Abstract
Background
Patient safety is a national priority. Patient Safety Indicators (PSIs) monitor potential adverse events during hospital stays. Surgical specialty PSI benchmarks do not exist, which are needed to account for differences in the range of procedures performed, reasons for the procedure, and differences in patient characteristics. A comprehensive profile of adverse events in vascular surgery was created.
Study Design
The Nationwide Inpatient Sample was queried for 8 vascular procedures using ICD-9-CM codes from 2005–2009. Factors associated with PSI development were evaluated in univariate and multivariate analyses.
Results
A total of 1,412,703 patients underwent a vascular procedure and 5.2% developed a PSI. PSIs were more frequent in female, non-white patients with public payers (p<.01). Patients at mid and low volume hospitals had greater odds of developing a PSI (Odds Ratio [OR], 1.17; 95% Confidence Interval [CI], 1.10–1.23 and OR, 1.69; CI, 1.53–1.87). Amputations had highest PSI risk-adjusted rate (RAR) and carotid endarterectomy and endovascular abdominal aortic aneurysm (AAA) repair had lower RAR (p<.0001). PSI RAR increased linearly by severity of patient indication: claudicants (OR, 0.40, CI, 0.35–0.46), rest pain patients (OR, 0.78, CI 0.69–0.90), ulcer (OR: 1.20, CI: 1.07–1.34) and gangrene patients (OR:1.85, CI: 1.66–2.06).
Conclusions
Patient safety events in vascular surgery were high and varied by procedure, with amputations and open AAA having substantially more potential adverse events. PSIs were associated with black race, public payer, and procedure indication. It is important to note the overall higher rates of PSIs occurring in vascular patients and appropriately adjust benchmarks for this surgical specialty.
Introduction
Patient safety has become a national priority since the Institute of Medicine’s 1999 landmark report, To Err is Human.1 Preventable adverse events (PAE) are associated with increased mortality rates, longer length of stay, and more frequent readmissions.2–6 The Agency for Healthcare Research and Quality (AHRQ) has established a set of quality indicators to monitor PAE during hospitalization, known as Patient Safety Indicators (PSIs).7 Data generated from these quality indicators can help evaluate hospital performance, safety improvement efforts, and may be used for hypothesis generation. To ensure these data produce meaningful rates, a system of risk adjustment must be in place that fully accounts for differences in patient demographics and co-morbidity.
Vascular surgery practices vary in the range of procedures they offer, which in turn vary in complexity and peri-procedural risk of death and complications. Recent studies have shown that adverse events are concentrated in a small number of procedure types and rates vary across patient characteristics. 5,8 Any one vascular procedure can be performed for different reasons that reflect different levels of disease severity, which can influence unadjusted rates of events. For instance, the same lower extremity bypass (LEB) may be performed for gangrene of the foot to prevent amputation, or alternatively for intermittent claudication, an ambulation limiting condition. Comparison of outcomes among different vascular surgery practices must therefore account for potential differences in the range of procedures being performed, the reason (indication) for the procedures, as well as differences in patient characteristics.
Given the increasing use of PSIs to evaluate hospital performance,9–11 it is important to have a comprehensive profile of adverse events within different surgical specialties. PSI risk-adjusted rates in vascular surgery are needed to profile quality healthcare delivery in this domain, given the different levels of complexity seen within this group. The aim of our research was to develop patient safety benchmarks for vascular surgery using PSIs to estimate a typical risk-adjusted profile. In this article we report our efforts to characterize patterns of PSIs after vascular surgery. We chose 8 major surgical procedures commonly performed by vascular surgeons. These procedure-specific profiles may be used as benchmarks and highlight areas where quality improvement efforts may be focused.
Methods
Data Source
Our source of data was the Nationwide Inpatient Sample database (NIS).12 AHRQ’s Healthcare Cost and Utilization Project (HCUP) distributes this database. The NIS represents approximately 20% of all hospitalizations across the US. The NIS includes up to 25 diagnosis codes, 15 procedure codes, and both patient and hospital demographics. These data are weighted to provide a nationally representative sample of all-payer inpatient hospitalizations in the United States.
Patient Safety Indicators
PSIs were developed to screen large administrative databases for PAE following surgeries, medical procedures, and childbirth.7 These indicators are based on ICD-9-CM codes and Medicare Severity Diagnosis-Related Groups (MS DRGs) and each PSI has specific inclusion and exclusion criteria.11,13 The PSI software (version 4.1b) was used to identify PAEs in our dataset and provide risk-adjustments; each PSI includes a unique set of adjustors.14,15 The numerator for PSI#4, Death Among Surgical Inpatients with Serious Treatable Complications (previously known as Failure to Rescue), includes surgical patients with a secondary diagnosis of potential complications of care, such as pneumonia, sepsis, deep vein thrombosis or pulmonary embolism, shock/cardiac arrest, or gastrointestinal hemorrhage/acute ulcer. Several PSIs were excluded from the analysis due to lack of applicability in our selected procedures that include: PSI#2 (Death in Low Mortality DRG), PSI#5 (Foreign Body Left During Procedure), PSI#6 (Iatrogenic Pneumothorax), PSI#8 (Postoperative Hip Fracture), PSI#14 (Postoperative Wound Dehiscence) PSI#16 (Transfusion Reaction) and Obstetric PSIs (#18 and #19).
