Pulmonary arterial hypertension (PAH) is a rare but progressive and deadly disease caused by functional and structural changes in the pulmonary vasculature which lead to an increase in pulmonary vascular resistance (PVR). Regardless of the initial pathogenic trigger, the major causes of increased PVR in patients with PAH are sustained pulmonary vasoconstriction, pulmonary vascular remodeling, in situ thrombosis, and increased pulmonary vascular wall stiffness. Despite expanding research into the diagnosis and treatment of pulmonary hypertension, death rates from pulmonary hypertension have continued to increase 2.5% per year for women and 0.9% per year for men during the past decade1. Patients with PAH, if untreated, die mainly due to progressive right heart failure, and the response of the right ventricle (RV) to the increased afterload is an important determinant of outcome in patients2. During the development of pulmonary hypertension, an initial adaptive response of the RV to the increased afterload is to increase its wall thickness and contractility with varying degrees of RV hypertrophy3. However, with disease progression, sustained long-term pressure overload of the RV can lead to progressive contractile dysfunction and eventually causes RV failure with further RV dilation. Little is known about the molecular and cellular mechanisms which underlie the development of RV failure. The mechanism that determines the transition of RV function from compensated hypertrophy to decompensated failure is also uncertain.
microRNAs (miRNAs), as crucial regulators of cardiovascular development and cardiac remodeling, have attracted increasing interest in recent years. Drake et al. compared the gene expression patterns between RV hypertrophy in hypoxia-induced moderate pulmonary hypertension and RV failure in hypoxia/SU5416-induced severe pulmonary hypertension4. A global increase in the expression of miRNA (miR) was found in the failing RV, while a specific decrease of the expression of miR133a was found in the hypoxia/SU5416 and PAB/Cu2+ depletion pulmonary hypertension models. Another group investigated dynamic miRNA expression during the transition from RV hypertrophy to failure using the pulmonary artery constriction model5. During the stage of hypertrophy, there was dysregulated expression of miRNAs including miR199a-3p, miRLet-7, miR223, miR143/145, miR181a, and miR30. During RV failure, miR208b increased and miR208a decreased, which are associated with an increase in the expression of β-myosin heavy chain (MHC) and a decrease in α-MHC, respectively. These studies suggest that differential expression of miRNAs contribute to alteration in genes in RV hypertrophy and the progression to RV failure. Continued efforts in elucidating the role of miRNA in regulating target genes that are involved in the development of RV failure may lead to novel RV specific therapies.
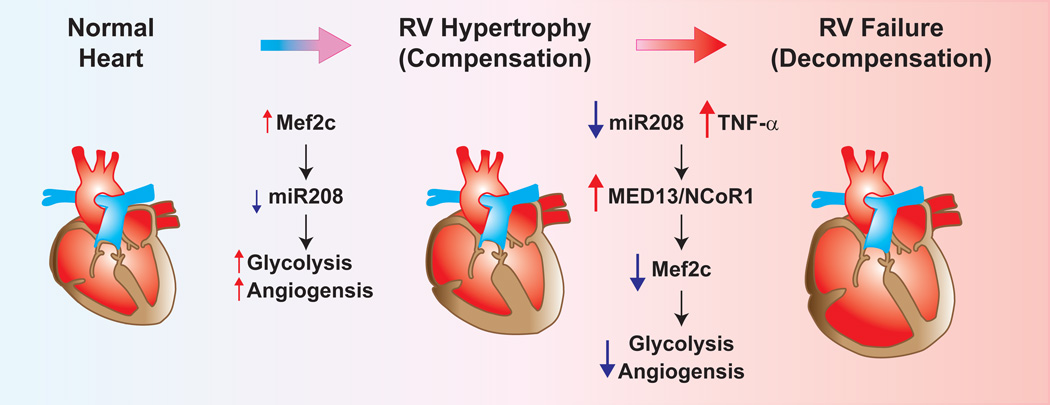
In this issue of Circulation Research, Paulin et al., a very productive research team led by Dr. Evangelos Michelakis, propose a novel approach in which the miR208 and myocyte enhancer factor 2 (Mef2) are targeted in monocrotaline (MCT)-rat animal models of pulmonary hypertension6. The authors show that the stage of RV hypertrophy in a rat model of MCT-mediated pulmonary hypertension can be divided into either “compensated” or “decompensated” according to hemodynamic and structural differences. Using this information, as well as several in vitro assays with fetal and adult cardiomyocytes, the authors then sought to determine a molecular signature of the RV tissue during these different stages. They focused on expression of the cardiogenic transcription factor, Mef2, which has been implicated in cardiomyocyte differentiation, proliferation, morphogenesis and contractility by regulating the expression of a number of miRNAs and genes. The authors found that these stages have a distinct molecular pattern, with miR208 being down-regulated as RV failure progressed, and Mef2c expression increasing in the compensatory stage but decreasing in the decompensatory stage. Furthermore, the authors found that the expression levels of MED13 and NCoR1 are increased in RV decompensatory stage. MED13, a regulatory subunit of the Mediator complex, is a direct target of miR208 in the heart and the modulation of MED13 expression in the heart by miR208a controls systemic metabolic homeostasis and energy expenditure in mice7. The data of this study suggest that the down-regulation of miR208 triggers the MED13/NCoR1 pathway, providing a negative feed-back mechanism for Mef2 that drives the RV toward decompensation (Figure 1).
Figure 1.
