Abstract
Engineered tissue constructs have the potential to augment or replace whole organ transplantation for the treatment of liver failure. Poly(ethylene glycol) (PEG)‐based systems are particularly promising for the construction of engineered liver tissue due to their biocompatibility and amenability to modular addition of bioactive factors. To date, primary hepatocytes have been successfully encapsulated in non‐degradable hydrogels based on PEG‐diacrylate (PEGDA). In this study, we describe a hydrogel system based on PEG‐diacrylamide (PEGDAAm) containing matrix‐metalloproteinase sensitive (MMP‐sensitive) peptide in the hydrogel backbone that is suitable for hepatocyte culture both in vitro and after implantation. By replacing hydrolytically unstable esters in PEGDA with amides in PEGDAAm, resultant hydrogels resisted non‐specific hydrolysis, while still allowing for MMP‐mediated hydrogel degradation. Optimization of polymerization conditions, hepatocellular density, and multicellular tissue composition modulated both the magnitude and longevity of hepatic function in vitro. Importantly, hepatic PEGDAAm‐based tissues survived and functioned for over 3 weeks after implantation ectopically in the intraperitoneal (IP) space of nude mice. Together, these studies suggest that MMP‐sensitive PEGDAAm‐based hydrogels may be a useful material system for applications in tissue engineering and regenerative medicine. © 2015 Wiley Periodicals, Inc. J Biomed Mater Res Part A: 103A: 3331–3338, 2015.
Keywords: polyethylene glycol diacrylamide, PEGDAAm, liver, tissue engineering, matrix metalloproteinase
INTRODUCTION
Cell‐based therapies promise to provide therapeutic alternatives or bridges to whole organ transplantation to treat a variety of liver diseases. For example, direct cellular injection of graft hepatocytes improves survival in animals with acute liver failure and end stage liver failure due to cirrhosis,1, 2, 3, 4, 5 and can correct metabolic deficiencies in several models of liver‐based metabolic diseases.1, 2 Despite these and other encouraging results following hepatic cell transplantation in animal models, disappointing clinical results to date demonstrate the need for further improvements in cell‐based liver therapies.6 Currently, these studies have been limited by inefficient engraftment and survival of transplanted hepatocytes as well as a “lag phase” between cell injection and successful cellular integration of the grafted cells with host tissue.7 These limitations might be addressed by constructing engineered liver tissue ex vivo, which would enable preservation of the engineered hepatocellular microenvironment upon implantation.
Indeed, previous work has demonstrated that delivery of hepatocytes in aggregates with intact intercellular junctions (e.g., “spheroids”) or on microcarriers coated with extracellular matrix results in superior engraftment compared to isolated suspensions of only hepatocytes.8, 9 Such studies have led to the development of a variety of natural (e.g., hyaluronic acid, collagen, alginate)10, 11, 12, 13, 14 and synthetic [e.g., polyesters, poly‐l‐lactic acid (PLA) with polyvinyl alcohol (PVA)]15, 16, 17 biomaterial matrices for the construction of three‐dimensional liver constructs. Of these, we believe that Polyethylene glycol (PEG) hydrogels are particularly interesting because of their biocompatibility, neutral charge, resistance to nonspecific protein absorption, and amenability for precise chemical modification via the addition of bioactive factors such as adhesive peptides and degradable linkages. Primary hepatocytes have been previously encapsulated in non‐degradable PEG‐based material systems and used for 3D microenvironmental optimization and humanized mouse model systems.16, 17, 18, 19, 20 Extension of this work for clinically relevant liver repair applications, however, will likely require the use of degradable material systems that enable long‐term engraftment and integration with the host.
Bioactive degradable PEG‐based systems have been developed which incorporate matrix‐metalloproteinase (MMP)‐sensitive peptides into the hydrogel backbone,21, 22, 23, 24, 25 thereby allowing for cell‐initiated degradation of the matrix and facilitating 3D cell migration. However, in most of these systems, MMP‐degradable moieties are attached through hydrolytically unstable ester bonds and therefore may experience nonspecific cleavage from the material backbone via hydrolysis.26 In this work, we built engineered liver tissue with a hydrogel system based on PEG‐diacrylamide (PEGDAAm), which replaces unstable ester bonds with hydrolytically stable amides. We first describe the synthesis of PEGDAAm, followed by its reaction with bis‐cysteine MMP‐sensitive peptides via step‐growth polymerization to create photoactive macromers of the form acrylamide‐PEG‐(peptide‐PEG)n‐acrylamide. Hydrogels were formed from these macromers via radical‐initiated photopolymerization and then characterized for MMP‐susceptibility and base‐catalyzed hydrolysis. To form functional hepatic tissue constructs, we identified parameters for hepatocyte density, polymerization conditions, and multicellular composition that maximized engineered hepatic tissue function in vitro. Moreover, these engineered tissues exhibited robust hepatic function and engraftment in vivo for several weeks in rodents.