Study Sample
We identified all patients who underwent the following vascular surgery procedures between 2005–2009: carotid endarterectomy, (CEA, 38.12); lower extremity endarterectomy (LEE, 38.18); open abdominal aortic aneurysm repair, (AAA, 38.44); aortobifemoral bypass (AFB, 39.25); lower extremity bypass (LEB 39.29); endovascular abdominal aortic aneurysm repair (EVAR, 39.71); below knee amputation (BKA 84.15); and above knee amputation (AKA, 84.17). Patients were assigned to only one vascular procedure group based on their principal procedure. Other endovascular procedures, such as angioplasty and insertion of stents were not included in the analyses, as the majority of these procedures were not coded as the principal procedure and they were mainly performed in combination with other vascular procedures included in the study. In addition, non-vascular surgeons, such as cardiologists and interventional radiologists, perform many of these endovascular procedures. Patients younger than 18 and non-elective admissions were excluded, including patients with ruptured aneurysms. Patients undergoing amputation for cancer or trauma were also excluded.
We defined indication for surgery for patients who underwent any of the 3 procedures designed to correct lower extremity arterial occlusive disease (LEE, AFB and LEB) with ICD-9-CM diagnosis codes: claudication, 440.21; rest pain, 440.22; ulceration, 440.23; or gangrene, 440.24. Categories of hospital volume were based on the average annual number of vascular surgery procedures performed during the 5-year study period; a vascular procedure was defined as one of the eight surgical procedures included in our study. Hospital volume was divided into tertiles and the average of the lower and upper tertiles were taken across the 5 study years, a methodology previously reported.16 Low-volume hospitals were defined as those performing <9 vascular procedures per year, mid-volume 9–79 per year, and high-volume >79 per year.
Statistical Analysis
Since the NIS is a 20% sample of hospitalizations in the US, sample weights were applied to the raw data to produce nationally representative estimates. Patient and hospital characteristics were compared between patients with at least one PSI versus those without a PSI using Rao-Scott χ² for categorical variables and Student’s t-test for continuous variables. The Wilcoxon-Rank Sum test was used for analyses of length of stay and total hospital charges because these data are not normally distributed. All rates reported are per 1,000 patients (hospital admissions), unless otherwise specified. The association between hospital volume and hospital risk-adjusted PSI rates was analyzed using a negative binomial regression model. Multivariate logistic regression models were performed to evaluate the association of PSI development with patient and hospital characteristics, taking into account hospital random effects.
The AHRQ PSI software flags patient discharges with ICD-9-CM codes corresponding to each PSI, applies external cause of injury codes (e-codes), and calculates crude, estimated, and risk-adjusted incidence rates.17 For this study we report PSI RAR generated by the PSI software, which are adjusted for age, sex, age-sex interactions, diagnostic related group (DRG), and comorbidities as assessed using the Elixhauser Comorbidity Index.18 PSI software was developed to ensure each PSI denominator includes only patients at risk for each individual event and therefore PSI rates give the number of cases divided by the number of patients at risk. The number of patients at risk for each PSI varies according to its specific inclusion and exclusion criteria.19
We first compared patient and hospital characteristics associated with the development of any PSI using univariate analysis, this dichotomous variable included any PSI development. Next, we generated a multivariate regression model, fitted by hospital random effects using GLIMMIX, to determine the association between PSI development and different patient and hospital characteristics from the univariate analyses. As a third step in the analyses, we used the PSI software to generate standardized PSI risk-adjusted rates. Next, we ran the PSI software on all other surgical patients in the 2009 NIS database to obtain standardized PSI RAR for these patients. These data were used to compare PSI RAR between vascular surgery patients and all other surgical patients. Finally, we examined the association between procedure indication, reasons for having a procedure, and odds of developing a PSI using an additive multivariate regression model, taking into account hospital random effects. The first model in this final analysis included only procedure indication as an independent variable (model 1). We next added patient demographics to the model (model 2) and then added patient comorbidities (model 3). The final model contained model 3 plus hospital characteristics, including hospital volume, teaching status, metropolitan area, and region (model 4).
All analyses accounted for the survey-design nature of the NIS data and were performed using SAS software 9.2 (SAS Institute Inc. Cary, NC).
Results
A total of 1,412,703 patients underwent one of the selected vascular procedures between 2005 and 2009 (Table 1). CEA was the most frequently performed operation, accounting for 40% of the sample. Procedures to treat lower extremity arterial occlusive disease (LEE, AFB, and LEB) together accounted for 33%, major amputations (BKA and AKA) comprised 18%, and treatments for aortic aneurysm 14%, with EVAR more than twice as common as open AAA. Overall, 5.2% of vascular patients developed at least one PSI. Both CEA and EVAR had a lower percentage of patients developing a PSI compared to the other vascular procedures (2.0% and 2.8%, respectively). In contrast, AKA and open AAA had higher percentages of patients develop a PSI during hospitalization compared to the other vascular procedures (15.9% and 10.9%, respectively).
Table 1.
