. 1991;8:10–25. doi: 10.1097/00004691-199101000-00004. [DOI] [PubMed] [Google Scholar]
- Karlstrom EF, Lundstrom R, Stensson O, Mild KH. Therapeutic staff exposure to magnetic field pulses during TMS/rTMS treatments. Bioelectromagnetics. 2006;27:156–158. doi: 10.1002/bem.20194. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Aref EE. Electrophysiological study of vocal-fold mobility disorders using a magnetic stimulator. Eur J Neurol. 2002;9:259–267. doi: 10.1046/j.1468-1331.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Papagiannopoulos S, Kazis DA, Sotirakoglou K, Vasiliadis G, Zara F, et al. Lorazepam-induced effects on silent period and corticomotor excitability. Exp Brain Res. 2006;173:603–611. doi: 10.1007/s00221-006-0402-1. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Papagiannopoulos S, Sotirakoglou K, Kazis DA, Kazis A, Mills KR. Silent period to transcranial magnetic stimulation: construction and properties of stimulus-response curves in healthy volunteers. Exp Brain Res. 2005;163:21–31. doi: 10.1007/s00221-004-2134-4. [DOI] [PubMed] [Google Scholar]
- King NK, Kuppuswamy A, Strutton PH, Davey NJ. Estimation of cortical silent period following transcranial magnetic stimulation using a computerised cumulative sum method. J Neurosci Methods. 2006;150:96–104. doi: 10.1016/j.jneumeth.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Kloppel S, Baumer T, Kroeger J, Koch MA, Buchel C, Munchau A, et al. The cortical motor threshold reflects microstructural properties of cerebral white matter. Neuroimage. 2008;40:1782–1791. doi: 10.1016/j.neuroimage.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Kloten H, Meyer B, Britton T, Benecke R. Normwerte und altersabhängige Veränderungen magnetoelektrisch evozierter Muskelsummenpotentiale. EEG–EMG. 1992;23:29–36. [PubMed] [Google Scholar]
- Lissens MA. Motor evoked potentials of the human diaphragm elicited through magnetic transcranial brain stimulation. J Neurol Sci. 1994;124:204–207. doi: 10.1016/0022-510x(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Lissens MA, Vanderstraeten GG. Motor evoked potentials of the respiratory muscles in tetraplegic patients. Spinal Cord. 1996;34:673–678. doi: 10.1038/sc.1996.122. [DOI] [PubMed] [Google Scholar]
- Ludolph A, Wenning G, Masur H, Füratsch N, Elger C. Electromagnetic stimulation of the nervous system. I. Normal values in the central nervous system and a comparison with electric stimulation. EEG-EMG. 1989;20:153. [PubMed] [Google Scholar]
- Maccabee PJ, Amassian VE, Eberle LP, Cracco RQ. Magnetic coil stimulation of straight and bent amphibian and mammalian peripheral nerve in vitro: locus of excitation. J Physiol. 1993;460:201–219. doi: 10.1113/jphysiol.1993.sp019467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistris MR, Rosler KM, Truffert A, Landis T, Hess CW. A clinical study of motor evoked potentials using a triple stimulation technique. Brain. 1999;122(Pt 2):265–279. doi: 10.1093/brain/122.2.265. [DOI] [PubMed] [Google Scholar]
- Magistris MR, Rosler KM, Truffert A, Myers JP. Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials. Brain. 1998;121(Pt 3):437–450. doi: 10.1093/brain/121.3.437. [DOI] [PubMed] [Google Scholar]
- Maskill D, Murphy K, Mier A, Owen M, Guz A. Motor cortical representation of the diaphragm in man. J Physiol. 1991;443:105–121. doi: 10.1113/jphysiol.1991.sp018825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis J, de Quervain D, Hess CW. Dependence of the transcranially induced silent period on the ‘instruction set’ and the individual reaction time. Electroencephalogr Clin Neurophysiol. 1998;109:426–435. doi: 10.1016/s0924-980x(98)00042-3. [DOI] [PubMed] [Google Scholar]
- McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, et al. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry. 2001;49:454–459. doi: 10.1016/s0006-3223(00)01039-8. [DOI] [PubMed] [Google Scholar]
- Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–171. doi: 10.1097/MOO.0b013e32832b255e. [DOI] [PubMed] [Google Scholar]
- Mills KR, Nithi KA. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve. 1997;20(Suppl):570–576. doi: 10.1002/(sici)1097-4598(199705)20:5<570::aid-mus5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mishory A, Molnar C, Koola J, Li X, Kozel FA, Myrick H, et al. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J ECT. 2004;20:160. doi: 10.1097/00124509-200409000-00007. [DOI] [PubMed] [Google Scholar]
- Molinuevo JL, Cruz-Martinez A, Graus F, Serra J, Ribalta T, Valls-Sole J. Central motor conduction time in patients with multifocal motor conduction block. Muscle Nerve. 1999;22:926–932. doi: 10.1002/(sici)1097-4598(199907)22:7<926::aid-mus17>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Nezu A, Kimura S, Uehara S, Kobayashi T, Tanaka M, Saito K. Magnetic stimulation of motor cortex in children: maturity of corticospinal pathway and problem of clinical application. Brain Dev. 1997;19:176–180. doi: 10.1016/s0387-7604(96)00552-9. [DOI] [PubMed] [Google Scholar]
- Opsomer R, Caramia M, Zarola F, Pesce F, Rossini P. Neurophysiological evaluation of central-peripheral sensory and motor pudendal fibres. Electroencephalogr Clin Neurophysiol/Evoked Potentials Section. 1989;74:260–270. doi: 10.1016/0168-5597(89)90056-7. [DOI] [PubMed] [Google Scholar]
- Ormerod IE, Waddy HM, Kermode AG, Murray NM, Thomas PK. Involvement of the central nervous system in chronic inflammatory demyelinating polyneuropathy: a clinical, electrophysiological and magnetic resonance imaging study. J Neurolog Neurosurg Psychiatry. 1990;53:789–793. doi: 10.1136/jnnp.53.9.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso GO, Cunic DI, Gunraj CA, Chen R. Representation of facial muscles in human motor cortex. J Physiol. 2005;567:323–336. doi: 10.1113/jphysiol.2005.088542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton HD, Amassian VE. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- Paulus W, Classen J, Cohen LG, Large CH, Di Lazzaro V, Nitsche M, et al. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008;1:151–163. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Pelliccioni G, Scarpino O, Piloni V. Motor evoked potentials recorded from external anal sphincter by cortical and lumbo-sacral magnetic stimulation: normative data. J Neurol Sci. 1997;149:69–72. doi: 10.1016/s0022-510x(97)05388-4. [DOI] [PubMed] [Google Scholar]
- Petersen NT, Pyndt HS, Nielsen JB. Investigating human motor control by transcranial magnetic stimulation. Exp Brain Res. 2003;152:1–16. doi: 10.1007/s00221-003-1537-y. [DOI] [PubMed] [Google Scholar]
- Pineda AA, Ogata K, Osoegawa M, Murai H, Shigeto H, Yoshiura T, et al. A distinct subgroup of chronic inflammatory demyelinating polyneuropathy with CNS demyelination and a favorable response to immunotherapy. J Neurol Sci. 2007;255:1–6. doi: 10.1016/j.jns.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal Function and Voluntary Movement. Oxford University Press; 1993. p. 448. [Google Scholar]
- Priori A, Berardelli A, Inghilleri M, Accornero N, Manfredi M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson’s disease and drug-induced parkinsonism. Brain. 1994;117(Pt 2):317–323. doi: 10.1093/brain/117.2.317. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Mercuri B, Inghilleri M, Manfredi M. The effect of hyperventilation on motor cortical inhibition in humans: a study of the electromyographic silent period evoked by transcranial brain stimulation. Electroencephalogr Clin Neurophysiol. 1995;97:69–72. doi: 10.1016/0924-980x(94)00224-u. [DOI] [PubMed] [Google Scholar]
- Qi F, Wu AD, Schweighofer N. Fast estimation of transcranial magnetic stimulation motor threshold. Brain Stimul. 2011;4:50–57. doi: 10.1016/j.brs.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Quintana H. Transcranial magnetic stimulation in persons younger than the age of 18. J ECT. 2005;21:88–95. doi: 10.1097/01.yct.0000162556.02720.58. [DOI] [PubMed] [Google Scholar]