MATERIALS AND METHODS
Synthesis and characterization of poly(ethylene glycol) diacrylamide (PEGDAAm)
The reaction scheme for the synthesis of Polyethylene glycol diacrylamide (PEGDAAm; MW, 3400) from polyethylene glycol (PEG) is given in Figure 1(A).26,27 Anhydrous triethylamine (TEA, 6 molar excess to PEG, 34.4 mL, 0.2471 mol) was added to a solution of dry PEG (MW 3400, 140 g, 0.0412 mol) and 4‐dimethylaminopyridine (DMAP, 0.1 molar equivalent to mesyl chloride, .0247 moles, 3.0183 g) in anhydrous dichloromethane (DCM, 150 mL) under argon. After mixing for 10 min, a concentrated solution of mesyl chloride (MsCl, 6 molar excess to PEG, 19.1 mL, 0.2471 mol) in DCM was added dropwise with rapid stirring. The reaction proceeded overnight under argon. PEG dimesylate was purified by filtering the solution through filter article under vacuum, followed by precipitation in diethyl ether (1 L). The product was again filtered and dried under vacuum to yield PEG dimesylate. To synthesize PEG diamine from PEG dimesylate, the entire PEG dimesylate product was added to 800 mL 25% aqueous ammonia solution within 2 days of completing the previous reaction. The container was closed and sealed tightly with Parafilm, and the reaction proceeded for 4 days with vigorous stirring at room temperature. The container was then opened to atmosphere to allow the ammonia to evaporate over 3 days. To remove remaining ammonia, NaOH was used to raise the pH of the solution to 13, and the solution was extracted with DCM (1:5 DCM volume to ammonia solution) three times. The DCM washes were pooled and concentrated under rotary evaporation. The product was then precipitated in diethyl ether, filtered, and dried under vacuum. Yields were typically ∼80%, and percent amination was 99% as verified by 1H NMR for the characteristic peak (3.1 ppm) of the PEG methylene protons adjacent to the amine end group. To synthesize PEG diacrylamide from PEG diamine, anhydrous DCM (75 mL) was added to PEG diamine (70 g, 0.0206 mol) and stirred until the solution became clear. The mixture was cooled to 4 °C on ice. To this cooled solution was added Diisopropylethylamine (DIPEA, 2 molar excess to PEG diamine, 5.7 mL, .0412 mol), followed by acryloyl chloride (4 molar excess to PEG diamine, 6.5 mL, 0.083 mol) dropwise with rapid stirring. The reaction proceeded overnight under argon protected from light and allowed to warm to room temperature. Aqueous reaction byproducts were removed by using aqueous 2M K2CO3 (2 molar excess to acryloyl chloride, 82.4 mL, 0.164 mol) to phase separate the solution overnight. The lower organic phase was dried over MgSO4 to remove residual aqueous solution, filtered, precipitated in diethyl ether and dried under vacuum to yield PEG diacrylamide. Yields were typically ∼70%, and percent amidation was >90% as verified by 1H NMR for the characteristic peaks (5.6, 6.1, and 6.3 ppm) of the vinyl protons on the acrylamide end groups.
Figure 1.
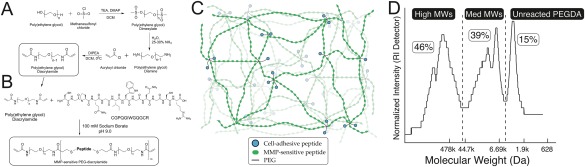
(A) Synthesis of polyethylene glycol diacrylamide (PEGDAAm) from PEG. Reaction of PEG under anhydrous conditions with mesyl chloride yields PEG‐dimesylate, which is aminated under aqueous conditions to yield PEG‐diamine. Reaction of PEG‐diamine with acryloyl chloride in the presence of DIPEA yields PEG diacrylamide (PEGDAAm). (B) Synthesis of MMP‐sensitive PEGDAAm is effected in aqueous conditions (100 mM sodium borate, pH 9.0) by reacting the MMP‐sensitive peptide CGPQGIWGQGCR with PEGDAAm. (C) Schematic of hydrogel mesh network resulting from photopolymerization of MMP‐sensitive PEGDAAm from (B) with cell‐adhesive peptides conjugated to PEG and tethered as pendant chains (see text for details). (D) Reaction of MMP‐degradable peptides with a 1.6 molar excess of PEGDAAm resulted in ∼85% conjugation (sum of medium and high molecular weights). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Synthesis and characterization of MMP‐sensitive PEGDAAm‐peptide hydrogels
To make degradable photoactive hydrogel precursors, PEGDAAm was reacted in 1.6 molar excess with the collagenase‐sensitive peptide CGPQGIWGQGCR (Aapptec, Louisville, KY; 95% pure by HPLC) by dissolution in sodium borate (100 mM, pH 9.0). The reaction was sterile filtered (0.22 μm PVDF membrane, Millipore, Billerica, MA), protected from light, and incubated at 37 °C to yield macromers of the type acrylamide–PEG–(peptide–PEG)n–acrylamide. Reaction products were dialyzed, frozen and lyophilized, and stored at −80 °C until use.