Index Elective Vascular Procedures in the US from 2005–2009 and Frequency of Any PSI Development during Hospitalization
| ICD-9 | Name | Abbreviation | n | ≥1 PSI, n (%) |
|---|---|---|---|---|
| 38.12 | Carotid endarterectomy | CEA | 570,333 | 11,157 (2.0) |
| 38.18 | Lower extremity endarterectomy | LEE | 55,788 | 2,308 (4.1) |
| 38.44 | Open aaa repair | AAA | 55,772 | 6,092 (10.9) |
| 39.25 | Lower extremity bypass | LEB | 266,387 | 12,254 (4.6) |
| 39.29 | Aortobifemoral bypass | AFB | 62,230 | 5,191 (8.3) |
| 39.71 | Endovascular AAA repair | EVAR | 143,706 | 3,971 (2.8) |
| 84.15 | Amputation, below knee | BKA | 142,635 | 13,755 (9.6) |
| 84.17 | Amputation, above knee | AKA | 115,853 | 18,407 (15.9) |
| Total | 1,412,703 | 73,135 (5.2) |
Demographic characteristics of the study population stratified by presence of any PSI are presented in Table 2. We examined the data in both univariate and multivariate analyses, with development of any PSI(s) (yes or no) the dependent variable for the model. The 73,135 vascular patients who developed at least one PSI were older than those without a PSI: 71.4% vs. 69.4% were >65 years of age (Table 2). In the multivariate model, each year of age was associated with a small increase in odds of developing a PSI (Odds Ratio [OR] 1.01, 95% Confidence Intervals [CI] 1.00–1.09). Patients with a PSI were more often female (45% vs. 37%, p<.0001; OR 1.18, CI 1.11–1.22). Black race comprised 14% of those with a PSI, but only 7% of those without one (p<.0001) and was a strong predictor of PSI development, with Blacks have 76% greater odds of developing a PSI compared to whites (OR 1.60, CI 1.71–1.80). The PSI group was less likely to include those with private insurance (15.2% vs. 21.4%, p<.0001). Not surprisingly for vascular patients, more than 90% of the entire sample was assigned at least one code for a co-morbid condition, based on Elixhauser designations. This was even more common in patients who developed a PSI (p=0.0068), who also had a significantly greater number of secondary diagnoses (12.7 versus 8.6, p<.0001). Patient outcomes also significantly differed by presence of a PSI. A higher percentage of patients who developed a PSI died in-hospital compared to other vascular patients without a PSI (13.7% vs. 1.3%, p<.0001). Patients with a PSI also stayed in the hospital longer, 15.9 days vs. 5.5 days (p<.0001) and accumulated substantially greater total hospital charges, $116,400 vs. $48,600 (p<.0001).
Table 2.
Characteristics of the Study Population Stratified by PSI Development during Hospitalization
| Patient Characteristics | No PSI n=1,338,568 |
Any PSI n=73,135 |
Univariate p value |
Multivariate OR* |
|---|---|---|---|---|
| Age, y, % | <0.0001 | 1.01† | ||
| 18–39 | 1.0 | 1.5 | ||
| 40–64 | 29.6 | 27.1 | ||
| 65–74 | 32.8 | 29.6 | ||
| 75+ | 36.6 | 41.8 | ||
| Female, % | 37.4 | 44.6 | <0.0001 | 1.18† |
| Race | ||||
| White | 62.6 | 55.0 | <0.0001 | Ref |
| Black | 7.4 | 14.1 | 1.76† | |
| Hispanic | 4.4 | 5.8 | 1.19† | |
| Asian/Pacific Islander | 0.8 | 1.0 | 1.05 | |
| Non-Specified | 24.4 | 23.6 | 1.11† | |
| Primary payer | <0.0001 | |||
| Medicare | 70.0 | 74.6 | Ref | |
| Medicaid | 4.8 | 6.5 | 1.14† | |
| Private | 21.4 | 15.2 | 0.75† | |
| Other | 3.8 | 2.7 | 0.95 | |
| Comorbidity‡ | ||||
| No comorbidity | 6.5 | 5.8 | 0.0068 | |
| No. of comorbidities, mean (SD) |
2.4 (1.4) | 2.8(1.5) | <0.0001 | |
| No. of diagnoses, mean (SD) | 8.6 (4.3) | 12.7 (5.5) | <0.0001 | |
| Outcomes | ||||
| Risk-Adjusted Mortality | 1.3 | 13.7 | <0.0001 | |
| LOS, mean (SD) | 5.5 (7.4) | 15.9 (15.9) | <0.0001 | |
| Total charges, mean (SD) |
$48.6K ($56K) |
$116.4K ($128K) |
<0.0001 | |
| Hospital characteristics | ||||
| Hospital volume | <0.0001 | |||
| Low | 1.2 | 2.1 | 1.69† | |
| Mid | 17.2 | 18.9 | 1.17† | |
| High Teaching Urban |
83.7 48.0 91.7 |
79.0 52.4 92.3 |
<0.0001 0.1136 |
Ref 1.06 1.15† |
| Region | 0.0040 | |||
| Northeast | 17.7 | 19.6 | 1.05 | |
| Midwest | 24.1 | 24.1 | 0.87† | |
| South | 42.0 | 41.2 | 1.00 | |
| West | 16.1 | 16.7 | Ref |
OR: Odds Ratios. The multivariate model includes age, gender, race, primary payer, number of comorbidities, hospital volume, hospital teaching status and hospital region were included for risk adjustment.
Indicates significance at p<0.05.
Comorbidity data was not included in the model, as PSIs and associated complications are among these codes..