Proposed model of miR208/Mef2c and TNF-α in the transition from compensated RV hypertrophy to decompensated RV failure. During RV hypertrophy (compensation) miR208 expression is decreased, and Mef2c expression is slightly increased. Mef2c regulates genes involved in increased glycolysis and angiogenesis. During the progression of RV hypertrophy to RV failure (de-compensation) miR208 continues to decrease in combination with increased TNF-α, leading to increased expression of MED13/NCoR1 complex which inhibits Mef2c through a negative feedback loop leading to decreased glycolysis and angiogenesis.
Although the first trigger for RV adaptation in patients with pulmonary hypertension is the increased afterload2, inflammation may also contribute to the development of RV failure. A number of studies have demonstrated the elevated expression levels of TNF-α in cardiomyocytes and increased plasma concentrations of TNF-α in patients with end-stage heart failure8, 9. Notably, one study on the expression of TNF-α in donor myocardium and the subsequent development of RV failure early after transplantation indicated that TNF-α expression in the donor heart is an important predictor of the development of right heart failure10. Experimental animal studies also showed that TNF-α plays a pivotal role in adverse myocardial remodeling and inhibition of TNF-α with its antagonist, etanercept, attenuated the progression to heart failure in experimental volume overload model11. Cardiac specific overexpression of TNF-α results in the development of a dilated cardiomyopathy with ventricular hypertrophy, ventricular dilation and other cardiomyopathy-like phenotype12. The Michelakis team also demonstrated in their study that treatment with TNF-α increased the expression of MED13 in RV, but not left ventricular, cardiomyocytes that were associated with an increase of NCoR1 expression in RV cardiomyocytes6. The authors proposed that TNF-α may also serve as a “second hit” for the RV failure. This is another important finding of their study. As the authors mentioned, TNF-α as a further trigger for RV dysfunction, in combination with the decreasing miR208, may potentially explain why, when the RV is exposed to strong inflammatory environments, it is much more prone to decompensation6.
This study advances our understanding of the molecular signatures which are present in the stages of RV failure following MCT-induced pulmonary hypertension in rats. It would be interesting to determine whether a similar concept can be applied to other pulmonary hypertension models, such as hypoxia/SU5416-induced pulmonary hypertension models, where the initial trigger for RV failure is still unknown. There are no data presented in this study to show the importance of modulating the miR208/Mef2 axis in vivo. Especially, inhibition of miR208a by systemic delivery of Locked Nucleic Acid (LNA) modified antisense oligonucleotides has been shown to prevent cardiac remodeling and improve cardiac function, overall health, and survival in rat model of LV failure13. Furthermore, it has been suggested that inhibition of miR208a has been shown to improve LV dysfunction in vivo in LV failure-rodent models, concurrently, it may be of interest to simulate this response with miR208 and apply it to RV failure14. Additionally, human tissue or plasma measurements from patients with PAH would also dramatically strengthen the conclusions drawn in their study, especially with the authors suggesting that these findings could be useful as potential biomarkers. Therefore, more studies are needed to provide better understanding of the progression of RV failure, which has distinct functional and molecular patterns in different stages, and which could be promising in the generation of stage-specific, novel biomarkers or therapeutic targets for this severe condition in the patient with PAH.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported, in part, by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL066012, HL115014, HL125208 and HL098053).
Footnotes
Disclosures
None
References
- 1.George MG, Schieb LJ, Ayala C, Talwalkar A, Levant S. Pulmonary hypertension surveillance: United states, 2001 to 2010. Chest. 2014;146:476–495. doi: 10.1378/chest.14-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: Cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]
- 3.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM. Right heart adaptation to pulmonary arterial hypertension: Physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45:1239–1247. doi: 10.1165/rcmb.2010-0412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reddy S, Zhao M, Hu DQ, Fajardo G, Hu S, Ghosh Z, Rajagopalan V, Wu JC, Bernstein D. Dynamic microrna expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics. 2012;44:562–575. doi: 10.1152/physiolgenomics.00163.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulin R, Sutendra G, Gurtu V, Dromparis P, Haromy AS, Provencher S, Bonnet S, Michelakis ED. A mir-208-mef2 axis drives the de-compensation of right ventricular function in pulmonary hypertension. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.115.303910. [DOI] [PubMed] [Google Scholar]
- 7.Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, Gautron L, Elmquist JK, Bassel-Duby R, Olson EN. A cardiac microrna governs systemic energy homeostasis by regulation of med13. Cell. 2012;149:671–683. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 9.Mann DL. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 10.Birks EJ, Owen VJ, Burton PB, Bishop AE, Banner NR, Khaghani A, Polak JM, Yacoub MH. Tumor necrosis factor-alpha is expressed in donor heart and predicts right ventricular failure after human heart transplantation. Circulation. 2000;102:326–331. doi: 10.1161/01.cir.102.3.326. [DOI] [PubMed] [Google Scholar]
- 11.Jobe LJ, Melendez GC, Levick SP, Du Y, Brower GL, Janicki JS. TNF-alpha inhibition attenuates adverse myocardial remodeling in a rat model of volume overload. Am J Physiol Heart Circ Physiol. 2009;297:H1462–H1468. doi: 10.1152/ajpheart.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedberg MK, Redington AN. Right versus left ventricular failure: Differences, similarities, and interactions. Circulation. 2014;129:1033–1044. doi: 10.1161/CIRCULATIONAHA.113.001375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.