PEG‐peptide conjugates were analyzed by GPC with a refractive index detector and DMF solvent using three tandem styrene‐divinylbenzene (SDVB) columns spanning a linear molecular weight range from 1 to 500 kDa for polystyrene. PEG molecular weight standards (628 Da to 478 kDa; Sigma) were utilized to assess molecular weight of the PEG‐peptide conjugates.
MMP‐sensitive PEGDAAm‐based hydrogels were created by photopolymerization of aqueous solutions of PEGDAAm (5–40 wt %) with 0.1% (w/v) Irgacure 2959 photoinitiator (I‐2959, Ciba) at 100 mW cm−2 (320–520 nm, 60 s, EXFO). Hydrogels were swollen to equilibrium for 24 h in 20 mM HEPES‐buffered saline (HBS) containing 0.2 mg mL−1 NaN3 (to prevent microbial growth), and then assessed for wet weight changes over time in either 0.2 mg mL−1 collagenase (Sigma–Aldrich) to assess MMP‐sensitivity or in 0.1N NaOH to assess sensitivity to base‐catalyzed hydrolysis.
Cell isolation and culture
Rat hepatocytes were isolated from 2‐ to 3‐month old female Lewis rats (Charles River) by collagenase perfusion as described previously.28, 29, 30 Briefly, animals were anesthetized and the portal vein was cannulated. The liver was then perfused with buffers and digested with collagenase. The resultant digest was purified using Percoll centrifugation. Hepatocytes were cultured in DMEM with high glucose (Cellgro), 10% (v/v) fetal bovine serum (Gibco), 0.5 U mL−1 insulin (Lilly), 7 ng mL−1 glucagons (Bedford Laboratories), 7.5 µg mL−1 hydrocortisone (Sigma), and 1% penicillin‐streptomycin (“Hepatocyte media,” Invitrogen). J2‐3T3 fibroblasts, a gift of Dr. Howard Green (Harvard Medical School), were cultured in DMEM with high glucose, 10% bovine serum, and 1% penicillin–streptomycin. Liver endothelial cell line TMNK‐1 (“LEC”), a gift from Dr. Naoya Kobayashi (Okayama University), were cultured in DMEM with high glucose, 10% FBS, and 1% penicillin‐streptomycin. Cells were used between passages 7 and 19.
Cell encapsulation
MMP‐degradable PEGDAAm “pre‐polymer solution” was created with final concentrations of 5% (w/v), PEGDAAm, 0.1% (w/v) Irgacure 2959 photoinitiator (I‐2959, Ciba), and 10 µmol mL−1 acrylate‐PEG‐RGDS.25 Hepatocytes and J2 fibroblasts were co‐cultured for 6–9 days and then trypsinized and suspended at a final density of 4–8 × 106 hepatocytes/ml in pre‐polymer solution. For hydrogels containing LECs, LECs were trypsinized and encapsulated at a density of 6 × 106 cells mL−1 of pre‐polymer (in addition to hepatocytes and J2 fibroblasts). Cells in pre‐polymer were exposed to UV light from a spot curing system (320–390 nm, 10–100 mW cm−2, 50–500 s, EXFO lite) to produce hydrogels. Hydrogels were washed with PBS and cultured in hepatocyte media with media changes every other day.
In vitro assays to assess hepatic function
Cell culture media from hydrogels containing encapsulated cells was collected and stored at −20 °C until analyzed. Hepatic function was measured by two surrogate measures, albumin production and urea secretion. A rat albumin enzyme‐linked immunosorbent assay (ELISA) kit (Bethyl labs) was used to quantify albumin production in cell culture media. To measure urea in cell culture media samples, acid‐catalyzed condensation of urea with diacetylmonoxime was used to yield a quantifiable colored product (Urea Nitrogen Kit, StanBio Labs).
Live/dead imaging
To label cells for in vitro live cell imaging, hydrogels containing cells were incubated with calcein AM (“Live” cell stain, 5 μg mL−1, Invitrogen) and ethidium homodimer (“Dead” cell stain, 2.5 μg mL−1, invitrogen) in hepatocyte media for 30 min at 37 °C and then washed three times. Images were obtained using a Nikon Eclipse TE200 inverted fluorescence microscope and CoolSnap‐HQ Digital CCD Camera.
Immunostaining
For immunostaining, hydrogels containing cells were collected at day 14, washed in PBS and fixed in a solution containing 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Fixed gels were processed, embedded in paraffin, and section for histology. Sections were stained using a primary antibody against cytokeratin (1:800, Sigma) and a secondary Alexa 488‐conjugated goat‐antimouse antibody (Life Technologies, Invitrogen). Images were obtained using a Nikon Eclipse TE200 inverted fluorescence microscope configured with a CoolSnap‐HQ Digital CCD Camera.