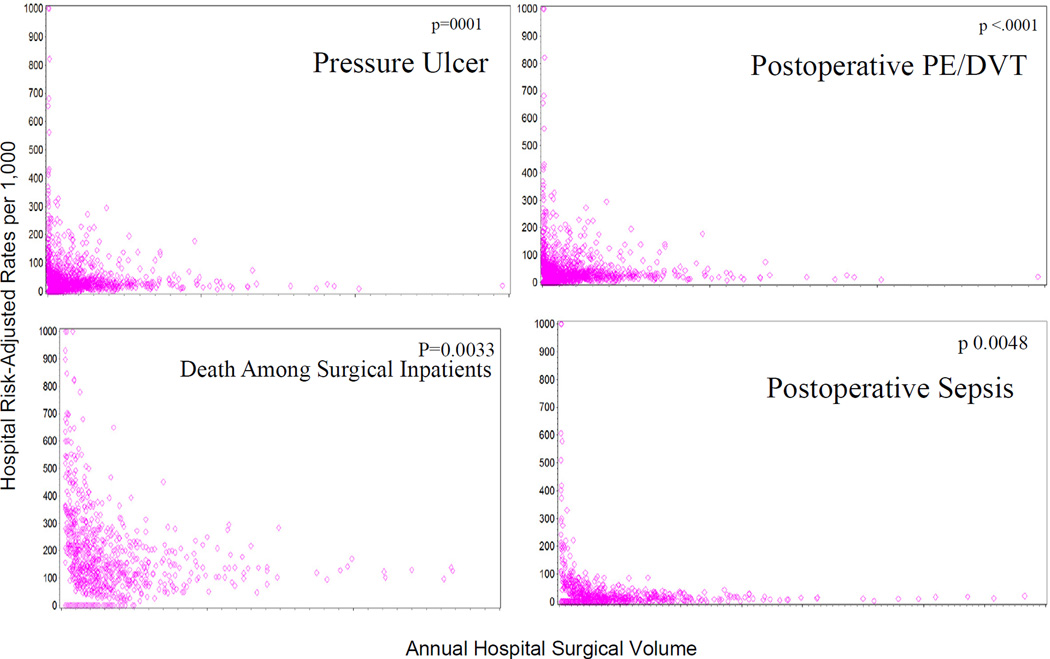
Hospital characteristics are also reported in Table 2. In the group of patients with at least one PSI, more patients went to low volume hospitals compared to patients without a PSI (p<.0001). Correspondingly, hospital volume predicted the likelihood of developing at least one PSI after a vascular operation (p<.0001), with mid and low volume hospitals having 17% and 69% greater odds of a PSI development, respectively. Figure 1 depicts the relationships between hospital volume and RAR of four individual PSIs in vascular surgery: PSI#3 (Pressure Ulcer), PSI#4 (Death Among Surgical Inpatients with Serious Treatable Complications), PSI#12 (Postoperative Pulmonary Embolism/Deep Vein Thrombosis), and PSI#13 (Postoperative Sepsis). These four PSIs were displayed, as they have the highest RAR in vascular surgery. These data illustrate the negative correlation between hospital volume and risk-adjusted PSI rates, a linear increase in volume was associated with lower hospital PSI risk-adjusted rates. For each of these PSIs the negative correlation between hospital volume and event rate was statistically significant. Coefficients from the negative binomial distribution include: PSI#3 estimate: −0.0089, p<.0001; PSI#4 estimate: −0.0245, p=0.0033; PSI#12 estimate: −0.0028, p<.0001; PSI#13 estimate: −0.0061, p=0.0048). These coefficients relate to the slope of the relationship between hospital vascular volume and PSI rate, with the steepest gradient for PSI#4, Death Among Surgical Inpatients with Serious Treatable Complications.
Figure 1.
Distribution of hospital PSI risk-adjusted rates per 1,000 by number of patients at risk within each hospital, 2005–2009.
PSI risk-adjusted rates for patients undergoing the 8 selected vascular procedures were compared with all other surgical inpatients from the 2009 NIS in Table 3. Vascular patients had significantly higher risk-adjusted PSI rates for pressure ulcers (#3), central-line bloodstream infections (#7), and postoperative PE/DVT (#12) and lower PSI rates for death among surgical inpatients with serious treatable complications (#4), postoperative hemorrhage/hematoma (#9), postoperative respiratory failure (#11), and accidental puncture/laceration (#15). However, postoperative respiratory failure had significantly higher RAR for open AAA, AFB, and AKA (p<.001). We next looked at PSI risk-adjusted rates in vascular patients by surgical procedure. Above knee amputations had significantly higher risk-adjusted PSI rates compared to all other vascular procedures. Carotid endarterectomy and EVAR had significantly lower PSI rates compared to other vascular procedures.
Table 3.
Risk-Adjusted PSI Rates per 1,000 At-Risk Inpatients from 2005–2009 for Vascular Patients
| Name | Pressure Ulcer |
Death Among Surgical Inpatients |
CLABSI1 | PO Hemorrhage or Hematoma |
PO Physiologic Metabolic Derangement |
PO Respiratory Failure |
PO PE/DVT |
PO Sepsis |
Accidental puncture laceration |
|---|---|---|---|---|---|---|---|---|---|
| Surgical inpatients 20092 |
5.6 (5.4–5.7) |
124.3 (123.1–125.6) |
0.8 (0.8–0.8) |
4.1 (4.1–4.2) |
1.2 (1.2–1.2) |
10.6 (10.5–10.7) |
10.2 (10.1–10.3) |