- Reutens DC, Berkovic SF, Macdonell RA, Bladin PF. Magnetic stimulation of the brain in generalized epilepsy: reversal of cortical hyperexcitability by anticonvulsants. Ann Neurol. 1993;34:351–355. doi: 10.1002/ana.410340308. [DOI] [PubMed] [Google Scholar]
- Riepe M, Ludolph AC. Untersuchung kortikobulbärer Bahnen und peripherer Hirnnerven bei Normalpersonen und Patienten mit multipler Sklerose: ergebnisse nach nichtinvasiver elektromagnetischer Reizung. Klin Neurophysiol. 1993;24(269):73. [Google Scholar]
- Rödel RMW, Olthoff A, Tergau F, Simonyan K, Kraemer D, Markus H, et al. Human cortical motor representation of the larynx as assessed by transcranial magnetic stimulation (TMS) The Laryngoscope. 2004;114:918–922. doi: 10.1097/00005537-200405000-00026. [DOI] [PubMed] [Google Scholar]
- Rosler KM, Hess CW, Schmid UD. Investigation of facial motor pathways by electrical and magnetic stimulation: sites and mechanisms of excitation. J Neurolog Neurosurg Psychiatry. 1989;52:1149–1156. doi: 10.1136/jnnp.52.10.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosler KM, Nirkko AC, Rihs F, Hess CW. Motor-evoked responses to transcranial brain stimulation persist during cataplexy: a case report. Sleep. 1994;17:168–171. doi: 10.1093/sleep/17.2.168. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Screening questionnaire before TMS: an update. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Desiato MT, Caramia MD. Age-related changes of motor evoked potentials in healthy humans: non-invasive evaluation of central and peripheral motor tracts excitability and conductivity. Brain Res. 1992;593:14–19. doi: 10.1016/0006-8993(92)91256-e. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Desiato MT, Lavaroni F, Caramia MD. Brain excitability and electroencephalographic activation: non-invasive evaluation in healthy humans via transcranial magnetic stimulation. Brain Res. 1991;567:111–119. doi: 10.1016/0006-8993(91)91442-4. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68:484–488. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- Saypol JM, Roth BJ, Cohen LG, Hallett M. A theoretical comparison of electric and magnetic stimulation of the brain. Ann Biomed Eng. 1991;19:317–328. doi: 10.1007/BF02584306. [DOI] [PubMed] [Google Scholar]
- Schmid UD, Moller AR, Schmid J. Transcranial magnetic stimulation of the facial nerve: intraoperative study on the effect of stimulus parameters on the excitation site in man. Muscle Nerve. 1992;15:829–836. doi: 10.1002/mus.880150712. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Benecke R. The silent period after transcranial magnetic stimulation is of exclusive cortical origin: evidence from isolated cortical ischemic lesions in man. Neurosci Lett. 1994;180:41–45. doi: 10.1016/0304-3940(94)90909-1. [DOI] [PubMed] [Google Scholar]
- Sharshar T, Hopkinson NS, Jonville S, Prigent H, Carlier R, Dayer MJ, et al. Demonstration of a second rapidly conducting cortico-diaphragmatic pathway in humans. J Physiol. 2004;560:897–908. doi: 10.1113/jphysiol.2004.061150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharshar T, Ross E, Hopkinson NS, Dayer M, Nickol A, Lofaso F, et al. Effect of voluntary facilitation on the diaphragmatic response to transcranial magnetic stimulation. J Appl Physiol. 2003;95:26–34. doi: 10.1152/japplphysiol.00918.2002. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sims HS, Yamashita T, Rhew K, Ludlow CL. Assessing the clinical utility of the magnetic stimulator for measuring response latencies in the laryngeal muscles. Otolaryngology-Head and Neck. Surgery. 1996;114:761. doi: 10.1016/S0194-59989670099-2. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M, Groppa S, Siebner H, Stephani U. A single dose of sulthiame induces a selective increase in resting motor threshold in human motor cortex: a transcranial magnetic stimulation study. Epilepsy Res. 2006;72:18–24. doi: 10.1016/j.eplepsyres.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Stokes MG, Chambers CD, Gould IC, English T, McNaught E, McDonald O, et al. Distance-adjusted motor threshold for transcranial magnetic stimulation. Clin Neurophysiol. 2007;118:1617–1625. doi: 10.1016/j.clinph.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Szelenyi A, Kothbauer K, de Camargo AB, Langer D, Flamm ES, Deletis V. Motor evoked potential monitoring during cerebral aneurysm surgery: technical aspects and comparison of transcranial and direct cortical stimulation. Neurosurgery. 2005;57:331–338. doi: 10.1227/01.neu.0000176643.69108.fc. [DOI] [PubMed] [Google Scholar]
- Tabaraud F, Hugon J, Salle J, Boulesteix J, Vallat J, Dumas M. Study of central motor pathways using cortical magnetic stimulation and spinal electrical stimulation: results in 20 normal subjects. Rev Neurol (Paris) 1989;145:690. [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol. 2004;96:1496–1503. doi: 10.1152/japplphysiol.01116.2003. [DOI] [PubMed] [Google Scholar]
- Tharayil BS, Gangadhar BN, Thirthalli J, Anand L. Seizure with single-pulse transcranial magnetic stimulation in a 35-year-old otherwise-healthy patient with bipolar disorder. J ECT. 2005;21:188–189. doi: 10.1097/01.yct.0000177516.38421.9c. [DOI] [PubMed] [Google Scholar]
- Tranulis C, Gueguen B, Pham-Scottez A, Vacheron M, Cabelguen G, Costantini A, et al. Motor threshold in transcranial magnetic stimulation: comparison of three estimation methods. Neurophysiol Clin. 2006;36:1–7. doi: 10.1016/j.neucli.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the ‘affected’ and ‘unaffected’ hemispheres in human stroke. Brain Res. 1998;803:1–8. doi: 10.1016/s0006-8993(98)00505-8. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation of corticospinal pathways at the foramen magnum level in humans. Ann Neurol. 1994;36:618–624. doi: 10.1002/ana.410360410. [DOI] [PubMed] [Google Scholar]
- Urban P, Beer S, Hopf H. Cortico-bulbar fibers to orofacial muscles: recordings with enoral surface electrodes. Clin Neurophysiol. 1997;105:8–14. doi: 10.1016/s0924-980x(96)96584-4. [DOI] [PubMed] [Google Scholar]
- Urban PP, Vogt T. Conduction times of cortical projections to paravertebral muscles in controls and in patients with multiple sclerosis. Muscle Nerve. 1994;17:1348–1349. doi: 10.1002/mus.880171116. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Alvarez R, Tolosa ES. Responses of the soleus muscle to transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1994;93:421–427. doi: 10.1016/0168-5597(94)90148-1. [DOI] [PubMed] [Google Scholar]
- von Giesen HJ, Roick H, Benecke R. Inhibitory actions of motor cortex following unilateral brain lesions as studied by magnetic brain stimulation. Exp Brain Res. 1994;99:84–96. doi: 10.1007/BF00241414. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113:1165–1171. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(Pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SA, Lockwood RJ, Thickbroom GW, Mastaglia FL. The muscle silent period following transcranial magnetic cortical stimulation. J Neurol Sci. 1993a;114:216–222. doi: 10.1016/0022-510x(93)90301-e. [DOI] [PubMed] [Google Scholar]
- Wilson SA, Thickbroom GW, Mastaglia FL. Transcranial magnetic stimulation mapping of the motor cortex in normal subjects. The representation of two intrinsic hand muscles. J Neurol Sci. 1993b;118:134–144. doi: 10.1016/0022-510x(93)90102-5. [DOI] [PubMed] [Google Scholar]
- Yang S, Xu G, Wang L, Chen Y, Wu H, Li Y, et al. 3D realistic head model simulation based on transcranial magnetic stimulation. Conf Proc IEEE Eng Med Biol Soc. 2006:6469–6472. doi: 10.1109/IEMBS.2006.260877. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]