In vivo implantation studies and imaging
To label cells for noninvasive in vivo imaging of hepatic function of engineered tissues, hepatocyte co‐cultures were transduced in 2D culture with a lentivirus in which firefly luciferase is expressed under a hepatocyte‐specific albumin promoter (pTRIP.Alb.IVSb.IRES.tagRFP‐DEST, gift of Charles Rice, The Rockefeller University) in “viral media” (20% v/v packaged virus, 4 μg mL−1 polybrene, 20 mM HEPES in hepatocyte media) for 6 h at 37 °C. Cells were then washed three times with hepatocyte media and cultured until encapsulation in hydrogel.
All animal procedures were approved by the Committee for Animal Care in the Department of Comparative Medicine at Massachusetts Institute of Technology. NIH guidelines for the care and use of laboratory animals have been observed. For intraperitoneal implantation of hydrogels containing cells, NCr nude mice (Taconic) were anesthetized with isofluorane. Hydrogels containing either 8 × 106 hepatocytes + J2 and 6 × 106 liver endothelial cells or no cells (sham) were placed into the intraperitoneal space via a 1 cm incision. Animals were closed aseptically, placed under a heat lamp for recovery, and administered 0.1 mg mL−1 buprenorphine every 12 h for pain for 3 days after surgery. Bioluminescence imaging was utilized to monitor engineered tissue function non‐invasively over time. Specifically, mice with implanted tissues were injected intraperitoneally with 250 μL of 15 mg mL−1 d‐Luciferin (Caliper Life Sciences) and imaged using the IVIS Spectrum (Xenogen) system and Living Image software (Caliper Life Sciences).
Statistical analysis
Data are expressed as the mean ± standard error. Statistical significance was determined using one‐way ANOVA followed by Tukey's post hoc test.
RESULTS
Synthesis and characterization of PEGDAAm‐based macromers and hydrogels
Overall, the method used to generate PEGDAAm from PEG is straightforward (Fig. 1), and the synthesis step that produces PEG‐diamine from PEG yields a 20× cost savings relative to commercially available sources for PEG‐diamine. GPC analysis demonstrated that approximately 85% of the PEGDAAm reacted with the peptide [Fig. 1(D)]. Exposure of MMP‐sensitive PEGDAAm‐based hydrogels to collagenase yielded a degradation curve (Fig. 2) similar to that seen previously for MMP‐sensitive PEGDA‐based hydrogels.25 However, MMP‐sensitive PEGDAAm‐based hydrogels demonstrated complete stability over several weeks in 0.1N NaOH compared to PEGDA‐based hydrogels, whose degradation was complete within a few hours [Fig. 1(B)]. These results further confirm 1H NMR characterization of synthesized PEGDAAm precursors and the lack of hydrolytically unstable ester bonds in our PEGDAAm‐based hydrogels.
Figure 2.
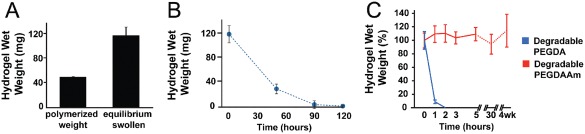
(A) MMP‐sensitive hydrogels were polymerized at 40% w/w and then swollen to equilibrium over 24 h (n = 3). (B) Degradation of MMP‐sensitive PEGDAAm hydrogels in collagenase (0.2 mg mL−1 with 0.2 mg mL−1 NaN3). (C) Assessment of the stability of MMP‐sensitive PEGDAAm hydrogels in 0.1 N NaOH compared to MMP‐sensitive PEGDA‐based hydrogels.25 Bars indicate standard deviation. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
PEGDAAm‐based hydrogels support hepatocyte function in vitro
To test the suitability of PEGDAAm for use in engineered liver tissues, we used them to encapsulate hepatocytes and assayed for maintenance of functional liver traits. We isolated rat hepatocytes and cocultured them for 6–9 days in the presence of supporting stromal cell line, J2‐3T3 fibroblasts (Fib), which have been shown previously to aid in the maintenance of hepatic function in vitro.19, 31, 32, 33 After this stabilization period, the cultures were trypsinized and resuspended in pre‐polymer solution prior to exposure to UV light to produce cell‐laden hydrogels. Embedded hepatocytes maintained the capacity to produce albumin and secrete urea, two surrogate measures of hepatic function, for at least 15 days (Fig. 3). In these studies, we found that hepatocyte density impacts hepatic function on a per cell level after encapsulation in the MMP‐degradable PEGDAAm hydrogels. Specifically, both albumin secretion and urea production were greater on a per cell level in hydrogels containing 8 × 106 hepatocytes per ml as compared to 4 × 106 hepatocytes per mL of prepolymer solution (Fig. 3).
Figure 3.
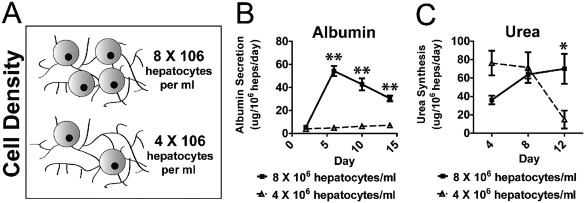
Cellular concentration (A) impacts hepatic tissue function, as measured by albumin production (B) and urea synthesis (C) (**p < 0.01, *p < 0.5, 5% PEGDAAm, 10 mmol RGDS. UV 10 mW cm−2 for 210 s).