13.7 (13.5–14.0) |
3.6 (3.6–3.6) |
| Vascular patients |
21.5 (20.9–22.0) |
111.2 (103.3–120.2) |
1.7 (1.6–1.8) |
3.6 (3.4–3.7) |
1.1 (1.0–1.2) |
8.6 (8.1–9.2) |
13.3 (12.9–13.7) |
11.7 (10.0–13.5) |
2.4 (2.2–2.5) |
| CEA | 2.3 (0.7–3.8) |
117.6 (99.9–135.3) |
0.4 (0.1–0.6) |
3.8 (3.6–4.0) |
0.3 (0.1–0.5) |
7.9 (7.3–8.5) |
6.2 (5.3–7.3) |
8.4 (6.0–10.9) |
1.3 (1.0–1.5) |
| LEE | 16.3 (12.4–20.1) |
202.3 (158.5–246.1) |
0.6 (0–1.4) |
3.6 (2.9–4.2) |
0.8 (0.1–1.0) |
16.6 (0–33.4) |
14.6 (12.7–16.5) |
6.5 (2.0–11.0) |
3.3 (2.7–3.8) |
| AAA | 7.1 (4.6–9.5) |
97.6 (84.8–110.3) |
3.4 (2.9–3.9) |
6.2 (5.6–6.9) |
3.3 (3.0–3.5) |
79.7 (42.9–116.6) |
15.6 (14.0–17.3) |
14.7 (13.0–16.4) |
5.5 (5.1–6.0) |
| LEB | 20.4 (19.0–21.9) |
137.4 (115.9–159.9) |
1.4 (1.1–1.7) |
3.9 (3.6–4.2) |
1.4 (1.1–1.7) |
13.4 (6.9–19.8) |
15.9 (15.0–16.8) |
6.3 (4.5–7.9) |
1.8 (1.5–2.1) |
| AFB | 12.3 (9.6–15.0) |
88.4 (74.0–102.9) |
2.8 (2.3–3.3) |
4.4 (3.8–5.0) |
1.4 (1.1–1.7) |
94.2 (73.8–114.5) |
14.6 (12.9–16.3) |
11.6 (9.9–13.4) |
3.8 (3.4–4.3) |
| EVAR | 8.6 (5.5–11.8) |
61.3 (45.4–77.1) |
0.5 (0.1–0.9) |
1.7 (1.3–2.1) |
0.5 (0.3–0.6) |
* | 3.9 (2.8–4.9) |
9.0 (6.9–11.1) |
2.1 (1.8–2.4) |
| BKA | 22.7 (21.7–23.6) |
96.9 (79.8–114.0) |
2.7 (2.3–2.7) |
2.1 (1.6–2.7) |
2.7 (2.2–3.3) |
9.8 (7.3–12.2) |
16.2 (15.2–17.2) |
22.8 (19.9–25.6) |
2.3 (1.4–3.1) |
| AKA | 35.5 (34.5–35.3) |
181.2 (165.8–196.5) |
3.4 (3.0–3.8) |
3.0 (2.4–3.6) |
4.2 (3.5–4.8) |
19.9 (17.1–22.8) |
21.5 (20.5–22.5) |
25.0 (21.8–28.2) |
2.7 (1.8–3.6) |
Data are presented as: Risk-Adjusted Rate per 1,000 (95% Confidence Interval).
There were no identified cases in the group.
Central Line Associated Blood Stream Infections.
All other surgical inpatients in the NIS 2009 database.
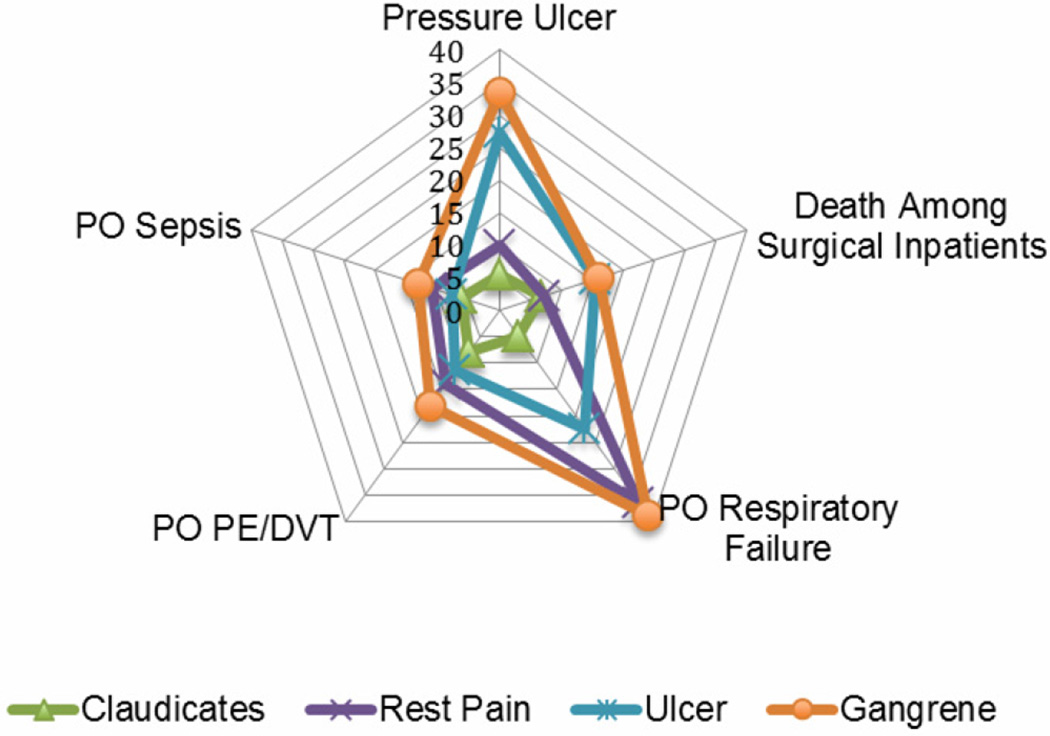
We compared the frequency of any PSI by procedure indication for procedures designed to correct lower extremity arterial occlusive disease (LEE, AFB and LEB) (Table 4). In the group of patients with a PSI, more patients had gangrene compared to the group of patients without a PSI (17% vs. 11%, p<.0001) and a significantly lower proportion were claudicants compared to patients without any PSI (15% vs. 28%, p<.0001). We examined PSIs in the relevant procedures by procedure indication. PSI risk-adjusted rates increased linearly by severity of patient indication: claudication, rest pain, ulcers and gangrene (Figure 2). Overall risk-adjusted rates were lowest in claudicants, followed by rest pain and then ulcer patients, with gangrene patients having the highest risk-adjusted rates for PSIs.
Table 4.
Procedure Indication for Vascular Patients undergoing Bypass and Endarterectomy Procedures, Stratified by PSI Development during Hospitalization
| Procedure Indication* | Total n=642,893 |
No PSI n=590,977 |
1+ PSI N=51,916 |
p Value |
|---|---|---|---|---|
| Claudicants, (%) | 103,407 (27) | 100,412 (28) | 2,996(15) | <.0001 |
| Rest pain, (%) | 50,410 (13) | 48,213 (13) | 2,197 (11) | 0.0003 |
| Ulcer, (%) | 39,364 (13) | 37,236 (10) | 2,128 (11) | 0.2501 |
| Gangrene, (%) | 42,277 (10) | 38,927 (11) | 3,350 (17) | <.0001 |
Data exclude patients undergoing AAA, EVAR, CEA, BKA, and AKA.