We hypothesized that modulation of polymerization conditions might also improve the activity of cell‐laden hydrogels, since primary hepatocytes are known to be sensitive to UV exposure. We varied the UV intensity and exposure time utilized to achieve polymerization, while keeping the total UV energy density constant in each case. Hydrogels polymerized using 100 mW cm−2 UV intensity for 50 s exhibited enhanced albumin secretion and urea synthesis compared to hydrogels that were polymerized using 10 mW cm−2 UV for 500 s (Fig. 4; 8 × 106 hepatocytes+Fib/ml). Together, these results demonstrate that MMP‐degradable PEGDAAm hydrogels support liver functions of encapsulated hepatocytes and that both polymerization conditions and intercellular signaling interactions modulate these traits upon encapsulation.
Figure 4.
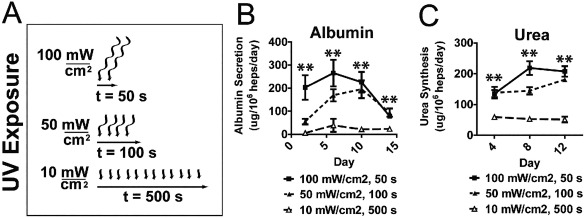
UV intensity and exposure duration (A) for material polymerization modulate albumin secretion (B) and urea synthesis (C) of encapsulated hepatocytes. (**p < 0.01, 5% PEGDDAAm, 10 mmol RGDS, 8 × 106 hepatocytes/mL).
Addition of liver endothelial cells improves engineered tissue longevity
“Angiocrine” signals provided by endothelial cells have been shown to be important for hepatocyte phenotype and function both in a liver regeneration34 setting as well as in in vitro engineered tissue model systems.17, 19, 35 We therefore tested whether further addition of immortalized liver endothelial cells (LECs) to hepatocyte cocultures upon encapsulation in MMP‐degradable PEGDAAm hydrogels altered hepatic function. Inclusion of LECs in tissues dramatically altered morphology of the tissue construct with time via the formation of a self‐supporting interconnected cellular network by 2 weeks in culture [Fig. 5(A), right]. Conversely, cells in hydrogels without LECs spread locally in the hydrogel over time in culture but remained dispersed throughout the hydrogel [Fig. 5(A)]. By 2 weeks in culture, hydrogels without LECs were completely degraded such that they could not be detected macroscopically in culture; cells in degraded hydrogels were presumably released into suspension in media and aspirated upon media changes [Fig. 5(B)]. Conversely, all tissues with LECs remained intact and produced albumin for at least 3 weeks in culture [Fig. 5(B,C)]. Immunohistochemistry confirmed that tissues encapsulated with hepatocyte co‐cultures and LECs maintained a population of cytokeratin‐positive hepatocytes at 3 weeks in culture [Fig. 5(D)]. Together, these results suggest that LEC‐mediated preservation of 3D tissue structure enabled maintenance of hepatic‐specific function for at least 3 weeks [Fig. 5(C)].
Figure 5.
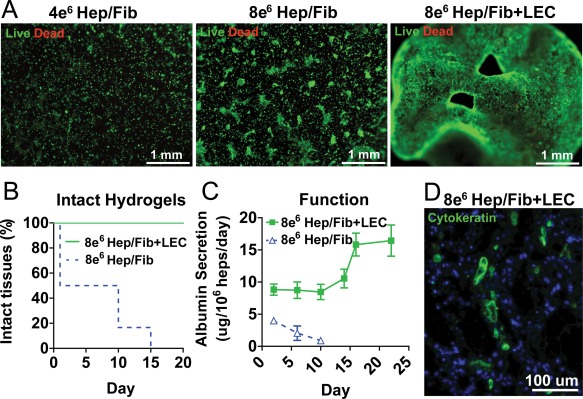
(A) Inclusion of LECs in hepatic hydrogels results in interconnected cellular network (green, calcein; red, ethidium homodimer, 5% PEGDAAm, 10 mmol RGDS. UV 10 mW cm−2 for 210 s, +LEC 6 × 106 heps/mL) and prolongs hydrogel lifetime (B) and hepatic tissue function (C). Hepatic hydrogels with LECs contain cytokeratin‐positive hepatocytes (green) after 3 weeks in culture (D). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Sustained hepatic function after implantation in rodents
We then investigated whether hepatocytes encapsulated in MMP‐degradable PEGDAAm hydrogels could be successfully engrafted into rodents (Fig. 6). Hepatocyte co‐cultures were transduced with a lentivirus in which luciferase is expressed under a modified albumin promoter17, 35 and encapsulated with LECs in MMP‐degradable PEGDAAm hydrogels. MMP‐degradable PEGDAAm hydrogels with or without encapsulated cells were inserted into the peritoneal space of immunodeficient mice. Individual mice were assayed over time for the production of bioluminescent signals. Mice bearing cell‐laden hydrogels exhibited detectable signals for at least 15 days after implantation, whereas mice grafted with cell‐free hydrogels did not generate any bioluminescence. These observations indicate that hepatocyte albumin promoter activity was maintained for over 2 weeks after implantation. Collectively, these findings indicate demonstrate that MMP‐degradable PEGDAAm hydrogels can be utilized as a biomaterial carrier for hepatocytes implanted in vivo.
Figure 6.
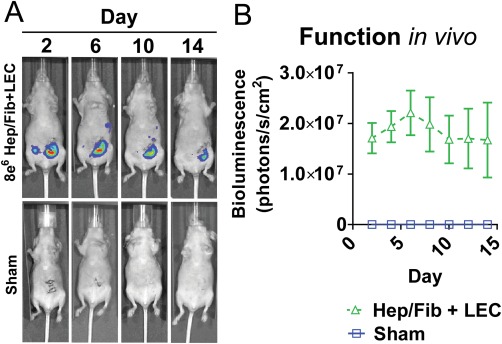
PEGDAAm‐based hepatic tissues survived and function after implantation in vivo to 2 weeks. (5% PEGDAAm, 10 mmol RGDS). UV 10 mW cm−2 for 210 s, 8 × 106 Hep/J2 +LEC 6 × 106 heps/mL in all animals w/cells. Sham animals had blank MMP‐degradable PEGDAAm hydrogels. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
In the work described here, we have constructed engineered liver tissue with precisely controlled chemical and biological parameters using a hydrolytically‐stable and MMP‐degradable PEG‐based hydrogel system. Screening of polymerization conditions, cellular density and cellular composition resulted in synthetic engineered hepatic tissue that exhibits robust hepatic function for several weeks following engraftment in rodents.
Our group and others have demonstrated that primary hepatocytes survive and function with culture on the surface of,35 within micromolded wells,36 or fully encapsulated within non‐degradable PEG‐based hydrogels.16, 17, 18, 19, 20 Our current work further demonstrates survival and function of primary hepatocytes after encapsulation in a degradable PEG‐based material system. It is known that hepatocytes are sensitive to the kinetics of photopolymerization,16 so it was not surprising that hepatocyte function varied with photopolymerization kinetics under constant light dosage.
The PEGDAAm material system used in these studies is an extension of our previous approaches, in which we created bioactive PEG‐based hydrogels using step‐growth polymerization via Michael‐type addition to create high MW bioactive macromers with the form acrylate–PEG–(peptide–PEG)n–acrylate.25 The PEG‐diacrylate‐based materials used in our previous studies were suitable for short timecourse (3 days) and low cell density angiogenic sprouting assays, but the presence of hydrolytically unstable ester bonds precluded applications in longer timecourse and higher cell density hepatic tissue engineering applications. Of particular note, it is known that serum‐containing media includes esterases which may structurally weaken PEGDA‐based or other hydrogel systems via non‐specific ester‐bond hydrolysis over time. In the studies described here, by reacting thiols on MMP‐sensitive peptides with the acrylamides in PEGDAAm, we abrogated non‐specific gel degradation and allowed for gels in which highly metabolically active cells—hepatocytes—could be encapsulated and allowed to remodel their extracellular microenvironment via MMP‐activity.
Importantly, the addition of both J2 stromal fibroblasts and LECs were essential for maximal function and longevity of hepatocytes encapsulated in PEGDAAm.37, 38 This finding is consistent with results reported in other systems, in which hepatic phenotypes can be at least partially rescued by providing supportive signals that may mimic aspects of the in vivo micoenvironment in vitro.19, 33 In particular, hepatic function was similarly enhanced upon encapsulation in non‐degradable PEG systems in the presence of microenvironmental factors such as non‐parenchymal cell types and bioactive moieties (e.g., RGD adhesion peptide, heparin).16, 17, 18 We postulate that augmented hepatic function we observe in the presence of non‐parenchymal cells is likely due to a combination of diverse cell‐cell contact, paracrine, and extracellular matrix interactions,19, 32, 33 such as via the release of “angiocrine factors” secreted by endothelial cells.34
Finally, we demonstrate that PEGDAAm‐based tissues containing hepatocytes, supportive stromal fibroblasts, and liver endothelial cells survive and function after ectopic implantation in the intraperitoneal space of immunocompromised mice. Similar to our own previous work in non‐degradable PEG‐based tissues, this work demonstrates that ectopic transplantation of hepatocytes is a viable option for the support of hepatocytes in vivo. Recent work by the Lagasse group, in which primary hepatocytes injected in the intraperitoneal space provided functional benefit in a model of the human liver disease tyrosinemia type I, suggests that such an ectopic approach may have therapeutic relevance.39 Such ectopic transplantation could be particularly useful for replacing lost hepatic function in patients with chronic hepatic damage due to cirrhosis and fibrosis, which limit the engraftment of hepatocytes in the host liver. These systems may ultimately be applied as a “bridge” or replacement for whole organ transplantation.