Figure 2.
Risk-adjusted rates per 1,000 for select PSIs by procedure indication, 2005–2009. Death among surgical inpatients rates are shown as risk-adjusted rates per 100 to fit on the graph.
We further examined the procedure indication relationship in a multivariate regression model for LEB, as an example. The baseline model contained only procedure indication as the independent variable and PSI development as the dependent variable (yes or no). An independent, negative association between procedure indicators claudicant and rest pain and development of a PSI was revealed (OR 0.40, CI 0.35–0.46; OR 0.78, CI 0.69–0.90, respectively). A strong, independent, positive association was revealed for ulcer and gangrene patients and development of a PSI (OR: 1.20, CI: 1.07–1.34; OR: 1.85, CI: 1.66–2.06, respectively). (Table 5, Model 1). Adjusting the models to account for both patient and hospital characteristics (Table 5, Models 2–4) revealed small effects in the odds ratios for the indicators. The final model, Model 4, contained procedure indicator, age, gender, race, payer, patient comorbidities, hospital volume, hospital teaching status, hospital urban location, and hospital region. Patient gender, race, primary payer, and hospital characteristics had minimal effect on the contribution of the procedure indicator and patient comorbidities had large effects on the overall point estimate of the procedure indicator. In the final adjusted models, procedure indicators remained significant (Claudicants OR: 0.49, CI: 0.46–0.52; Rest Pain OR: 0.84, CI 0.79–0.89; Ulcer OR: 1.06, CI: 1.00–1.12; Gangrene OR: 1.43, CI: 1.37–1.50). The regression model analyzing the association between procedure indication and PSI development was also performed on endarterectomy and aortobifemoral bypass procedures with similar results (data not reported).
Table 5.
Additive Regression Models of Developing a PSI during Hospitalization in Lower Extremity Bypass Procedures by Disease Indication
| Procedure Indicator | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Claudicant | 0.401 (0.35–0.46) |
0.417 (0.37–0.48) |
0.484 (0.43–0.55) |
0.490 (0.46–0.52) |
| Rest Pain | 0.784 (0.69–0.90) |
0.766 (0.68–0.89) |
0.842 (0.74–0.96) |
0.840 (0.79–0.89) |
| Ulcer | 1.197 (1.07–1.34) |
1.124 (1.00–1.26) |
1.063 (0.95–1.19) |
1.058 (1.00–1.12) |
| Gangrene | 1.847 (1.66–2.06) |
1.745 (1.57–1.94) |
1.45 (1.30–1.62) |
1.433 (1.37–1.50) |
Odds ratios with 95% confidence interval shown in parenthesis compare odds of developing at least one PSI.
Model 1 includes procedure indication alone; Model 2 includes Model 1 plus patient age, gender, race, and payer; Model 3 includes Model 2 plus patient comorbidities; Model 4 includes Model 3 plus hospital teaching status, urban hospital, hospital region, and hospital volume.
Discussion
This report characterizes the current status of preventable adverse events in patients undergoing vascular surgery in the US. For the majority of the PSIs in this report, risk-adjusted rates were significantly higher in vascular patients compared to 2009 national estimates for all other surgical at-risk patients. Our analyses indicate that adverse events, captured by PSIs, occur in 5% of patients undergoing a vascular procedure, and that there are large variations in rates of adverse events and complication rates by procedure type, amongst different patient characteristics, and by procedure indication, especially for open surgeries. In addition, these data highlight areas in vascular surgery, such as above knee amputations in gangrene patients, where quality improvement efforts can be focused.
Our data confirm previous studies that there are significant disparities in the development of any PSI by race, gender, and payer and these differences remain in vascular surgeries.20–22 In our study, PSIs were more frequent in female, non-white patients with public payers. Similar to other studies, 21 we found blacks had significantly higher odds of developing a PSI compared to whites. Clement et al. reported that payer is associated with the development of a PSI.22 Our data further investigate this relationship and show consistent results, with a higher percentage of Medicaid recipients developing an adverse event during their hospitalization for vascular surgery compared to patients with other payers. These associations remained after accounting for other known confounders. Some of these characteristics may track with less access to primary care and deferral of seeking medical services. Further investigation into these discrepancies is needed to better understand the reasons for these elevated rates in minority women with public payers before these differences can be eliminated.
Significant differences in risk-adjusted rates of PSIs by type of procedure were noted. The most commonly performed vascular procedure, CEA, had significantly lower PSI risk-adjusted rates compared to that of all other surgical inpatients, and the rates for EVAR were only slightly higher than CEA, still significantly lower than all other surgical patients. These procedures are performed with small incisions and are usually followed by short post-operative recoveries. In contrast, above and below knee amputations and open AAA had overall significantly higher PSI risk adjusted rates compare to other surgical patients, probably reflecting the high burden of comorbidities among vascular amputation patients that is not captured in current PSI risk-adjustment and an obligatory long post-operative stay. These patients’ arterial disease is so advanced that it is not remediable, or they have been judged to be too ill or frail to undergo attempts at limb salvage. They may have been hospitalized for days before amputation, are frequently malnourished and may not have walked for months because of foot wounds. These stark differences, also described by others,5 show that any quality benchmarks employing PSI rates for “vascular surgery” must account for the range and variety of procedures being performed.