CONCLUSIONS
In the work described here, we have constructed engineered liver tissue composed of primary hepatocytes and supporting non‐parenchymal cells encapsulated in a hydrolytically stable and MMP‐degradable PEGDAAm hydrogel system. We found that optimization of polymerization conditions, cellular density, and multicellular composition resulted in synthetic engineered hepatic tissue that exhibits robust hepatic function for several weeks following engraftment in rodents. This work may provide a new avenue for utilizing synthetic degradable materials for organ failure applications.
ACKNOWLEDGMENTS
This work was supported with grants from the NIH (R01EB008396, R01DK85713, and EB00262) and Skolkovo Institute of Science and Technology (022423‐003). The authors would like to thank Heather Fleming and Alice Chen for helpful thoughts during manuscript preparation and Lia Ingaharro for hepatocyte isolations. S.N.B. is a Howard Hughes Investigator.
How to cite this article: Stevens KR, Miller JS, Blakely BL, Chen CS, Bhatia SN. 2015. Degradable hydrogels derived from PEG‐diacrylamide for hepatic tissue engineering. J Biomed Mater Res Part A 2015:103A:3331–3338.
REFERENCES
- 1. Lee SW, Wang X, Chowdhury NR, Roy‐Chowdhury J. Hepatocyte transplantation: State of the art and strategies for overcoming existing hurdles. Ann Hepatol 2004;3:48–53. [PubMed] [Google Scholar]
- 2. Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, Najarian JS. Hepatocellular transplantation for metabolic deficiencies: Decrease of plasms bilirubin in gunn rats. Science 1976;192:892–894. [DOI] [PubMed] [Google Scholar]
- 3. Bernardi M, Tacconi C, Somaroli M, Gasbarrini G, Mazziotti A. Hyperammonemic, ammonia‐independent coma in experimental acute liver failure induced in the pig. Gastroenterology 1981;81:191–192. [PubMed] [Google Scholar]
- 4. Demetriou AA, Reisner A, Sanchez J, Levenson SM, Moscioni AD, Chowdhury JR. Transplantation of microcarrier‐attached hepatocytes into 90% partially hepatectomized rats. Hepatology 1988;8:1006–1009. [DOI] [PubMed] [Google Scholar]
- 5. Kobayashi N, Fujiwara T, Westerman KA, Inoue Y, Sakaguchi M, Noguchi H, Miyazaki M, Cai J, Tanaka N, Fox IJ, Leboulch P. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science 2000;287:1258–1262. [DOI] [PubMed] [Google Scholar]
- 6. Bhatia SN, Underhill GH, Zaret KS, Fox IJ. Cell and tissue engineering for liver disease. Sci Transl Med 2014;6:245sr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta S, Gorla GR, Irani AN. Hepatocyte transplantation: Emerging insights into mechanisms of liver repopulation and their relevance to potential therapies. J Hepatol 1999;30:162–170. [DOI] [PubMed] [Google Scholar]
- 8. Demetriou AA, Whiting JF, Feldman D, Levenson SM, Chowdhury NR, Moscioni AD, Kram M, Chowdhury JR. Replacement of liver function in rats by transplantation of microcarrier‐attached hepatocytes. Science 1986;233:1190–1192. [DOI] [PubMed] [Google Scholar]
- 9. Hamazaki K, Doi Y, Koide N. Microencapsulated multicellular spheroid of rat hepatocytes transplanted intraperitoneally after 90% hepatectomy. Hepatogastroenterology 2002;49:1514–1516. [PubMed] [Google Scholar]
- 10. Dixit V, Arthur M, Reinhardt R, Gitnick G. Improved function of microencapsulated hepatocytes in a hybrid bioartificial liver support system. Artif Organs 1992;16:336–341. [DOI] [PubMed] [Google Scholar]
- 11. Demetriou AA, Levenson SM, Novikoff PM, Novikoff AB, Chowdhury NR, Whiting J, Reisner A, Chowdhury JR. Survival, organization, and function of microcarrier‐attached hepatocytes transplanted in rats. Proc Natl Acad Sci USA 1986;83:7475–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hasirci V, Berthiaume F, Bondre SP, Gresser JD, Trantolo DJ, Toner M, Wise DL. Expression of liver‐specific functions by rat hepatocytes seeded in treated poly(lactic‐co‐glycolic) acid biodegradable foams. Tissue Eng 2001;7:385–394. [DOI] [PubMed] [Google Scholar]
- 13. Ranucci CS, Kumar A, Batra SP, Moghe PV. Control of hepatocyte function on collagen foams: Sizing matrix pores toward selective induction of 2‐D and 3‐D cellular morphogenesis. Biomaterials 2000;21:783–793. [DOI] [PubMed] [Google Scholar]
- 14. Dvir‐Ginzberg M, Gamlieli‐Bonshtein I, Agbaria R, Cohen S. Liver tissue engineering within alginate scaffolds: Effects of cell‐seeding density on hepatocyte viability, morphology, and function. Tissue Eng 2003;9:757–766. [DOI] [PubMed] [Google Scholar]
- 15. Mooney DJ, Park S, Kaufmann PM, Sano K, McNamara K, Vacanti JP, Langer R. Biodegradable sponges for hepatocyte transplantation. J Biomed Mater Res 1995;29:959–965. [DOI] [PubMed] [Google Scholar]
- 16. Underhill GH, Chen AA, Albrecht DR, Bhatia SN. Assessment of hepatocellular function within PEG hydrogels. Biomaterials 2007;28:256–270. [DOI] [PubMed] [Google Scholar]
- 17. Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci USA 2011;108:11842–11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim M, Lee JY, Jones CN, Revzin A, Tae G. Heparin‐based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials 2010;31:3596–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stevens KR, Ungrin MD, Schwartz RE, Ng S, Carvalho B, Christine KS, Chaturvedi RR, Li CY, Zandstra PW, Chen CS, Bhatia SN. InVERT molding for scalable control of tissue microarchitecture. Nat Commun 2013;4:1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li CY, Stevens KR, Schwartz RE, Alejandro BS, Huang JH, Bhatia SN. Micropatterned cell‐cell interactions enable functional encapsulation of primary hepatocytes in hydrogel microtissues. Tissue Eng A 2014;20:2200–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. West J, Hubbell J. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 1999;32:241–244. [Google Scholar]
- 22. Mann BK, Gobin AS, Tsai AT, Schmedlen RH, West JL. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: Synthetic ECM analogs for tissue engineering. Biomaterials 2001;22:3045–3051. [DOI] [PubMed] [Google Scholar]
- 23. Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end‐linked poly(ethylene glycol)‐co‐peptide hydrogels formed by Michael‐type addition. Biomacromolecules 2003;4:713–722. [DOI] [PubMed] [Google Scholar]
- 24. Aimetti AA, Machen AJ, Anseth KS. Poly(ethylene glycol) hydrogels formed by thiol‐ene photopolymerization for enzyme‐responsive protein delivery. Biomaterials 2009;30:6048–6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller JS, Shen CJ, Legant WR, Baranski JD, Blakely BL, Chen CS. Bioactive hydrogels made from step‐growth derived PEG‐peptide macromers. Biomaterials 2010;31:3736–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elbert DL, Hubbell JA. Conjugate addition reactions combined with free‐radical cross‐linking for the design of materials for tissue engineering. Biomacromolecules 2001;2:430–441. [DOI] [PubMed] [Google Scholar]
- 27. Legant WR, Miller JS, Blakely BL, Cohen DM, Genin GM, Chen CS. Measurement of mechanical tractions exerted by cells in three‐dimensional matrices. Nat Methods 2010;7:969–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dunn JC, Tompkins RG, Yarmush ML. Long‐term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog 1991;7:237–245. [DOI] [PubMed] [Google Scholar]
- 29. March S, Hui EE, Underhill GH, Khetani S, Bhatia SN. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology 2009;50:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol 1976;13:29–83. [DOI] [PubMed] [Google Scholar]
- 31. Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol 2008;26:120–126. [DOI] [PubMed] [Google Scholar]
- 32. Khetani SR, Szulgit G, Del Rio JA, Barlow C, Bhatia SN. Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling. Hepatology 2004;40:545–554. [DOI] [PubMed] [Google Scholar]
- 33. Hui EE, Bhatia SN. Micromechanical control of cell‐cell interactions. Proc Natl Acad Sci USA 2007;104:5722–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ding BS1, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, Sato TN, Rabbany SY, Rafii S. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 2010;468:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mehta G1, Williams CM, Alvarez L, Lesniewski M, Kamm RD, Griffith LG. Synergistic effects of tethered growth factors and adhesion ligands on DNA synthesis and function of primary hepatocytes cultured on soft synthetic hydrogels. Biomaterials 2010;31:4657–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Williams CM1, Mehta G, Peyton SR, Zeiger AS, Van Vliet KJ, Griffith LG. Autocrine‐controlled formation and function of tissue‐like aggregates by primary hepatocytes in micropatterned hydrogel arrays. Tissue Eng A 2011;17:1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Allen JW, Bhatia SN. Engineering liver therapies for the future. Tissue Eng 2002;8:725–737. [DOI] [PubMed] [Google Scholar]
- 38. Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: Hepatocytes cultured in a sandwich configuration. FASEB J 1996;10:1471–1484. [DOI] [PubMed] [Google Scholar]
- 39. Hoppo T, Komori J, Manohar R, Stolz DB, Lagasse E. Rescue of lethal hepatic failure by hepatized lymph nodes in mice. Gastroenterology 2011;140:656–666, e652. [DOI] [PMC free article] [PubMed] [Google Scholar]