In addition, our data suggest that vascular patients have higher risk-adjusted rates of PE/DVT compared to all other surgical patients, a finding also noted elsewhere.23 These differences were significant for all vascular procedures analyzed with the exception of CEA and EVAR. To date, vascular surgery patients have not been considered at high-risk for PE/DVT.24 However, our data suggest that the common view of low DVT risk in vascular patients should be challenged with more focused research and that more aggressive DVT prophylaxis treatment is warranted in patients undergoing vascular procedures, in particular patients having above knee amputations.
Data in our study show that the majority of patients undergoing vascular procedures have several listed comorbidities, with less than 10% of all patients with no coded secondary diagnosis. Current risk adjustments try to capture the case-mix, and we further refine vascular risk-adjustment with the addition procedure indication. Applicable to many surgical fields, this concept is especially important in vascular surgery, in which a given procedure can be performed for a variety of reasons, or indications. Three of our index procedures fall into this category. All (AFB, LEE and LEB) are performed to correct impairments in blood flow in the lower extremity, and all can be offered (at one extreme) to patients inconvenienced by calf cramping after a block of walking (claudication), or at the other extreme to a bedridden patient with gangrene of the foot. We have shown, not surprisingly, that the more dire indications strongly predict adverse events, with a significant linear increase in risk-adjusted PSI rates with disease severity. These differences in risk-adjusted rates may likely be an effect of disease severity that is not captured in the current risk-adjustments, thus “indication for surgery” is a critical component of case mix, and must be accounted for in benchmarks or prediction models for vascular surgery.
There have been mixed reports regarding the relationship between volume, teaching status, and urban hospitals and rates of PSIs.5,16,25 Our data support the volume-outcome relationship and provide further evidence that high volume hospitals have superior outcomes through the measurement of inpatient adverse event risk-adjusted rates. In vascular patients we show that lower hospital volume is associated with a higher PSI risk-adjusted rates. The reasons for this volume-event relationship are unclear. Higher volume hospitals may have more resources designed to promote patient safety. Alternatively, their practitioners may be more alert to potential complications because of greater previous experience with them. These findings merit further analysis and have broad implications for public policy, such as vascular surgery resource allocation.
It is important to have a holistic view of patient safety within a surgical specialty, such as vascular surgery. Evidence-based quality indicators, such as AHRQ’s PSIs, are becoming a standardized way to measure potential lapses of quality in healthcare systems and are a widely used surgical quality-benchmarking tool. The data presented in this report highlight important differences in risk-adjusted rates of adverse events in different procedures and subpopulations of patients receiving vascular surgery. These data can be used to benchmark the expected occurrence rates of different adverse events at a national level in vascular surgery and for individual vascular procedures.
Limitations
This study has important limitations. First, we identified adverse events and procedure indication using administrative data, which results in miscoded events and incomplete risk adjustment due to ICD-9 coding limitations and completeness for secondary diagnoses.11,26 Although principal diagnosis is accurately coded in administrative data, secondary or comorbid diagnoses are often underreported. However, this is indirectly controlled for in the software using the Elixhauser method of comorbidity adjustment. Our study did not compare hospital performance, a situation that requires more attention to the limitations of risk adjustment.
Another concern is the low positive predictive value of preventable adverse events due to the possibility of including present on admission (POA) conditions.6,27 Certain PSIs are greatly influenced by the inclusion of POA information and the validity of these rates is questionable in the absence of POA codes. 28–30 PSI software now imputes POA data at the hospital level in an attempt to address this concern, although patient-level data are not available in the NIS. Despite these limitations, PSIs are used for safety monitoring across the nation, and often considered an important first step in identifying clinical targets for more detailed clinical data exploration.
Conclusions
In conclusion, using AHRQ’s Patient Safety Indicators on administrative data to monitor potential adverse events in major vascular procedures can identify several important areas of focus for quality improvement efforts. Over the five-year study period we identified 1.4 million peripheral vascular surgeries and 73,135 potentially preventable adverse events. Data from this report reveal that the majority of patient safety events in vascular surgery occurred at significantly higher rates compared to all other surgical inpatients. We noted substantial disparities in PSI rates among vascular patients, with higher percentages of Blacks and Hispanics developing an event and those patients with a public payer, both Medicaid and Medicare. Procedure indication was highly associated with risk of developing a patient safety event in bypass surgeries. Patient safety events were also negatively associated with hospital volume. As in-hospital adverse events significantly increase a patient’s length of stay, associated hospital charges and substantially increase inpatient mortality, it is important to note the overall higher rates of adverse events occurring in vascular patients and appropriately adjust set benchmarks for this surgical specialty, with the inclusion of procedure indication in the risk-adjustments. It is apparent that better patient safety and higher surgical quality can be achieved by prioritizing quality improvement in procedures with the highest level of adverse events. The degree and type of adverse event in vascular surgery may be under-recognized particularly for amputation patients with gangrene and overall vascular surgery DVT rates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
References
- 1.Kohn KT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington DC: Institutes of Medicine, National Academy Press; 1999. [PubMed] [Google Scholar]
- 2.Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290:1868–1874. doi: 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]
- 3.Rivard PE, Luther SL, Christiansen CL, et al. Using patient safety indicators to estimate the impact of potential adverse events on outcomes. Med Care Res Rev. 2008;65:67–87. doi: 10.1177/1077558707309611. [DOI] [PubMed] [Google Scholar]
- 4.Friedman B, Encinosa W, Jiang HJ, Mutter R. Do patient safety events increase readmissions? Med Care. 2009;47:583–590. doi: 10.1097/MLR.0b013e31819434da. [DOI] [PubMed] [Google Scholar]
- 5.Vogel TR, Dombrovskiy VY, Haser PB, Graham AM. Evaluating preventable adverse safety events after elective lower extremity procedures. J Vasc Surg. 2011 doi: 10.1016/j.jvs.2011.03.230. [DOI] [PubMed] [Google Scholar]
- 6.Kaafarani HM, Borzecki AM, Itani KM, et al. Validity of selected patient safety indicators: opportunities and concerns. J Am Coll Surg. 2011;212:924–934. doi: 10.1016/j.jamcollsurg.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Guide to Patient Safety Indicators. Rockville, MD: Department of Health and Human Services; 2003. Feb, Agency for Healthcare Research and Quality. Version 3.1 (March, 2007) [Google Scholar]
- 8.Schilling PL, Dimick JB, Birkmeyer JD. Prioritizing quality improvement in general surgery. J Am Coll Surg. 2008;207:698–704. doi: 10.1016/j.jamcollsurg.2008.06.138. [DOI] [PubMed] [Google Scholar]
- 9.National Healthcare Disparities Report. Rockville, MD: Agency for Healthcare Research and Quality; 2007. [Google Scholar]
- 10.NQF Endorsed Standards. Washington, DC: National Quality Forum; 2011. [Google Scholar]
- 11.Romano PS, Geppert JJ, Davies S, Miller MR, Elixhauser A, McDonald KM. A national profile of patient safety in U.S. hospitals. Health Aff (Millwood) 2003;22:154–166. doi: 10.1377/hlthaff.22.2.154. [DOI] [PubMed] [Google Scholar]
- 12.Gagne JJ, Leas B, Lofland JH, Goldfarb N, Freitag F, Silberstein S. Quality of care measures for migraine: a comprehensive review. Dis Manag. 2007;10:138–146. doi: 10.1089/dis.2007.103639. [DOI] [PubMed] [Google Scholar]
- 13.McDonald KM, Romano PS, Geppert J, et al. Measures of Patient Safety Based on Hospital Administrative Data—The Patient Safety Indicators, Structured Abstract. Vol 02-0038. Rockville, MD: Agency for Healthcare Reseach and Quality; 2002. [PubMed] [Google Scholar]
- 14.Patient Safety Indicators, PSI. Version 4.1b ed. Rockville, MD: Agency for Healthcare Research and Quality; 2011. [Google Scholar]
- 15.AHRQ Quality Indicator: Risk Adjustment Coefficients for the PSI. 4.1a ed. Rockville, MD: Agency for Healthcare Research and Quality; 2010. [Google Scholar]
- 16.Hernandez-Boussard T, Downey JR, McDonald K, Morton JM. Relationship between Patient Safety and Hospital Surgical Volume. Health Serv Res. 2011 doi: 10.1111/j.1475-6773.2011.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patient Safety Indicators: Technical Specifications. Rockville, MD: Health and Human Services; 2003. Mar, Agency for Healthcare Research and Quality. Version 3.2 (February, 2008) [Google Scholar]
- 18.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 19. [Accessed February 2008];Patient Safety Indicators Overview, Quality Indicators. 2008 http://www.qualityindicators.ahrq.gov/psi_overview.htm.
- 20.Fiscella K, Franks P, Meldrum S, Barnett S. Racial disparity in surgical complications in New York State. Ann Surg. 2005;242:151–155. doi: 10.1097/01.sla.0000171031.08435.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimada SL, Montez-Rath ME, Loveland SA, et al. Racial Disparities in Patient Safety Indicator (PSI) Rates in the Veterans Health Administration. In: Henriksen K, Battles JB, Keyes MA, Grady ML, editors. Advances in Patient Safety: New Directions and Alternative Approaches (Vol. 1: Assessment) Rockville (MD): 2008. [PubMed] [Google Scholar]
- 22.Clement JPLR, Chukmaitov AS, Chen HF. Does patient payer matter in hospital patient safety? Medical Care. 2007;45:131–138. doi: 10.1097/01.mlr.0000244636.54588.2b. [DOI] [PubMed] [Google Scholar]
- 23.White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90:446–455. doi: 10.1160/TH03-03-0152. [DOI] [PubMed] [Google Scholar]
- 24.Schunemann HJ, Cook D, Guyatt G. Methodology for Antithrombotic and Thrombolytic Therapy Guideline Development*. Chest. 2008;133:113S–122S. doi: 10.1378/chest.08-0666. [DOI] [PubMed] [Google Scholar]
- 25.Vartak S, Ward MM, Vaughn TE. Do postoperative complications vary by hospital teaching status? Med Care. 2008;46:25–32. doi: 10.1097/MLR.0b013e3181484927. [DOI] [PubMed] [Google Scholar]
- 26.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32:700–715. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Bahl V, Thompson MA, Kau TY, et al. Do the AHRQ patient safety indicators flag conditions that are present at the time of hospital admission? Med Care. 2008;46:516–522. doi: 10.1097/MLR.0b013e31815f537f. [DOI] [PubMed] [Google Scholar]
- 28.Houchens RL, Elixhauser A, Romano PS. How often are potential patient safety events present on admission? Jt Comm J Qual Patient Saf. 2008;34:154–163. doi: 10.1016/s1553-7250(08)34018-5. [DOI] [PubMed] [Google Scholar]
- 29.Henderson KE, Recktenwald A, Reichley RM, et al. Clinical validation of the AHRQ postoperative venous thromboembolism patient safety indicator. Jt Comm J Qual Patient Saf. 2009;35:370–376. doi: 10.1016/s1553-7250(09)35052-7. [DOI] [PubMed] [Google Scholar]
- 30.Romano PS, Mull HJ, Rivard PE, et al. Validity of selected AHRQ patient safety indicators based on VA National Surgical Quality Improvement Program data. Health Serv Res. 2009;44:182–204. doi: 10.1111/j.1475-6773.2008.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]